Abstract
Adaptation and acclimation of metabolism and development to environmental conditions at the site of rooting requires nonmobile plants to memorize information introduced by external signals. These act at various spatiotemporal levels of structure and function and ecophysiological performance. There are different types of memory, among which are priming memory, store/recall memory (STO/RCL), where both the storage and the recall function as well as their combination have ecophysiological significance, and epigenetic memory. Timing is important. Therefore, ultradian, circadian and annual rhythms are underlying memory functions, where the circadian clock may represent a prominent component. Memorization associated with adaptation and acclimation needs implementation of memory as backbone. A plethora of ecological impacts require memory, some of which will be exemplified and critically examined, namely, molecular aspects of membrane transport, fitness, photosynthesis, osmotic stress and salinity, pollution events and priming by volatile organic compounds and by vibrations. Memory is not an occasional episode but a fundamental property of general importance in the life of plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Memory is not a straightforward concept. In the basic meaning, the term “memory” applies to animals (especially higher animals and humans). It is defined as an “ability to retain and recall information, ideas, images and thoughts” (Sinclair et al. 1987), and it is based on the activity and interactions of neurones, especially in the central nervous system (Dudai 2004; Lesburguères et al. 2011). However, the meaning has now broadened in two ways: (1) it is employed not only to animals but also to practically any sort of living organism including plants (Thellier et al. 1982) and prokaryotes (Thellier and Lüttge 2013). (2) It is not necessarily based on neuronal activity so that one may speak of genetic and epigenetic memories in living beings, the memory of an instrument (such as a computer or a pocket calculator) or even the memory of anything involving processes with a hysteretic behaviour (see the chapter “Hysteresis” in Wikipedia, the free encyclopaedia). The semantic difficulty is that one single word, “memory”, stands for all these different aspects. To elude this difficulty, one might say “memory sensu stricto”, i.e. when routinely speaking, for instance, of human memory, and “memory sensu lato”, i.e. for memories of any kind within more recent contexts of accepted understanding. However, such terminology would be quite cumbersome, so that we shall rather continue to use “memory”, as everybody does, although staying aware of the many different meanings this one notion may have.
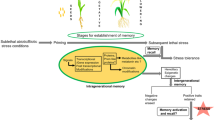
A tentative model of the network modules incorporated in the system of operations of memory is shown in Fig. 1. Its various features shall be revealed as we go along in this assay, where we aim at unravelling the role of memory in the autecological and synecological performance of plants. Autecological performance is determined by adaptation and/or acclimation of given species or individual plants to their environment, while synecological behaviour is characterized by the interactions of species or individuals among each other and with the environment at the community level. In two sections we shall first consider the different types of memory (Sect. 2) and relations to biorhythmicity and the biological clock (Sect. 3) with an ecological perspective in mind. Then, we shall assess concrete ecological functions which require memorization and, hence, implementation of memory and the biological clock as supporting grounds (Sect. 4). Eventually, we shall explore the ecophysiological potential of the priming and store/recall forms of plant memory (Sect. 5). Finally, we shall conclude considering plant memory in relation to that of other organisms as an essential means for persistence in the environment.
Tentative model of the network of modules incorporated in the system of operations of memory for ecophysiological performance of plants. Stimuli of environmental cues are received by molecular receptors, and their information is translated into signalling of various forms, such as electric, hydraulic, phytohormonal/chemical and small RNA (sRNA) signals and Ca2+ waves. These signals generate molecular effectors either directly or indirectly via the epigenetic memory or the biological clock. The effectors activate memory genes of priming and of store (STO) and recall (RCL) functions, where the STO and RCL boxes are independent of each other. With the operation of the activated priming genes, the instruction inherent in the original stimuli is effective directly. With the operation of the activated STO genes, the instruction is stored but becomes effective only via the operation of the activated RCL genes. The effective instruction leads to gene products of ecophysiological relevance
2 Types of Plant Memory
We will only briefly summarize those aspects of memory that are mainly studied in physics or engineering (computer’s memory and hysteresis). We shall develop in more detail the biological aspects with particular reference to plants (genetic and epigenetic memory, priming and store/recall forms of plant memory and the relations of memory to developmental phenological phases in plants).
2.1 Aspects of Memory Mainly Studied in Physics and Engineering
Briefly, “a computer’s memory is the part of the computer where information is stored, especially for a short time, before it is transferred to magnetic tapes or disks” (Sinclair et al. 1987). “Hysteresis is the dependence of the output of a system not only on its current input, but also on its history of past inputs; the dependence arises because the history affects the value of an internal state; to predict its future outputs, either its internal state or its history must be known; if a given input alternately increases and decreases, a typical mark of hysteresis is that the output forms a loop” (see the chapter “Hysteresis” in Wikipedia, the free encyclopaedia; for a possible application to plant systems, see, for instance, Sect. 4.2.4 or Desbiez et al. 1994).
2.2 Aspects of Memory Common to All Living Beings
2.2.1 Genetic Memory
Genomes reflect the history of organisms because the genotypes are selected in evolution. In a very broad and general sense, we may therefore think of this genetic information as being a kind of memory of past events that have affected evolutionary selection. In a similar vein we may think of the genomes of sexually produced organisms, being memory of mother and father having contributed their sets of chromosomes via the gametes. Reading the genetic information for transcription followed by translation and then regulating metabolic and physiological responses could be considered in recalling the genetic information. The processes of the control of reading genetic information will lead to more specific considerations of which we shall give examples later, for instance, the activation and inactivation of operons (Sect. 4.2.1). A dynamic process in reading the genetic information is epigenetics, which will lead us to more concrete memory functions in the next section.
2.2.2 Epigenetic Memory
Epigenetic modifications of the genome are induced by internal and external signals. They can be stored and affect gene expression beyond cell cycles and even generations. Therefore, we can speak of an “epigenetic memory” (Thellier and Lüttge 2013; Kinoshita and Seki 2014). Epigenetic modifications are currently the best understood molecular mechanism of memory.
Molecular epigenetics is a system of reading the genetic information of DNA where the structure and conformation properties of chromatin are modulated by acetylation and methylation, respectively, of DNA and nucleosomal histones. In the DNA methyl or acetyl groups are attached to the cytosine groups. In the histone proteins the lysine and arginine residues are post-translationally modified (Yaish et al. 2011), i.e. by acetylation/methylation (Grunstein 1997; Zhang and Reinberg 2001), ADP-ribosylation (Tanigawa et al. 1984), glycosylation (Cervantes-Laurean et al. 1996), phosphorylation (Lo et al. 2001) and ubiquitination (Sridhar et al. 2007). Acetylation allows access of regulator molecules of gene activation or deactivation due to the larger size of the acetyl group as compared to the smaller methyl group. Methylation leads to repression of gene transcription, and the genetic information is silenced (Chinnusamy and Zhu 2009). Under the perspective of ecological performance of plants, it is important that epigenetic modifications are triggered by environmental cues (Jablonka and Lamb 1989; Boyko and Kovalchuk 2008; Alvarez et al. 2010; Chen et al. 2010; Yaish et al. 2011; Kinoshita and Seki 2014). Both histone and chromatin methylation patterns are strongly modified by environmental stress (Molinier et al. 2006; Bond and Finnegan 2007; Chinnusamy and Zhu 2009; Adams 2010; Daxinger and Whitelaw 2010; Verhoeven et al. 2010).
The methylation status is not necessarily reset when the stress is relieved (Chinnusamy and Zhu 2009). In fact, it can even be transferred through cell divisions both mitotically and meiotically (Molinier et al. 2006). This means that the epigenetic memory is retained in somatic cell lines. With this it provides a more short-term epigenetic stress memory within a given organism. However, epigenetic memory can even last over generations. For a rather long-term trans-generational stress memory, it is remarkable that stress-induced methylation changes are not reset through the germ line and are mostly heritable, so that epigenetic information relative to stresses received by plants can be transferred through several subsequent generations (Jablonka and Lamb 1989; Bird 2002; Kakutani 2002; Molinier et al. 2006; Bond and Finnegan 2007; Saze 2008; Verhoeven et al. 2010). In brief, the epigenetic memory remains stable when stress is not continuous but occurs in episodes, and this can last for generations.
2.3 Memory Capacities in Plants
2.3.1 Plant Sensitivity to Stimuli and Types of Subsequent Response
Plants are sensitive to a variety of stimuli such as wind, rain, touch, drought, cold shock, heat shock, wounds inflicted by herbivorous animals, attack by fungi, bacteria or viruses and even electromagnetic irradiation in the approximate range 1–100 GHz (Tafforeau et al. 2002, 2004; Roux et al. 2006; Vian et al. 2006). There are basically two contrasting types of possible responses to environmental cues acting as signals or stimuli, namely, a direct immediate response and responses involving memory.
Usually, plants react almost immediately to a stimulus by generating a “calcium wave”, i.e. a transient invasion of the cytosol by calcium originating from Ca2+-rich internal and external pools (Knight et al. 1991; Trewavas 1999). This calcium wave triggers a chain of events, including the opening of ionic channels, the phosphorylation of existing proteins and changes in the genome expression (Dolmetsch et al. 1997; McAinsh and Hetherington 1998).
All of that eventually results in a final response that can be a modification of growth and/or metabolism and sometimes a macroscopic movement (Dionaea muscipula, Mimosa pudica). The response may be stereotyped and direct, i.e. independent of the previous history of the plant and involving no more delay than necessary for the intermediate events required to occur between the perception of the stimulus and the final response to this stimulus. The rapidity is advantageous for reacting to rare or unknown stimulations, especially those involving an attack by an herbivore or a pest.
However, if plants made such a direct, stereotyped response to each individual stimulus which they perceive, and if they responded with similar intensity to innocuous and harmful stimuli, erratic metabolic and growth behaviour would emerge, being unnecessarily costly in energy. Therefore, an apparent requirement exists for a mechanism that permits plants to adjust their response to the entirety of stimulations and their dynamics experienced in the past. This is achieved by means that functionally resemble animal memories, although the underlying mechanisms are very different (especially since plants neither have neurons nor anything comparable to a central nervous system).
