Abstract
Introduction
Many current guidelines on optimal target blood pressure (BP) for chronic kidney disease (CKD) patients are largely based on studies in diabetic and hypertensive patients. However, there have been few studies in patients with glomerular diseases.
Methods
We retrospectively studied the longitudinal association between BP and CKD progression in 1,066 biopsy-proven patients diagnosed with primary glomerular diseases, including IgA nephropathy, membranous nephropathy (MN), and focal segmental glomerulosclerosis (FSGS), between 2005 and 2017. The main predictor was time-updated systolic blood pressure (SBP) at every clinic visit. The primary outcome was a composite one including ≥ 50% decrease in estimated glomerular filtration rate (eGFR) from the baseline, and end-stage kidney disease (ESKD).
Results
During 5009 person-years of follow-up, the primary outcome occurred in 157 (14.7%) patients. In time-varying Cox model, the adjusted hazard ratios (HRs) (95% confidence interval (CI)) for the primary outcome were 1.48 (0.96–2.29), 2.07 (1.22–3.52), and 2.53 (1.13–5.65) for SBP of 120–129, 130–139, and ≥ 140 mmHg, respectively, compared with SBP < 120 mmHg. This association was particularly evident in patients with elevated proteinuria. However, there was no association between baseline SBP and adverse kidney outcomes. Finally, prediction models failed to show the improvement of predictive performance of SBP compared with that of remission status. Moreover, patients with remission and less controlled SBP had better kidney outcomes than those with non-remission and well-controlled SBP.
Conclusion
Among patients with glomerular disease, higher time-updated SBP was significantly associated with higher risk of CKD progression. However, the clinical significance of blood pressure was less powerful than remission status.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The goal of blood pressure (BP) control in patients with chronic kidney disease (CKD) is to not only reduce cardiovascular events and mortality but also to delay the progression of CKD. In general, target BP levels < 130/80 mmHg and < 140/90 mmHg have been suggested in patients with and without albuminuria, respectively [1], which are based on the results of many clinical randomized controlled trials. However, these studies did not exclusively examine the effects of BP control in CKD patients, and excluded patients with advanced CKD. Therefore, the optimal target BP level is yet to be determined in these patients.
Diabetes and hypertension are the two main causes of CKD. However, other diseases such as glomerular disease and polycystic kidney disease also constitute a major part of CKD. Interestingly, almost all studies have examined the effects of BP control in patients with diabetes and hypertension, while there have been no relevant studies in patients with other kidney diseases. A recent trial in autosomal polycystic kidney disease patients aged between 15 and 49 years with eGFR > 60 ml min−1 1.73 m−2 showed that a lower target BP between 95/60 and 110/75 mmHg, in comparison to the standard BP control of 120/70 to 130/80 mmHg, was associated with a slow increase in kidney volume but no overall change in glomerular filtration rate (GFR) [2]. From a mechanistic viewpoint, the glomerular disease is a unique entity because some circulating factors could play a major role in glomerular filtration barrier damage even in normotensive patients. Thus, BP may contribute little to kidney function decline in the early phase of glomerular disease. In addition, in heavy proteinuric disease, reduction of proteinuria and preservation of kidney function are largely affected by the success of immunosuppression and the achievement of complete or partial remission [3,4,5,6]. The prevalence of hypertension varies widely between 9 and 80% in patients with primary glomerular diseases [7,8,9,10]. However, the clinical implication of hypertension is unknown and studies on the association between BP levels and kidney outcomes are scarce in these patients. Herein, we examine the association between BP and kidney disease progression in patients with three representative glomerular diseases—IgA nephropathy, membranous nephropathy (MN), and focal segmental glomerulosclerosis (FSGS).
Materials and methods
Study population
We conducted an observational, retrospective study in 1323 patients who were diagnosed with biopsy-proven primary glomerular disease including IgA nephropathy, MN, FSGS, or a combination of two glomerular diseases at the Yonsei University Health System (YUHS) between 2005 and 2017. We excluded patients with the following criteria: (i) age < 18; (ii) history of dialysis or kidney transplantation; (iii) patients with less than two follow-up visits after kidney biopsy; (iv) less than BP two measurements; (v) history of cancer, viral hepatitis, or autoimmune disease (Fig. S1). None of the patients with diabetes in the analysis had evidence of diabetic kidney disease and all had good glycemic control.