At the beginning of the 1980s (see Thellier et al. 1982), it was discovered that plants possess memory capacities, which to some extent mimic our human “memory”. Since then, a number of publications have been devoted to the occurrence and characteristics of that memory (for reviews, see, e.g. Thellier et al. 2000, 2013; Trewavas 2003; Ripoll et al. 2009). It has also been recognized (Trewavas 2003) that two different kinds of plant memory can be distinguished, namely, “priming”, which resembles the animal “training” (Bailey and Chen 1983), and “store/recall (STO/RCL) memory” (resembling the animal “memorization/evocation”).
2.3.2 Priming Memory
In the priming memory, the first stimulus, or sequence of stimuli, changes the transduction of subsequent stimuli, thus tending to either diminish or enhance the intensity of the plant response (observations carried out at the level of the final response or as early as the generation of the calcium wave).
For instance, in Nicotiana plumbaginifolia seedlings, a wind stimulus causes cytosolic calcium to rapidly increase, but repeated wind stimuli within very short periods of time make the plant cells refractory to further calcium signalling for approximately 1 min (Knight et al. 1992). In Arabidopsis thaliana, cold pretreatments attenuate the increase of cytosolic calcium due to cold shock (Plieth et al. 1999). Again in Arabidopsis, a hyperosmotic-stress pretreatment increases the elevation of cytosolic calcium due to hyperosmosis (mimicking drought), while an oxidative-stress pretreatment reduces it (Knight et al. 1998).
2.3.3 Store/Recall Memory
In the STO/RCL memory, the perception of a stimulus is responsible for storage (STO) of information within the plant; then, that information may be recalled (RCL) at a later time. During the lapse of time between storage and recall (memorization time), the stored information remains latent, i.e. without any apparent effect on the plant behaviour. When an appropriate stimulus or change in internal or environmental conditions causes the RCL function to be switched from “off” to “on”, the plant is enabled to recall the stored information and to make it effective in the control of the its metabolism and growth.
Three experimental systems, which shall be termed here SR1 (Desbiez et al. 1983, 1987), SR2 (Desbiez et al. 1991) and SR3 (Verdus et al. 1997), have been mainly used in the basic original studies of STO/RCL memory (Table 1; for reviews, see, e.g. Thellier et al. 2000, 2013; Trewavas 2003; Ripoll et al. 2009). With system SR1, Bidens seedlings were stimulated by pricking one or both cotyledons, which caused the storage of “reduction of hypocotyl growth” information, acting as a kind of instruction governing the control of hypocotyl growth. However, it is only when the plants were grown on a very diluted medium that they were enabled to recall the stored information/instruction and let it take effect in reducing hypocotyl growth. (When the pricked and non-pricked plants were grown in a conventional nutrient solution, the growth of their hypocotyls was not significantly different). For brevity, see Table 1 for the description of the experiments with SR1 to SR3. These experiments were designed at the outset of memory investigations for testing and proving the very existence of memory under strictly controlled laboratory conditions. Before extrapolating from ecological observations to the involvement of memory functions in a framework of environmental conditions, the ground laying operation of STO/RCL functions had to be shown in readily reproducible experimental approaches. These experiments clearly revealed the sequence of events relevant for any kind of ecological responses as exemplified in Sect. 4, i.e. > external stimulus > process of information/instruction storage > state of information/instruction being stored > triggering induction to put stored information/instruction into action (Table 1). The main results obtained from the compilation of data yielded with SR1 to SR3 are as follows.
2.3.3.1 The Storage Function
With SR1, it has been observed that a signal migrates from the stimulated area (here the pricked cotyledons) to the reactive area (here the hypocotyl) where information storage finally occurs. The rate of signal migration is of the order of one to a few tenths of a millimetre per second (Desbiez et al. 1983). Electric depolarization signals in phloem cells are involved in signal migration, but the mechanisms in action are different from those in animal nerves. At SR3, the application of pharmaceutical agents blocking calcium movements, during and shortly after the occurrence of the calcium wave, prevents information storage (Verdus et al. 2007). This means that these agents have blocked information storage either directly or indirectly by blocking the migration of the signal from the stimulated to the reactive area. In any case, the information induced by the initial stimulus becomes firmly stored in the responding tissue after a few minutes at the most.
When a stimulus has been perceived, the shape, amplitude and duration of the calcium wave (Dolmetsch et al. 1997; McAinsh and Hetherington 1998; Knight et al. 1998) and the early and transient modifications of existing proteins or of genome expression (Tafforeau et al. 2006) are specific of the stimulus perceived. However, the memory of the stimulus is finally lost and what is memorized is mere instruction. More precisely, it is a sort of instruction, which addresses the final response that has to be performed in reaction to the specific stimulus (SR1, SR2 and SR3).
Comparing SR1 and SR2, it appears that the application of the same stimulus (pricking one of the two plant cotyledons) stores two different pieces of instruction in the hypocotyl and in the cotyledonary buds, i.e. percentage of reduction of hypocotyl growth and specification of bud dominance (measured by the percentage of dominant buds at the axil of the non-pricked cotyledon), respectively. There is an apparent discrepancy in the storage behaviour in SR1 and SR2 because the percentage of reduction of hypocotyl elongation in SR1 is quasi proportional to the number of pricks, whereas, in SR2, the percentage of dominant buds at the axil of the non-pricked cotyledon is independent of the number of pricks. It is likely that this discrepancy can be explained by assuming that, in SR2, the application of a single prick suffices to saturate the storage capacity of the system, and therefore delivering one or several pricks has exactly the same effect. The reason is that, when taking into account much weaker stimuli, such as the small gradients of temperature or light that inevitably exist in the culture rooms, the behaviour in SR2 is fairly similar to that in SR1. In brief, as long as there is no saturation effect, after a stimulus the intensity of the stored instruction depends on the intensity of the stimulus (Thellier 2015).
Once a first stimulus has been perceived and an instruction for a response has been stored accordingly, subsequent stimuli can modulate quantitatively (i.e. in its intensity) this programmed response (SR3). Though a direct experimental test is still lacking, it may be reasonably inferred from the preceding paragraph that the instruction stored after a first stimulus can also be modulated qualitatively as a consequence of the perception of subsequent stimuli. This would mean that the very nature of the information for performance of a response may be modified.
2.3.3.2 The Recall Function
The recall function can usually be switched “off/on” or “on/off” reversibly, thus enabling/disabling the plant to recall instruction stored after the perception of a stimulus. However, cases exist when recall can be blocked in status “on” or in that of “off”, thus always permitting or preventing, respectively, the plant to recall stored instruction. There is no universal way to enable/disable a plant to recall stored instruction: with the three systems studied, enabling/disabling plants to recall stored instruction was accomplished by (1) using a dilute/normal growth medium (SR1), (2) decapitating the seedlings at the onset/middle of daylight (SR2) or (3) imposing a transient Ca2+ depletion/excess (SR3). Stored instruction can be repetitively recalled (at least twice in SR2 and SR3). Recalling stored instruction, whether once or at several times, does not seem to alter the stored information. The functioning of the RCL box is apparently independent of the functioning of the STO box.
2.3.3.3 Hypothetical Mechanism of Functioning
It may be that various substances play a part in the memorization process in plants. Such substances are “memory metabolites” (Ueda and Nakamura 2006), molecules involved in the control of the cell cycle (Desbiez et al. 1998) or small bundles of messenger RNA termed “stress granules” (Alain Vian, personal communication, Davies et al. 2012). However, it is possible to account for the main facts observed by interpreting plant memory (especially the “store/recall” type) via an interaction, involving epigenetics, between a few genes (Thellier 2015). The perception of a stimulus would modify the histone and/or chromatin methylation patterns (see Sect. 2.2.2), thus unlocking a few locked genes (and/or locking a few unlocked genes): this is the storage function, and the genes involved are termed “STO genes”. However, the unlocked genes would remain silent, until being activated by an appropriate ligand. Other genes would be unlocked and activated (on perception of an appropriate stimulus, after an appropriate treatment and/or depending on the external conditions), and their products would be the activators of the unlocked STO genes: this is the recall function, and the genes involved are termed “RCL genes” (for details see Thellier 2015). Hence, only the unlocked and activated STO genes would be functional, thus permitting the corresponding metabolic pathways to function, while the metabolic pathways depending on the locked STO gene would remain non-functional. Thanks to this “store/recall memory”, the plant would be able to adjust its metabolism to the external conditions and stimuli.
In brief, a change of methylation/acetylation equilibrium means storage (STO) of stimulus-information. A changed access of transcription factors is modulating recall (RCL). These are mechanisms of the epigenetic memory (Sect. 2.2.2; Fig. 2 in Thellier and Lüttge 2013), which is one of the possible pathways of signal transduction in the priming and in the STO/RCL memory (Fig. 1; Fig. 3 in Thellier and Lüttge 2013; Hütt et al. 2015; Thellier 2015). Moreover, it is likely that “priming memory” can be interpreted using a similar conceptual model of functioning (Thellier 2015).
2.3.4 Memory and Developmental Phenological Phases
In rhythmic phenomena which normally are oscillations, a phase is a rhythmically occurring specific point or state be it in developmental cycles or any other shorter type of oscillation. Phenology relates developmental phases of plants to the times when they are expressed. Stress treatments in particular phenological phases during earlier stages of development can become effective at later stages or phases with transition periods in between such stages. This means that development is not simply a cumulative expression of genetically preprogrammed events (Amzallag 2002, 2005). Complex links exist between development and adaptation. Evidently memory is involved. An example is the memory-regulated meristem formation in flax seedlings particularly active during April through June (Verdus et al. 1997; see also section “Annual Fitness”). A fascinating challenge is posed for a thorough assessment of the role of memory in development as modified by the impact of environmental signals. Clearly the experience of stress in an early developmental phenological phase is stored. The signal for recalling it is the transition into a later developmental phenological phase. The phenomenon has been particularly studied with NaCl salinity as the stress signal and is of eminent ecological significance (Sect. 4.2.5).
3 Rhythmicity and Memory
3.1 Ultradian, Circadian and Annual Rhythmicity
Rhythms of plants as of other organisms including man in time can cover a vast range of period lengths. Basically we define ultradian rhythmicity having period lengths shorter than the 24 h of the day, diurnal rhythmicity with the period length of a day and infradian rhythmicity with period lengths longer than a day. When diurnal rhythms run endogenously, i.e. under constant conditions independent of external rhythms of environmental parameters, they normally do not have an exact 24 h period length but just a little shorter or longer than that. Therefore, these rhythms are called circadian rhythms (from Latin circa and dies = day).
In the SR2 example of pricking cotyledons of Bidens in Table 1 and looking at specific bud dominance, an inherent ultradian rhythmicity can be observed. When a plant is subjected to two successive stimuli (one of which is dissymmetrical, consisting of pricking only one of the two cotyledons), the RCL function exhibits a damped oscillation “on/off” according to whether the delay between the two stimuli is close to 1 h or 8.5 h or larger than 14 h (RCL “on”) or close to 3 h or 12 h (RCL “off”; Desbiez et al. 1991; and see Hütt et al. 2015 for a theoretical approach). Again with SR2 under the experimental conditions used, the RCL function is “on” or “off” according to whether plant decapitation has been carried out in the morning or in the middle of the day (Desbiez et al. 1986, 1991; Thellier and Lüttge 2013). Finally, with SR3 (Verdus et al. 1997), when using plants all subjected to transient Ca2+ depletion, a very significant increase of the production of meristems was observed to take place in the period of April to June whether these plants were stimulated or non-stimulated. However, the number of meristems produced remained at least 5–10 times larger in the stimulated than in the non-stimulated plants. By contrast, when using plants nonsubjected to transient Ca2+ depletion, the number of meristems produced was always close to zero, whether the plants were stimulated or not and whatever the period of the year. From all these data, it may be inferred that the RCL function is linked (1) with an ultradian rhythm of the plant, reset by the dissymmetric stimulus, (2) with a circadian rhythm and (3) with an infradian annual rhythm (Fig. 2).
Schematic overview of OFF and ON responses of RCL in SR2 and SR3 experiments of Table 1. Relations (a) to short ultradian periods, (b) to the time of the day and (c) to the months of the year. Experiments are (a) SR2 and pricking stimuli, (b) SR2 and decapitation and (c) SR3 and Ca2+ depletion
3.2 Circadian Clock and Memory
Memory is related to functions of timing. Therefore, it should be expected that the biological clock is part of mechanisms of memory. What are the relations between clocks and memory? A clock allows measuring the flow of time. However, as such this has nothing to do with memory. However, a clock becomes part of the structure and function of memory if it contains specific points set at a certain time at which recall functions are alerted. A familiar example of such set points is an alarm clock set on a specific point in time. It is a reminder because it causes us to remember. In the biological circadian clocks reflecting the natural day–night rhythms with period lengths close to 24 h when running free under constant environmental conditions (Lüttge 2003), genes are involved that label set points. Among the master genes of the clock CIRCADIAN CLOCK ASSOCIATED (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) are expressed in the morning (morning genes) and TIMING OF CHLOROPHYLL a/b BINDING (TOC1) in the evening (evening genes). There are further morning and evening elements functioning as transcription factors (e.g. Kikis et al. 2005; Harmer and Kay 2005; McClung 2006; Nakamichi 2011). Downstream of these genes, a vast number of other genes are controlled by the clock, the so-called clock-controlled genes (CCGs). A plethora of plant functions are under the regime of the clock including complex processes such as growth (Farré 2012). Thus, there is a complex machinery of set points determining phases in the rhythmic oscillations of the clock.
The set points are affected by storage in the memory. Setting the clock is a result of entrainment (Dixon et al. 2014) by environmental ecologically relevant factors. The most obvious example is the exact operation of clock-dependent functions in the entrainment by the natural 24 h rhythm of the day. When the phases of external environmental rhythms change, new entrainment will change the set points. The recall function of the STO/RCL memory breaking the symmetry of bud growth after stimulation of cotyledons is dependent on diurnal timing (Table 1).
The molecular basis has to be assessed at the level of genes involved in the phase relations of resetting the clock. Genes giving phase information (Michael and McClung 2002) and phase mutants (Onai et al. 2004) have been identified. In Arabidopsis an OUT OF PHASE 1 gene has been characterized (Salomé et al. 2002). Phase variations in populations are studied (Darrah et al. 2006). However, much more work is needed, precisely addressing the links between memory, clock and environmental responses at the molecular level.
The most relevant signals eliciting phase shifting, entrainment and resetting the circadian clock in plants are light, temperature and for rhythms of photosynthesis also CO2 (Lüttge 2003). The responses to the signals in resetting are dependent on the phase of the rhythms, i.e. it matters at which time during oscillations the phase shifting signals are applied. This means that there is the so-called gating, i.e. that the input pathways to the clock are not constantly open over the entire 24-hour period of the day for the reception of the environmental signals (Edmunds and Tamponnet 1990; Rikin 1991; Johnson 1992; Millar and Kay 1996; Millar 1999; McWatters et al. 2000; Covington et al. 2001). The predominant light signals are blue and red light with cryptochrome and phytochrome, respectively, as their photoreceptors (see Table 2 in Lüttge 2003 with references; Devlin 2002; Wenden et al. 2011) and green light in Chlamydomonas reinhardtii (Forbes-Stovall et al. 2014). Marking new set points for the memory by clock resetting under the influence of these environmental control parameters demonstrates close relations between clock and memory in ecophysiological performance.
4 Ecophysiological Functions That Require Memory and Clock
4.1 Adaptation, Acclimation and Memory Functions
In conditioning for ecological and ecophysiological performance of plants, we distinguish adaptation and acclimation (Wilhelm and Wirth 2015). Adaptation is a long-term process. It builds up during evolution and is secured in the form of genetically stored information. Acclimation is based on expression of present instruction and modulation of phenotype via transcription, translation and the control by metabolites. This requires induction by perception of signals. If we accept that acclimation in general includes memory, of course, this leads us to consider memory as a fundamental quality in ecology. Such a general relation implies that there will be a plethora of examples for memory in the ecological performance of plants.
Induction can result in immediate responses by direct activation. However, induction can also involve the priming and the STO/RCL memory. This evidently is a matter of temporal dynamics. An example illustrating this is given by responses of defence. They can be activated directly upon attack. Then, obviously no memory functions are involved. Conversely, activation of defence can follow induction and priming indirectly after a certain lapse of time when an attack of predators, herbivores or parasites is a later event, but plants appear prepared or acclimatized to it (Conrath et al. 2001; Conrath 2009, 2011). An example is the cell-content-feeder Tupiocoris notatus, which by its feeding on tobacco plants elicits greater mortality of attacking hornworm (Manduca sexta) (Kessler and Baldwin 2004; Voelckel and Baldwin 2004; Gális et al. 2009). Similarly, chronically enhanced tropospheric ozone impact can prime against intermittent pathogenic interference, delaying or reducing infestation under controlled and field conditions in woody plants, irrespective of ontogenetic stage (Bahnweg et al. 2005; Luedemann et al. 2005, 2009; Olbrich et al. 2010), as both kinds of stress act through oxidant release (Matyssek and Sandermann 2003; Matyssek et al. 2008). In such context authors have also spoken of “immunity” and “vaccination” as a certain kind of memory.
4.2 Examples of Ecophysiological Performance Requiring Memory and Clock
In this section we address some specific ecophysiological examples at various scales beginning with the molecular level. Circadian clock genes act in stress responses (Kant et al. 2008). The epigenetic memory (Sect. 2.2.2) is explicitly involved in many ecophysiological functions. While we have seen that memory and clock can be intimately related (Sect. 3.2), it is noteworthy also to outline their participation in conveying fitness. Fitness is much more than Darwinian reproductive success. Among many aspects of plant performance, it also requires competitiveness given by growth (see zu Castell et al. 2016), where timing is essential on the levels of both diurnal and annual entrainment. Furthermore, other selected phenomena of memory functions in ecological performance will be addressed.
4.2.1 Examples for the Molecular Level: Induction of Mechanisms of Membrane Transport
Transport across membranes is of great ecophysiological importance, e.g. for the acquisition of substrates. A well-known example worked out at the molecular level is the regulator-operator model of François Jacob and Jacques Monod (Nobel Prize 1965; see textbooks, e.g. Lüttge et al. 2010). When cells of the bacterium Escherichia coli are grown in the absence of β-galactosides, e.g. lactose, a capacity to use these as substrate for growth is not expressed. When lactose is added, a membrane transporter lactose-permease and a β-galactosidase that splits the lactose in its hexose moieties glucose and galactose for further metabolism are induced. This occurs because lactose inactivates a repressor of the operator blocking the transcription of the genes of the operon coding the permease and the β-galactosidase. When the substrate lactose is removed, the cells will remember that they can metabolize lactose for a certain time as seen when the substrate is added again. The repressor is coded by a regulator gene. In the absence of lactose after some time, this gene will lead again to the production of active repressor, so that the previous experience of lactose will be forgotten. Hence, even prokaryotes have memory. In the eukaryotic cells of Chlorella vulgaris, a memory of glucose uptake was demonstrated (Tanner 1969; Tanner et al. 1970). The capacity for uptake is not expressed in the absence of glucose. When glucose was added to the medium, an uptake was induced within 20 min. When glucose was subsequently removed, 10 h later glucose-uptake capacity was still active. However, the memory did not last for much longer. After 13 h glucose-uptake capacity had been forgotten and needed to be induced again.
Both examples illustrate a molecular memory important for acclimation to the use of substrates available in the environment. Expression of products of transcription (mRNA), translation (proteins, enzymes) and regulatory metabolites constitutes store functions of memory. As long as the respective products are present and active, the events which led to their production will be remembered. When turnover results in their disappearance, the functions of induction, priming and storage will be lost with them.
4.2.2 Examples of Epigenetic Changes in Response to Environmental Cues and Their Inheritance
Epigenetic modifications of methylation patterns of histone and chromatin and, hence, the epigenetic memory have been shown to be involved in responses to stresses by salt (Wang et al. 2010), drought (Bruce et al. 2007; Baek et al. 2011; Ding et al. 2012; Kinoshita and Seki 2014), heat (Kinoshita and Seki 2014; Li et al. 2014), nutritional limitation, e.g. nitrogen deficiency (Kou et al. 2011), as well as herbivores and pathogens each inducing biochemical defences (Gális et al. 2009; Verhoeven et al. 2010), and virus infection (Kathiria et al. 2010).
The regulation of methylation patterns following stress reception involves chemical signals such as by phytohormones, electrical signals and calcium waves (Trewavas 2003; Thellier et al. 2013). These signals may transcribe into particular RNA signals by stress-induced expression of microRNAs, for example, under salinity, drought, low relative air humidity, cold and herbivore stress (Matzke et al. 2001, 2007; Sunkar and Zhu 2004; Gális et al. 2009; Shen et al. 2010; Yaish et al. 2011; Kinoshita and Seki 2014). Small interfering RNAs (siRNAs) of a length of 24–26 nucleotides direct DNA methylation and histone modifications (Richards 2006; Zhang et al. 2006; Bond and Finnegan 2007; Saze 2008; Zhang 2008; Chinnusamy and Zhu 2009). Small RNAs are mobile in the symplast via plasmodesmata and in the phloem. They can be transmitted within plants and function as systemic signals produced by stress (Saze 2008). Rasmann et al. (2012) demonstrated that Arabidopsis and tomato plants that experienced herbivory were more resistant to subsequent attack in the next generation. The induction of the defence process is dependent on the phytohormone jasmonic acid and the biogenesis of siRNA priming progeny plants for enhanced resistance.
4.2.3 Fitness
4.2.3.1 Diurnal Fitness
Logical common sense takes endogenous circadian rhythmicity as being essential for fitness because it provides preparedness or alertness for regularly changing conditions in the day–night rhythm. Some studies support this argument. Plants take advantage from circadian control of photosynthesis and physiological performance in general (Dodd et al. 2005; Hotta et al. 2007; Yerushalmi and Green 2009). The advantage of fitness to possess the suitable endogenous period length of rhythmicity matching with external rhythmicity was suggested by some work using period mutants showing entrainment of competitive fitness. Golden and collaborators obtained mutants of the cyanobacterium Synechococcus elongatus PCC7942 having different circadian periods of their endogenous clocks. They grew them in coculture under different external light-dark rhythms. The mutants having the correct endogenous period, i.e. closest to the imposed external light-dark rhythms, outcompeted the others during growth in the cultures. This competitive advantage disappeared in constant environments where the selective pressure of external light-dark rhythms was removed (Ouyang et al. 1998; Johnson and Golden 1999; Woelfle et al. 2004). Yerushalmi et al. (2011) crossed Arabidopsis thaliana mutants with different circadian period lengths and studied the F2 and F3 generations which they subjected to the selective pressure of altered external light-dark-cycle periods. Endogenous circadian rhythms that resonated with the environmental ones were positively selected. Nevertheless, a match of internal circadian period with external rhythmicity does not appear to be sufficient for guaranteeing positive clock effects on growth and competitiveness. The relationships are much more complex, which results from an interplay between the clock and metabolism. Metabolites can affect resetting of the clock where especially sucrose plays a dual role as signal and metabolite. In a feedback loop the circadian clock controls metabolism and is controlled by metabolites (Müller et al. 2014).
4.2.3.2 Annual Fitness
At latitudes north and south from the equator, the duration of the light period (or photoperiod) during the 24 h day changes over the year; the phases of the photoperiod change between short and long days or long and short nights. Changing entrainment in response of the transition between short days and long days allows flexibility of responses to phases (Dixon et al. 2014). For obtaining annual fitness plant growth and development, frost hardiness, flowering and seed production are subject to regulation by the photoperiod. Phase adjustment of the biological clock is an essential mechanism in photoperiod perception, where phytochrome acts as the photoreceptor (Frankhauser and Staiger 2002; Roden et al. 2002; Love et al. 2004; Ogudi et al. 2004; Fujiwara et al. 2008; Lüttge and Hertel 2009; Niwa et al. 2009; Ibáñez et al. 2010). The overwhelming evidence for the absolute necessity of the biological clock for plant fitness is provided by a huge volume of literature on photoperiodism and phenology. In experiments on epidermal meristem induction in flax seedlings, it was shown that the STO/RCL memory is involved. A stimulus induces the storage of meristem-production information (STO function), and a transient depletion of calcium enables the seedlings to recall stored information and let it take effect in the promotion of meristem production (RCL function). This is subject to seasonal modulations. The memory-controlled meristem formation is particularly active in April to June (Verdus et al. 1997; Sect. 3.1; Fig. 2), suggesting annual rhythmicity to be involved and ecologically relevant for growth and competitiveness. Hence, overall we can conclude that seasonal phenological memory is stored in the clock.
4.2.4 Photosynthesis
Photosynthesis is the foremost ecophysiological function of plants under the influence of primarily the impact of photosynthetically active radiation (PAR) and secondarily a broad array of almost all other prevailing environmental cues. In the context of memory in ecology, it is worthwhile to explore the involvement of memory functions in the ecophysiology of photosynthesis. Photosynthetic memory, as far as we can see, has not been addressed in the literature, but we can refer to some phenomena in which priming or storage and recall of information must obviously be involved.
In general activation–deactivation equilibria of metabolic activities, e.g. of enzymes, may imply memorization. This is the case if there is turnover with on and off states, respectively, being stable for some time in the absence of activation–deactivation elicitors (see also examples of membrane transporters in Sect. 4.2.1). In photosynthesis ribulose-bisphosphate carboxylase/oxygenase (RUBISCO) may be an interesting example for exploration in memory studies. The enzyme is active in the form of RUBISCO-carbamate-Mg2+ and needs carbamylation by binding of CO2 plus Mg2+ as catalysed by RUBISCO activase (Buchanan et al. 2000; Portis 2003).
When we take a photosynthetically active plant sample from darkness and subject it to gradually increasing PAR, we can record hyperbolic light saturation curves of photosynthesis saturating at a certain level of high PAR. When we then decrease PAR again, we may observe hysteresis where the rates of photosynthesis are lower during the descent of PAR than at any given PAR during the ascent. The plant memorizes that it has been at these PARs before but responds in a different way. The reason is that it has become subject to photoinhibition at high PAR. This elicited protective molecular changes within the photosynthetic apparatus (Osmond and Grace 1995), which may be stable for some time at low PAR and in darkness reminding to the high PAR experienced before.
Photoinhibition can be acute, i.e. reversible within short periods of time up to the length of a nocturnal dark period, or chronic, i.e. irreversible or only reversible after long periods of repair. Chronic photoinhibition at high irradiance is due to photodestruction. However, not always photoinhibitory reactions are right out destructive. By contrast, there is a cascade of mechanisms leading to acute photoinhibition but functioning in protection of the photosynthetic apparatus under high irradiance (Figs. 10–14 in Lüttge et al. 2010). Some of the photoprotective mechanisms are based on conformational changes of the photosynthetic apparatus, providing acclimation which is subject to turnover. Thus, acute photoinhibition appears as an instructive case for being viewed under the perspective of memory, demanding for further unravelling priming and STO/RCL functions:
-
Spill over of excitation from photosystem II (PSII) to photosystem I (PSI) is a protective mechanism based on the reversible separation of the light-harvesting complex LHII from PSII and its transfer from the grana thylakoids to the stroma thylakoids towards PSI. The reaction is mediated by a kinase phosphorylating LHII. Reversibility and turnover are given by dephosphorylation catalysed by a phosphatase (Jennings et al. 1986).
-
Another mechanism with turnover is aggregation/dissociation of the LHII complex. This is based on the binding of the xanthophyll zeaxanthin replacing lutein, under involvement of the thylakoid protein PsbS. Conformational changes modify the structure of LHII so that it switches from a light harvesting to a heat dissipation state dispersing harmful excess of excitation energy (Bilger and Björkman 1994; Horton et al. 1994, 1996; Gilmore and Yamasaki 1998; Gilmore et al. 1996, 1998; Gilmore 1997; Gilmore and Govindjee 1999; Holt et al. 2005; Horton and Ruban 2005; Goss and Lepetit 2015).
-
The D1 protein of LHCII is involved in transferring excitation to plastoquinone. The protein is damaged and destroyed under harmfully high irradiance. The repair requires protein synthesis. The protein is under continuous turnover, although only at low irradiance destruction and repair are balanced. At high irradiance destruction exceeds repair (Critchley and Russell 1994; Tyystjärvi and Aro 1996; He and Chow 2003).
A conspicuous example of memory in photosynthesis is the acclimation to light flecks on the floor of forests. Under the trees the solar irradiance reaching the forest floor is only a few percent of the irradiance at the upper canopy. However, light flecks occur when movements of leaves in the wind or the changing angle of irradiance allow direct light penetration through gaps in the canopy cover for intermittent periods of time. In tropical rain forests, light flecks may provide up to 80 % of the total irradiation reaching the forest floor, and their intensity varies between 10 % and 70 % of that of full sunlight (Lüttge 2008). Ecologically they are highly important for photosynthesis of the forest floor vegetation. When a sudden light fleck arrives after plants were at low-background irradiance, the photosynthetic apparatus must be activated and stomata must open for CO2 uptake. Excitation of the photosynthetic apparatus is immediate, and activation of the electron transport reactions occurs within seconds to minutes. However, activations of stomatal opening for CO2 uptake, of the reactions of CO2 assimilation through RUBISCO and of Calvin-cycle enzymes, and the filling of pools of intermediates are sluggish with time constants of 10–30 min (Sassenrath-Cole and Pearcy 1992). The advantage of the slower induction processes is that they also are subject to slower decay, and this is the mechanism of memory in this case. The use of the energy from light flecks by photosynthesis accelerates with time when short light flecks alternate with low-background irradiance. Conditioned or induced leaves have considerably higher light use efficiency than non-induced ones (Pearcy et al. 1985; Sassenrath-Cole and Pearcy 1992; Valladares et al. 1997). Such memorizing of previous light flecks under the dynamic light environment on the forest floor is essential for the persistence of plants under closed canopies.
4.2.5 Tolerance of Osmotic Stress and Salinity
The clock and memory have been shown to be involved in plant responses to osmotic stress and salinity. Osmotic stress at the level of barley roots up-regulated expression of clock genes which control the expression of stress response genes (Habte et al. 2014). When young seedlings of different species, such as tomato and Sorghum bicolor, are subjected to sublethal salinity, stress response will be modulated during subsequent phenophases in development. Amzallag and coworkers (Amzallag et al. 1993, Amzallag 2005) found that in S. bicolor, there was a developmental window during the 5th and the 10th day following germination. After being treated during this particular period with mild salinity of 150 mM NaCl, the plants remembered this at later stages by proving to be resistant to 300 mM NaCl. This also changed other aspects of development, e.g. perturbing reproductive development. Signal and response occurring during a critical period may be adaptive for some aspects of development and disturbing for others.
4.2.6 Memory of Pollution Events
Some perennial plants like conifers have been shown to memorize events of pollution such as the effects of acid rain and ozone on photosynthesis. When seedlings of loblolly pine (Pinus taeda L.) were exposed to such stress, their ozone memory was expressed in the following season before they experienced any further stress (Sasek et al. 1991). Similar memorizing of ozone exposure was recorded in Pinus sylvestris (L.) and Picea abies ((L.) Karst.). The new flush appearing during spring after ozone exposure in the previous growing season showed visible stress symptoms including premature shedding of needles (Langebartels et al. 1998).
4.2.7 Signalling by Volatile Organic Compounds
Signalling triggers induction. Both are elements in the functioning of memory. Among the signals eliciting induction are volatile organic compounds (VOCs) of plants. There is a large variety of such compounds, including gaseous phytohormones (pheromones) like ethylene and methyl jasmonate, as recently surveyed in a special issue of “Plant, Cell and Environment” (Loreto et al. 2014). VOCs have signalling functions between different plant species, between individuals of given species and even within given individual plants avoiding vascular constraints of signal transport (Conrath 2009; Gols 2014; Karban et al. 2014). VOC signals play a prominent role in plant defence. Herbivore-triggered plant VOCs lead to induced defences (Kessler and Baldwin 2002; Conrath 2009; Dicke 2009; Pierik et al. 2014). Inducible defences are of eminent relevance in ecological contexts. In cases of induction of defence genes and “increased accumulation, and/or posttranslational modification of inactive cellular signalling proteins”, a molecular basis of memory in ecology becomes accessible (Conrath 2009). “Dormant signalling proteins” thus are memory molecules.
4.2.8 Signalling by Vibrations
The VOC systems of long-distance signalling pathways can be complemented by other signals, e.g. vibrations caused by caterpillar feeding. In Arabidopsis thaliana such signalling induced the production of increased levels of anti-herbivore compounds such as glucosinolate and anthocyanin when subsequently attacked by caterpillars. The plants can even distinguish between different specific vibration signals and discriminate between the chewing vibrations and wind or insect song (Appel and Cocroft 2014).
5 Ecophysiological Potential of Plant Priming and Store/Recall Memory
5.1 Potential of the “Priming” Form of Plant Memory
Plants are subject to many signals in their environment that occur repeatedly. Some of these signals are relatively innocuous, and reactions to them would be quite unnecessary and detrimental as wasting resources and energy. Thus, an ecological memory function is to remember irrelevant signals for filtering them out. Priming memory is suited to that since the repetition of a stimulus can change the transduction of subsequent stimuli of the same type in a way tending to diminish the intensity of the plant response (see the examples of wind stimulus and cold shock in Sect. 2.3.2). By analogy with the animal case, such a plant behaviour may be termed “familiarization” or “habituation”. As an example, the leaf-folding behaviour of Mimosa pudica elicited by the mechanic signals from trampling herbivores is thought to be a defensive reflex of the plants to avoid being seen and readily exposed to the herbivores. However, it is costly because in the folded state, photosynthesis is drastically reduced. Thus, there is a trade-off between protection and productivity at low and limiting photosynthetically active radiation (PAR). At high PAR this may be less inflictive than at low PAR. To test this, Gagliano et al. (2014) compared the response of plants kept under low- and high-PAR environments, respectively. They found that the low-PAR plants learned faster to ignore the stimulus and retained the memory of this longer, i.e. for up to a month when undisturbed, than the high-PAR plants. For the low-PAR plants, the response to the stimulus leading to a reduction in photosynthesis is more severely disadvantageous and non-adaptive than for the high-PAR plants.
Conversely, memory is important for remembering grave signals to react more violently to them (Lodish et al. 2000). Again, priming memory is suited since the repetition of a stimulus can change the transduction of subsequent stimuli of the same type in a way tending to increase the intensity of the plant response. See the example with Arabidopsis, in which hyperosmotic-stress pretreatment amplifies the increase of cytosolic calcium due to hyperosmosis (Sect. 2.3.2). By analogy with the animal case, such a plant behaviour may be termed “sensitization”. Many examples come from priming in defence physiology (Conrath 2009; Pastor et al. 2013) where the signals are mechanical and chemical injury associated with herbivory and phytopathology (Baluška and Ninkovic 2010; Bruce 2010; Heil 2010; van Hulten et al. 2010). Defence priming involves enhancement of molecular mechanisms having many analogies with immunology (Conrath 2011; Rasmann et al. 2012).
Signals can also become grave when the repetition of stimuli changes the response to other types of stimuli. The intensity of a reaction to new types of stimuli can be altered by previous stimuli of another nature. An example is when an oxidative-stress pretreatment reduces the elevation of cytosolic Ca2+ due to hyperosmosis (see Sect. 2.3.2).
Thus, the ecophysiological advantage for a plant to possess priming memory is evident. When such kind of memory is involved, the response is rapid, similar to a direct response (see Sect. 2.3.1), although negative or positive modulation is possible. Familiarization-like effects permit the plant to ignore harmless stimuli and thus to economize the cost of elaborating a full defence response to a non-dangerous stimulation. Conversely, sensitization-like effects can produce increasingly rapid and violent responses to harmful stimuli. More sophisticated effects occur when the perception of a first stimulus modulates the intensity of the response to a subsequent stimulus of different nature.
5.2 Potential of the “Store/Recall” Memory Functions
The ecophysiological advantage for a plant to possess store/recall memory lays in the individual properties of the two functions STO and RCL and their combined effect.
5.2.1 Ecophysiological Significance of the Storage Function
Concerning the STO box, two facts are of primary importance: (1) that what is stored after the perception of a first stimulus is a sort of instruction for the response to that stimulus and (2) that subsequent stimuli will modulate the intensity, and perhaps also the nature, of the programmed response. The STO/RCL memory can thus progressively elaborate an integrated, updated programme of response to the ensemble of stimuli that the plant has perceived during the preceding course of time. Such integration will be much more efficient than responding separately to each individual stimulus. It will efficaciously contribute to plant acclimation to the climatic conditions prevailing at the place where it has rooted.
However, the ecological advantage of the programmed responses to stimuli is sometimes questionable in systems such as SR1 to SR3 (cf. Table 1). For instance, what can be the advantage of specifying the dominance between the cotyledonary buds in response to cotyledon pricking or of responding to manipulation stimulus by producing meristems in the hypocotyl? The reason is that at the outset of plant-memory research, simple and strictly controllable conditions had to be used to be able to prove the very existence of memory and describe its basic functions in view of the great reservations of many people to accept such a property of plants. Plants thus have made unexpected responses to unusual environmental conditions, i.e. differing from natural site scenarios.
Ecologists have stressed the importance for a plant to adjust the allocation of its (usually limited) resources to its main living activities in order to optimize its probability of survival and reproduction (Herms and Mattson 1992; Gayler 2010; Gayler et al. 2006, 2008; Matyssek et al. 2012). We may assume as a working hypothesis that the stored information, permitting the elaboration of an integrated, updated response to the variety of stimuli perceived by the plant in the course of time, is in fact an instruction for an optimized allocation of the plant resources to its principal living activities. It will be rewarding to explore if stored information vanishes after each generation or if cases of trans-generational information conservation exist.
5.2.2 Ecophysiological Significance of the Recall Function
Thanks to the RCL function, a stored instruction of response will not be expressed at any arbitrary time but only when an appropriate stimulus or external conditions have enabled the plant to recall the stored instruction and let it take effect. The RCL function can thus synchronize the release of the memorized response with the occurrence of a particular environmental event. The RCL function being linked with ultradian, circadian and annual rhythms of the plant (Sect. 3.1) means that it can similarly synchronize the release of the memorized response with the occurrence of a particular internal event. Since a plant can repeatedly recall a stored instruction of response, the RCL function is able to synchronize the release of the memorized response with different external and/or internal events occurring at different times.
5.2.3 Ecophysiological Significance of the Combined Effect of the Storage and Recall Functions
We have seen above (section “The Storage Function”) that (1) information storage induced by cotyledon pricking was corresponding in fact to the storage of a sort of instruction of growth reduction in the hypocotyl of very young Bidens seedlings and to the storage of an instruction of dominance specification in the cotyledonary buds of slightly older Bidens seedlings and (2) the plants were enabled to recall the stored instructions by being subjected to different external events or conditions. Hence, same types of stimulus can induce the storage of different instructions of response in the different plant tissues, so that plants are enabled to recall these stored instructions of responses at different times in different tissues. A store/recall memory thus supplies an extreme variety of possibilities to a plant to adjust its response to stimuli. Hence, the plant can keep track of the progress in internal and external processes possibly occurring at different times across different tissues.
5.3 Ecophysiological Significance of the Combined Effect of Priming and Store/Recall Memory
Although published data usually deal with the study of either the priming or store/recall forms of memory, it is likely that plants operate both forms simultaneously. In that case, with the priming memory plants would be able to adjust their response intensity to the dangerousness of the signals perceived. Simultaneously with the store/recall memory, they could elaborate an integrated, updated response to the entirety of the environmental stimuli (especially the climatic stimuli) and synchronize the response to any particular internal or external event including ultradian, circadian or annual rhythms of the plant. Instead of appearing as a sort of cock-and-bull story, the equipment of plants with priming and store/recall memories could thus play a major part in optimizing plant response to aggressions and climatic hazards. Experimental clarification is required for strengthening such interpretation of plant memory. However, a straight explanation is offered already right now of the fact that plants can manage to survive under possibly awkward conditions that may prevail at rooting sites.
6 Conclusions
Originally, researchers have been mainly interested in proving that memory capacities exist in plants and in unravelling the main characteristics of plant memory (Thellier et al. 1982). Incidentally, with this work it has been observed early that these properties of the plant memory enabled them to produce a sophisticated and efficacious mode of adapting to the conditions at the places of rooting. Now, time has come to assess specific ecological advantages that the possession of a memory can confer to a plant for the adaptation and acclimation to the environment. Exploring such dimensions was the aim of this assay. Plants have a variety of ways to store ecologically relevant information for memorization. Turnover of pools of metabolites, of conformation states of individual enzymes or multi-protein-subunit enzymes and of transcript levels provides mechanisms of memory. This particularly holds true for the priming memory. The complex STO/RCL memory involves STO and RCL genes as incorporated in our model of memory (Thellier and Lüttge 2013), and epigenetic modifications are substantial mechanisms of memory (Fig. 1). The more we survey adaptations and acclimations to environmental signals and stress which are often stable over non-stressful intervals and even generations, the more examples we find of memory functions which are controlling and regulating ecological performance of plants. It is clear that memory of plants is not an occasional episode but a fundamental property of plant life governing ecological comportment. This is also a challenge for future research on specific examples of physiological and biochemical ecology for unravelling molecular mechanisms of memory. The ultimate aim is the understanding of functional linkages resulting in theory building and computer simulations of plant internal networks with the functions of memory and the biological clock (Hütt et al. 2015).
Natural selection has equipped animals and plants with memory. The underlying mechanisms are completely different, based on the existence and lack of a nervous system, respectively. Strategies of information management are different. Animals are able to change their location to feed, escape predators and, more generally, look for environmental conditions suited to their metabolism and mode of life: their memory helps them to orientate in space and time during migratory explorations. Evolution yielded sophisticated results as in the case of higher species and humans. By contrast, plant strategies consist of adapting their metabolism and development to the environmental conditions at rooting sites. To do that plants must be able (1) to perceive the stimuli and stresses issued from their environment and (2) to create a response optimizing probabilities of survival and reproduction, which requires memorization at various spatiotemporal levels of structure and function. Many processes at the genetic and molecular, the metabolic and the physiological levels are involved in plant memorization and make up parts of the hardware structure as well as the operational software functioning of memory.
All living beings that have biomembranes, polynucleotides and proteins have the molecular basis to develop memory, and this includes prokaryotes (Thellier and Lüttge 2013). Memory is a fundamental property of all life. Therefore, we should not be surprised that being equipped with memory is essential for the ecological performance and persistence of plants in their environment.
References
Adams KL (2010) Dandelions ‘remember’ stress: heritable stress-induced methylation patterns. New Phytol 185:867–868
Alvarez ME, Nota F, Cambiagno DA (2010) Epigenetic control of plant immunity. Mol Plant Pathol 11:563–576
Amzallag GN (2002) The adaptive potential of plant development: evidence from the response to salinity. In: Läuchli A, Lüttge U (eds) Salinity: environment—plants—molecules. Kluver, Dordrecht, pp 291–312
Amzallag GN (2005) Perturbed reproductive development in salt-treated Sorghum bicolor: a consequence of modifications in regulation networks? J Exp Bot 56:2821–2829
Amzallag GN, Seligmann H, Lerner HR (1993) A developmental window for salt-adaptation in Sorghum bicolor. J Exp Bot 44:645–652
Appel HM, Cocroft RB (2014) Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 175:1257–1266
Baek D, Jiang J, Chung JS, Wang B, Chen J, Xin Z, Shi H (2011) Regulated AtHKT1 gene expression by a distal enhancer and DNA methylation in the promoter plays an important role in salt tolerance. Plant Cell Physiol 52:149–161
Bahnweg G, Heller W, Stich S, Knappe C, Betz G, Heerdt C, Kehr RD, Ernst D, Langebartels C, Nunn AJ, Rothenburger J, Schubert R, Müller-Starck G, Werner H, Matyssek R, Sandermann H Jr (2005) Beech leaf colonization by the endophyte Apiognomonia errabunda dramatically depends on light exposure and climatic conditions. Plant Biol 7:659–669
Bailey C, Chen M (1983) Morphological basis of long-term habituation and sensitization in Aplysia. Science 220:91–93
Baluška F, Ninkovic V (2010) Plant communication from an ecological perspective. Springer, Berlin, p 252
Bilger W, Björkman O (1994) Relationships among violaxanthin deepoxidation, thylakoid membrane conformation, and non-photochemical chlorophyll fluorescence quenching in leaves of cotton (Gossypium hirsutum L.). Planta 193:238–246
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16:6–21
Bond DM, Finnegan EJ (2007) Passing the message on: inheritance of epigenetic traits. Trends Plant Sci 12:211–216
Boyko A, Kovalchuk I (2008) Epigenetic control of plant stress response. Environ Mol Mutagen 49:61–72
Bruce TJA (2010) Exploiting plant signals in sustainable agriculture. In: Baluška F, Ninkovic V (eds) Plant communication from an ecological perspective. Springer, Berlin, pp 215–227
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful ‘memories’ of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Buchanan RB, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. American Society Plant Physiologists, Rockville, MD, 1367p
Cervantes-Laurean D, Jacobson EL, Jacobson MK (1996) Glycation and glycoxidation of histones by ADP-ribose. J Biol Chem 271:10461–10469
Chen M, Lv S, Meng Y (2010) Epigenetic performers in plants. Dev Growth Differ 52:555–566
Chinnusamy V, Zhu J-K (2009) Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12:133–139
Conrath U (2009) Priming of induced plant defense responses. Adv Bot Res 51:361–395
Conrath U (2011) Molecular aspects of defence priming. Trends Plant Sci 16:524–531
Conrath U, Thulke O, Katz V, Schwindling S, Kohler A (2001) Priming as a mechanism in induced systemic resistance of plants. Eur J Plant Pathol 107:113–119
Covington MF, Panda S, Liu XL, Strayer CA, Wagner DR, Kay SA (2001) ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 13:1305–1315
Critchley C, Russell AW (1994) Photoinhibition of photosynthesis in vivo: the role of protein turnover in photosystem II. Physiol Plant 92:188–196
Darrah C, Taylor BL, Edwards KD, Brown PE, Hall A, McWatters HG (2006) Analysis of phase of LUCIFERASE expression reveals novel circadian quantitative trait loci in Arabidopsis. Plant Physiol 140:1464–1474
Davies E, Stankovic B, Vian A, Wood AJ (2012) Where has all the message gone? Plant Sci 185–186:23–32
Daxinger L, Whitelaw E (2010) Transgenerational epigenetic inheritance: more questions than answers. Genome Res 20:1623–1628
Desbiez MO, Champagnat P, Boyer N, Frachisse JM, Gaspar T, Thellier M (1983) Inhibition correlative de la croissance de l’hypocotyle de Bidens pilosus L. par des traumatismes cotylédonaires légers. Bull Soc Bot Fr (Actual Bot) 130:67–77
Desbiez MO, Champagnat P, Thellier M (1986) Mécanismes de mise en mémoire et de rappel de mémoire chez Bidens pilosus. CR Acad Sci Paris 302:573–578
Desbiez MO, Gaspar T, Crouzillat D, Frachisse JM, Thellier M (1987) Effect of cotyledonary prickings on growth, ethylene metabolism and peroxidase activity in Bidens pilosus. Plant Physiol Biochem 25:137–143
Desbiez MO, Tort M, Thellier M (1991) Control of a symmetry-breaking process in the course of the morphogenesis of plantlets of Bidens pilosa L. Planta 184:397–402
Desbiez MO, Mikulecky D, Thellier M (1994) Growth messages in plants: principle of a possible modeling and further experimental characteristics. J Biol Syst 2:127–136
Desbiez MO, Tort M, Monnier C, Thellier M (1998) Asymmetrical triggering of the cell cycle in opposite buds of a young plant, after a slight cotyledonary wound. CR Acad Sci Paris (Sciences de la Vie/Life Sciences) 321:403–407
Devlin PF (2002) Signs of the time: environmental input to the circadian clock. J Exp Bot 53:1535–1550
Dicke M (2009) Behavioural and community ecology of plants that cry for help. Plant Cell Environ 32:654–665
Ding Y, Fromm M, Avramona Z (2012) Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat Commun 3:740
Dixon LE, Hodge SK, van Ooijen G, Troein C, Akman OE, Millar AJ (2014) Light and circadian regulation of clock components aids flexible responses to environmental signals. New Phytol 203:568–577
Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival and competitive advantage. Science 309:630–633
Dolmetsch RE, Lewis RS, Goodnow CC, Healy JJ (1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386:855–858
Dudai Y (2004) The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55:51–86
Edmunds LN, Tamponnet C (1990) Oscillator control of cell division cycles in Euglena: role of calcium in circadian time-keeping. In: O’Day DH (ed) Calcium as an intracellular messenger in eucaryotic microbes. American Society for Microbiology, Washington, DC, pp 97–123
Farré EM (2012) The regulation of plant growth by the circadian clock. Plant Biol 14:401–410
Forbes-Stovall J, Howton J, Young M, Davis G, Chandler T, Kessler B, Rinehart CA, Jacobshagen S (2014) Chlamydomonas reinhardtii strain CC-124 is highly sensitive to blue light in addition to green and red light in resetting its circadian clock, with the blue-light photoreceptor plant cryptochrome likely acting as negative modulator. Plant Cell Physiol 75:14–23
Frankhauser C, Staiger D (2002) Photoreceptors in Arabidopsis thaliana: light perception, signal transduction and entrainment of the endogenous clock. Planta 216:1–16
Fujiwara S, Oda A, Yoshida R, Niinuma K, Miyata K, Tomozoe Y, Tajima T, Nakagawa M, Hayashi K, Coupland G, Mizoguchi T (2008) Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell 20:2960–2971
Gagliano M, Renton M, Depczynski M, Mancuso S (2014) Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 175:63–72
Gális I, Gaquerel E, Pandey SP, Baldwin IT (2009) Molecular mechanisms underlying plant memory in JA-mediated defense responses. Plant Cell Environ 32:617–627
Gayler S (2010) Modélisation de l’effet de facteurs de l’environnement sur la répartition des ressources dans un système végétal mixte. CR Acad Agric France 96:89–90
Gayler S, Grams TEE, Kozovits A, Luedemann G, Winkler JB, Priesack E (2006) Analysis of competition effects in mono- and mixed cultures of juvenile beech and spruce by means of the plant growth simulation model PLATHO. Plant Biol 8:503–514
Gayler S, Grams TEE, Heller W, Treutter D, Priesack E (2008) A dynamic model of environmental effects on allocation to carbon-based secondary compounds in juvenile trees. Ann Bot 101:1089–1098
Gilmore AM (1997) Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant 99:197–209
Gilmore AM, Govindjee (1999) How higher plants respond to excess light: energy dissipation in photosystem II. In: Singhal GS, Renger G, Sopory SK, Irrgang KD, Govindjee (eds) Concepts in photobiology: photosynthesis and photomorphogenesis. Narosa Publishing House, New Delhi, pp 513–548
Gilmore AM, Yamasaki H (1998) 9-Aminoacridine and dibucaine exhibit competitive interactions and complicated inhibitory effects that interfere with measurements of ΔpH and xanthophyll cycle-dependent photosystem II energy dissipation. Photosynth Res 57:159–174
Gilmore AM, Hazlett TL, Debrunner PG, Govindjee (1996) Comparative time-resolved photosystem II chlorophyll a fluorescence analyses reveal distinctive differences between photoinhibitory reaction center damage and xanthophyll cycle-dependent energy dissipation. Photochem Photobiol 64:552–563
Gilmore AM, Shinkarev VP, Hazlett TL, Govindjee (1998) Quantitative analysis of the effects of intrathylakoid pH and xanthophyll cycle pigments on chlorophyll a fluorescence lifetime distributions and intensity in thylakoids. Biochemistry 73:13582–13593
Gols R (2014) Direct and indirect chemical defenses against insects in a multitrophic framework. Plant Cell Environ 37:1741–1752
Goss R, Lepetit B (2015) Biodiversity of NPQ. J Plant Physiol 172:13–32
Grunstein M (1997) Histone acetylation in chromatin structure and transcription. Nature 389:349–352
Habte E, Müller LM, Shtaya M, Davis SJ, von Korff M (2014) Osmotic stress at the barley root affects expression of circadian clock genes in the shoot. Plant Cell Environ 37:1321–1337
Harmer SL, Kay SA (2005) Positive and negative factors confer phase-specific circadian regulation of transcription in Arabidopsis. Plant Cell 17:1926–1940
He J, Chow WS (2003) The rate coefficient of repair of photosystem II after photoinactivation. Physiol Plant 118:297–304
Heil M (2010) Within-plant signaling by volatiles triggers systemic defences. In: Baluška F, Ninkovic V (eds) Plant communication from an ecological perspective. Springer, Berlin, pp 99–112
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307:433–436
Horton P, Ruban A (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56:365–373
Horton P, Ruban AV, Walters RG (1994) Regulation of light harvesting in green plants. Indication by nonphotochemical quenching of chlorophyll fluorescence. Plant Physiol 106:415–420
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AAR (2007) Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ 30:333–349
Hütt M-T, Lüttge U, Thellier M (2015) Noise-induced phenomena and complex rhythms: a test scenario for plant systems biology. In: Mancuso S, Shabala S (eds) Rhythms in plants, 2nd edn. Springer, Berlin, pp 279–321
Ibáñez C, Kozarewa I, Johansson M, Ögren E, Rohde A, Eriksson ME (2010) Circadian clock components regulate entry and affect exit of seasonal dormancy as well as winter hardiness in Populus trees. Plant Physiol 153:1823–1833
Jablonka E, Lamb MJ (1989) The inheritance of acquired epigenetic variation. J Theor Biol 139:69–83
Jennings RC, Islam K, Zucchelli G (1986) Spinach-thylakoid phosphorylation: studies on the kinetics of changes in photosystem antenna size, spill-over and phosphorylation of light-harvesting chlorophyll a/b protein. Biochim Biophys Acta [Bioenergetics] 850:483–489
Johnson CH (1992) Phase response curves: what can they tell us about circadian clocks? In: Hiroshige T, Honma K (eds) Circadian clocks from cell to human. Hokkaido University Press, Sapporo, Japan, pp 209–249
Johnson CH, Golden SS (1999) Circadian programs in cyanobacteria: adaptiveness and mechanism. Annu Rev Microbiol 53:389–409
Kakutani T (2002) Epi-alleles in plants: inheritance of epigenetic information over generations. Plant Cell Physiol 43:1106–1111
Kant P, Gordon M, Kant S, Zolla G, Davydov O, Heimer YM, Chalifa-Caspi V, Shaked R, Barak S (2008) Functional-genomics-based identification of genes that regulate Arabidopsis responses to multiple abiotic stresses. Plant Cell Environ 31:697–714
Karban R, Wetzel WC, Shiojiri K, Ishizaki S, Ramirez SR, Blande JD (2014) Deciphering the language of plant communication: volatile chemotypes of sagebrush. New Phytol 204:380–385
Kathiria P, Sidler C, Golubov A, Kalischuk M, Kawchuk LM, Kovalchuk I (2010) Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial, and fungal pathogens in the progeny of infected tobacco plants. Plant Physiol 153:1859–1870
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328
Kessler A, Baldwin IT (2004) Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in wild tobacco Nicotiana attenuata. Plant J 38:639–649
Kikis EA, Khanna R, Quail PH (2005) ELF4 is a phytochrome-regulated component of a negative-feedback loop involving the central oscillator components CCA1 and LHY. Plant J 44:300–313
Kinoshita T, Seki M (2014) Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol 55:1859–1863
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effect of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352:524–526
Knight MR, Smith SM, Trewavas AJ (1992) Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA 89:4967–4971
Knight H, Brandt S, Knight MR (1998) A history of stress alters drought calcium signaling pathways in Arabidopsis. Plant J 16:681–687
Kou HP, Li Y, Song XX, Ou XF, Xing SC, Ma J, von Wettstein D, Liu B (2011) Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to stress in rice (Oryza sativa L.). J Plant Physiol 168:1685–1693
Langebartels C, Heller W, Führer G, Lippert M, Simons S, Sandermann H (1998) Memory effects in the action of ozone on conifers. Ecotoxicol Environ Saf 41:62–72
Lesburguères E, Gobbo OL, Alaux-Cantin S, Hambucken A, Trifilieff P, Bontempi B (2011) Early tagging of cortical networks is required for the formation of enduring associative memory. Science 331:924–928
Li S, Liu J, Liu Z, Li X, Wu F, He Y (2014) HEAT-INDUCED TAS1 TARGETG1 mediates thermotolerance via HEAT STRESS TRANSCRIPTION FACTOR A1-directed pathways in Arabidopsis. Plant Cell 26:1764–1780
Lo WS, Duggan L, Emre NCT, Belotserkovskya R, Lane WS, Shiekhattar R, Berger SL (2001) Snf1—a histone kinase that works in concert with the histone acetyltransferase Gcn5 to regulate transcription. Science 293:1142–1146
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000) Molecular cell biology, 4th edn. W. H. Freeman, New York, NY, Section 21-7: Learning and memory
Loreto F, Dicke M, Schnitzler J-P, Turlings TCJ (2014) Plant volatiles and the environment. Plant Cell Environ 37:1905–1908
Love J, Dodd AN, Webb AAR (2004) Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 16:956–966
Luedemann G, Matyssek R, Fleischmann F, Grams TEE (2005) Acclimation to ozone affects host/pathogen interaction and competitiveness for nitrogen in juvenile Fagus sylvatica and Picea abies trees infected with Phytophthora citricola. Plant Biol 7:640–649
Luedemann G, Matyssek R, Winkler JB, Grams TEE (2009) Contrasting ozone × pathogen interaction as mediated through competition between juvenile European beech (Fagus sylvatica) and Norway spruce (Picea abies). Plant Soil 323:47–60
Lüttge U (2003) Circadian rhythmicity: is the “biological clock” hardware or software. Progr Bot 64:277–319
Lüttge U (2008) Physiological ecology of tropical plants, 2nd edn. Springer, Berlin, 458p
Lüttge U, Hertel B (2009) Diurnal and annual rhythms in trees. Trees 23:683–700
Lüttge U, Kluge M, Thiel G (2010) Botanik. Die umfassende Biologie der Pflanzen. Wiley-VCH, Weinheim, 1215p
Matyssek R, Sandermann H (2003) Impact of ozone on trees: an ecophysiological perspective. Prog Bot 64:349–404
Matyssek R, Sandermann H, Wieser G, Booker F, Cieslik S, Musselman R, Ernst D (2008) The challenge of making ozone risk assessment for forest trees more mechanistic. Environ Pollut 156:567–582
Matyssek R, Koricheva J, Schnyder H, Ernst D, Munch JC, Osswald W, Pretzsch H (2012) The balance between resource sequestration and retention: a challenge in plant science. In: Matyssek R, Schnyder H, Osswald W, Ernst D, Munch JC, Pretzsch H (eds) Growth and defence in plants—resource allocation at multiple scales, Ecological studies 220. Springer, Heidelberg, pp 3–24
Matzke M, Matzke AJ, Pruss GJ, Vance VB (2001) RNA-based silencing strategies in plants. Curr Opin Genet Dev 11:221–227
Matzke M, Kanno T, Huettel B, Daxinger L, Matzke AJ (2007) Targets of RNA directed DNA methylation. Curr Opin Plant Biol 10:512–519
McAinsh MR, Hetherington AM (1998) Encoding specificity in Ca2+ signalling systems. Trends Plant Sci 3:32–36
McClung CR (2006) Plant circadian rhythms. Plant Cell 18:792–803
McWatters HG, Bastow RM, Hall A, Millar AJ (2000) The ELF3 zeitnehmer regulates light signaling to the circadian clock. Nature 408:716–720
Michael TP, McClung CR (2002) Phase-specific circadian clock regulatory elements in Arabidopsis. Plant Physiol 130:627–638
Millar AJ (1999) Biological clocks in Arabidopsis thaliana. New Phytol 141:175–197
Millar AJ, Kay SA (1996) Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis. Proc Natl Acad Sci USA 93:15491–15496
Molinier J, Ries G, Zipfel C, Hohn B (2006) Transgeneration memory of stress in plants. Nature 442:1046–1049
Müller LM, von Korff M, Davis SJ (2014) Connections between circadian clocks and carbon metabolism reveal species-specific effects on growth control. J Exp Bot 65:2915–2923
Nakamichi N (2011) Molecular mechanisms underlying the Arabidopsis circadian clock. Plant Cell Physiol 52:1709–1718
Niwa Y, Yamashino T, Mizuno T (2009) The circadian clock regulates the photoperiodic response of hypocotyl elongation through a coincidence mechanism in Arabidopsis thaliana. Plant Cell Physiol 50:838–854
Ogudi T, Sage-Ono K, Kamada H, Ono M (2004) Characterization of transcriptional oscillation of an Arabidopsis homolog of PnC401 related to photoperiodic induction of flowering in Pharbitis nil. Plant Cell Physiol 45:232–235
Olbrich M, Knappe C, Wenig M, Gerstner E, Häberle K-H, Kitao M, Matyssek R, Stich S, Leuchner M, Werner H, Schlink K, Müller-Starck G, Welzl G, Scherb H, Ernst D, Heller W, Bahnweg G (2010) Ozone Fumigation (twice ambient) reduces leaf infestation following natural and artificial inoculation by the endophytic fungus Apiognomonia errabunda of adult European beech trees. Environ Pollut 158:1043–1050
Onai K, Okamoto K, Nishimoto H, Morioka C, Hirano M, Kami-Ike N, Ishiura M (2004) Large-scale screening of Arabidopsis circadian clock mutants by a high-throughput real-time bioluminescence monitoring system. Plant J 40:1–11
Osmond CB, Grace SC (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot 46:1351–1362
Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH (1998) Resonating circadian clocks enhance fitness in cyanobacteria. Proc Nat Acad Sc USA 95:8660–8664
Pastor V, Luna E, Mauch-Mani B, Ton J, Flors V (2013) Primed plants do not forget. Environ Exp Bot 94:46–56
Pearcy RW, Osteryoung K, Calkin HW (1985) Photosynthetic responses to dynamic light environments by Hawaiian trees. Plant Physiol 79:896–902
Pierik R, Ballaré CL, Dicke M (2014) Ecology of plant volatiles: taking a plant community perspective. Plant Cell Environ 37:1845–1853
Plieth C, Hansen UP, Knight H, Knight MR (1999) Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J 18:491–497
Portis AR (2003) Rubisco activase—Rubisco’s catalytic chaperone. Photosyn Res 75:11–27
Rasmann S, de Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, Agrawal AA, Felton GW, Jander G (2012) Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol 158:854–863
Richards EJ (2006) Inherited epigenetic variation—revisiting soft inheritance. Nat Rev Genet 76:395–401
Rikin A (1991) Temperature-induced phase shifting of circadian rhythms in cotton seedlings as related to variations in chilling resistance. Planta 185:407–414
Ripoll C, Le Sceller L, Verdus M-C, Norris V, Tafforeau M, Thellier M (2009) Memorization of abiotic stimuli in plants: a complex role for calcium. In: Baluska F (ed) Plant-environment interactions. Springer, Berlin, pp 267–283
Roden LC, Song H-R, Jackson S, Morris K, Carré IA (2002) Floral responses to photoperiod are correlated with the timing of rhythmic expression relative to dawn and dusk in Arabidopsis. Proc Natl Acad Sci USA 99:13313–13318
Roux D, Vian A, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G (2006) Electromagnetic fields (900 MHz) evoke consistent molecular responses in tomato plants. Physiol Plant 128:283–288
Salomé PA, Michael TP, Kearns EV, Fett-Neto AG, Sharrock RA, McClung CR (2002) The out of phase 1 mutant defines a role for PHYB in circadian phase control in Arabidopsis. Plant Physiol 129:1674–1685
Sasek TW, Richardson CJ, Fendick EA, Bevington SR, Kress LW (1991) Carryover effects of acid rain and ozone on the physiology of multiple flushes of loblolly pine seedlings. For Sci 37:1078–1098
Sassenrath-Cole GF, Pearcy RW (1992) The role of ribulose-bisphosphate regeneration in the induction requirement of photosynthetic CO2 exchange under transient light conditions. Plant Physiol 99:227–234
Saze H (2008) Epigenetic memory transmission through mitosis and meiosis in plants. Semin Cell Dev Biol 19:527–536
Shen J, Xie K, Xiong L (2010) Global expression profiling of rice microRNAs by one-tube stem-loop reverse transcription quantitative PCR revealed important roles of microRNAs in abiotic stress responses. Mol Gen Genet 284:477–488
Sinclair J, Hanks P, Fox G, Moon R, Stock P (1987) Collins Cobuild English dictionary. Collins, London, 1703p
Sridhar VV, Kapoor A, Zhang K, Zhu J, Zhou T, Hasegawa PM, Bressan RA, Zhu JK (2007) Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature 447:735–738
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16:2001–2019
Tafforeau M, Verdus M-C, Norris V, White G, Demarty M, Thellier M, Ripoll C (2002) SIMS study of the calcium-deprivation step related to epidermal meristem production induced in flax by cold shock or radiation from a GSM telephone. J Trace Microprobe Techn 20:611–623
Tafforeau M, Verdus MC, Norris V, White GJ, Cole M, Demarty M, Thellier M, Ripoll C (2004) Plant sensitivity to low intensity 105 GHz electromagnetic radiation. Bioelectromagnetics 25:403–407
Tafforeau M, Verdus M-C, Norris V, Ripoll C, Thellier M (2006) Memory processes in the response of plants to environmental signals. Plant Signal Behav 1:9–14
Tanigawa Y, Tsuchiya M, Imai Y, Shimoyama M (1984) ADP-ribosyltransferase from hen liver nuclei. Purification and characterization. J Biol Chem 259:2022–2029
Tanner W (1969) Light-driven active uptake of 3-O-methylglucose via an inducible hexose uptake system of Chlorella. Biochem Biophys Res Comm 36:278–283
Tanner W, Grünes R, Kandler O (1970) Spezifität und Turnover des induzierbaren Hexose-Aufnahmesystems von Chlorella. Z Pflanzenphysiol 62:376–386
Thellier M (2015) Les plantes ont-elles de la mémoire? Editions Quae, Versailles, 111p
Thellier M, Lüttge U (2013) Plant memory: a tentative model. Plant Biol 15:1–12
Thellier M, Desbiez MO, Champagnat P, Kergosien Y (1982) Do memory processes also occur in plants? Physiol Plant 56:281–284
Thellier M, Le Sceller L, Norris V, Verdus M-C, Ripoll C (2000) Long-distance transport, storage and recall of morphogenetic information in plants: the existence of a primitive plant “memory”. CR Acad Sci Paris (Sciences de la Vie/Life Science) 323:81–91
Thellier M, Ripoll C, Norris V (2013) Memory processes in the control of plant growth and metabolism. Nova Acta Leopoldina NF 114(391):21–42
Trewavas A (1999) Le calcium c’est la vie: calcium waves. Plant Physiol 120:1–6
Trewavas A (2003) Aspects of plant intelligence. Ann Bot 92:1–20
Tyystjärvi E, Aro E-M (1996) The rate constant of photoinhibition measured in lincomycin-treated leaves is directly proportional to light intensity. Proc Natl Acad Sci USA 93:2213–2218
Ueda M, Nakamura Y (2006) Metabolites involved in plant movement and “memory”: nyctinasty of legumes and trap movement in the Venus flytrap. Nat Prod Rep 23:548–557
Valladares F, Allen MT, Pearcy RW (1997) Photosynthetic responses to dynamic light under field conditions in six tropical rainforest shrubs along a light gradient. Oecologia 111:505–514
van Hulten M, Ton J, Pieterse CMJ, van Wees SCM (2010) Plant defense signaling from the underground primes aboveground defenses to confer enhanced resistance in a cost-efficient manner. In: Baluška F, Ninkovic V (eds) Plant communication from an ecological perspective. Springer, Berlin, pp 43–60
Verdus M-C, Thellier M, Ripoll C (1997) Storage of environmental signals in flax: their morphogenetic effect as enabled by a transient depletion of calcium. Plant J 12:1399–1410
Verdus M-C, Le Sceller L, Norris V, Thellier M, Ripoll C (2007) Pharmacological evidence for calcium involvement in the long-term processing of abiotic stimuli in plants. Plant Signal Behav 2:212–220
Verhoeven KJF, Jansen JJ, van Dijk PJ, Biere A (2010) Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytol 185:1108–1118
Vian A, Roux D, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G (2006) Microwave irradiation affects gene expression in plants. Plant Signal Behav 1:67–70
Voelckel C, Baldwin IT (2004) Herbivore-induced plant vaccination. Part II. Array-studies reveal the transience of herbivore-specific transcriptional imprints and a distinct imprint from stress combinations. Plant J 38:650–663
Wang WS, Pan YJ, Zhao XQ, Dwivedi D, Zhu LH, Ali J, Fu BY, Li ZK (2010) Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J Exp Bot 62:1951–1960
Wenden B, Kozma-Bognár L, Edwards KD, Hall AJW, Locke JCW, Millar AJ (2011) Light inputs shape the Arabidopsis circadian system. Plant J 66:480–491
Wilhelm C, Wirth C (2015) Physiodiversity—New tools allow physiologist to embrace biodiversity and reconstruct the evolution of ‘physiologies’? J Plant Physiol 172:1–3
Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH (2004) The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol 14:1481–1486
Yaish MW, Colasanti J, Rothstein SJ (2011) The role of epigenetic processes in controlling flowering time in plants exposed to stress. J Exp Bot 62:3727–3735
Yerushalmi S, Green RM (2009) Evidence for the adaptive significance of circadian rhythms. Ecol Lett 12:970–981
Yerushalmi S, Yakir E, Green RM (2011) Circadian clocks and adaptation in Arabidopsis. Mol Ecol 20:1155–1165
Zhang X (2008) The epigenetic landscape of plants. Science 320:489–492
Zhang Y, Reinberg D (2001) Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev 15:2343–2360
Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW-L, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, Ecker JR (2006) Genome-wide high resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 126:1189–1201
zu Castell W, Fleischmann F, Heger T, Matyssek R (2016) Shaping theoretic foundations of holobiont-like systems. In: Cánovas FM, Lüttge U, Matyssek R (eds) Progress in botany, vol 77. Springer, Heidelberg
Acknowledgement
We thank Professor Dr. Rainer Matyssek for carefully reading the manuscript and very many useful suggestions and stimulating comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lüttge, U., Thellier, M. (2016). Roles of Memory and Circadian Clock in the Ecophysiological Performance of Plants. In: Lüttge, U., Cánovas, F., Matyssek, R. (eds) Progress in Botany 77. Progress in Botany, vol 77. Springer, Cham. https://doi.org/10.1007/978-3-319-25688-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-25688-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25686-3
Online ISBN: 978-3-319-25688-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)