Data collection and measurements
From the Glomerular Disease Registry database of YUHS, demographic, clinical, and biochemical data were retrieved. These data, along with data obtained at the time of renal biopsy, were considered baseline data. BP was measured after a 5-min rest in the sitting position at the clinic office by using an electronic sphygmomanometer. The mean of BP readings was used as the BP value for each visit. Data on BP were collected from the date of kidney biopsy up to the date of an outcome event or the last follow-up. Demographic and clinical data included age, sex, BP, body mass index (BMI), prior history of hypertension, diabetes, cardiovascular disease, and smoking status. Laboratory measurements included serum creatinine, hemoglobin, serum albumin, serum calcium, serum phosphorus, total cholesterol, serum bicarbonate, and random urine protein-to-creatinine ratio (UPCR) levels. Serum creatinine was measured by the isotope dilution mass spectrometry (IDMS) method after April 2011. Thus, non-IDMS creatinine was converted to IDMS creatinine using the following equation for the serum creatinine values measured until April 2011; non-IDMS Cr (mg/dl) = Cr − IDMS (mg/dl) × 1.065 + 0.067 [11, 12]. Based on this, we determined the estimated glomerular filtration rate (eGFR) by CKD-EPI equation [13]. During follow-up, patients were treated with various immunosuppressive drugs based on the KDIGO (Kidney Disease Improving Global Outcomes) guideline and disease status. Immunosuppression users were defined as individuals who had been administered any immunosuppressive drugs for ≥ 1 month. Complete remission was defined as UPCR < 0.3 g/g and partial remission was defined as a reduction in proteinuria by ≥ 50% from the baseline with a UPCR between ≥ 0.3 g/g and < 3.5 g/g, irrespective of whether it was achieved due to treatment drugs or spontaneously.
The main exposure of interest and primary outcome
The main predictor of this study was systolic BP (SBP), which was analyzed as a continuous variable per 10 mmHg increase and as a categorical variable with 10 mmHg increments. SBP was categorized into four groups; < 120, 120–129, 130–139, and ≥ 140 mmHg. For time-updated SBP, we used averaged SBP determined by averaging the mean of SBP readings at any given visit and those from all prior visits. The primary outcome was CKD progression, which was a composite of a ≥ 50% decrease in eGFR from the baseline, or the onset of end stage kidney disease (ESKD). This endpoint was defined as a sustained decrease of eGFR by ≥ 50% in at least two consecutive measurements. The first of these consecutive measurements was retrospectively designated as the study endpoint. EKSD was defined as the initiation of maintenance dialysis or kidney transplantation. The study observation closed on December 31st, 2018.
Statistical analysis
Continuous variables were presented as mean and standard deviation for normally distributed variables or median with interquartile ranges (IQRs) for skewed variables. Categorical variables were expressed as count and percentage. Missing values were imputed by the most recent values for all laboratory measurements. To explore the association between blood pressure and CKD progression, time-varying Cox proportional-hazard model was used for primary analysis since BP was not static but highly variable during follow-up. In these analyses, all repeated measures such as SBP, total cholesterol, phosphorus, serum concentrations of albumin, and UPCR, and all drugs were treated as time-varying exposures. We further used the conventional Cox proportional-hazard regression model to analyze the baseline SBP and other laboratory parameters. Model 1 represented unadjusted hazard ratios (HRs). We also created model 2 after adjustment of important factors that can affect kidney outcomes. These includes age, sex, BMI, smoking status, comorbid disease, glomerular disease type, laboratory measurements (eGFR, UPCR, total cholesterol, phosphorus, and albumin), medications (renin–angiotensin–aldosterone system (RAAS) blockers, diuretics, statins, immunosuppressive drugs), and remission status. The results from the hazard models are presented as HR and 95% confidence interval (CI). The survival curves represent the cumulative survival function for baseline SBPs and time-updated SBPs. The survival time was defined as the time between kidney biopsy and the primary outcome or last recorded visit. Restricted cubic splines were used to show any association between the cumulative mean SBP as a continuous variable and the HR of kidney outcomes. To explore effect modification on the relationship between time-updated SBP and CKD progression, subgroup analyses were performed after stratification by age (< 45 or ≥ 45 years old), sex, BMI (< 25 or ≥ 25 kg/m2), eGFR (< 45 or ≥ 45 ml min−1·1.73 m−2), proteinuria (< 1.0 or ≥ 1.0 g/g), hypertension, diabetes, and primary disease.
To compare the predictability of time-updated SBP, we used the Harrell C-statistics [14] in the following models. The base model included conventional factors such as age, sex, BMI, smoking status, history of hypertension, and baseline eGFR and UPCR. The remission model included remission status. Finally, the SBP model was constructed after SBP was added to the base model. All statistical analyses were performed using the R and Stata 15. P-values < 0.05 were considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of all patients in relation to the baseline SBP category are presented in Table 1. The median age was 41.0 (IQR 32.0–52.0) years, and baseline eGFR was 89.1 ± 30.7 ml min−1 1.73 m−2. Seven hundred and twenty-nine (68.4%) patients had a history of hypertension. In total, 1,066 patients were diagnosed with at least one of the following glomerular diseases; 761 (71.4%) patients with IgA nephropathy, 128 (12.0%) with MN, 133 (12.5%) with FSGS, and 44 (4.1%) with two of the above three glomerular diseases. At baseline, patients with higher SBP were older, more likely to be men, and had a higher BMI and more comorbidities than patients with lower SBP. In addition, eGFR was lower and RAAS blockers were more prescribed in patients with higher SBP.
SBP and risk of CKD progression
During 5,009 person-years follow-up, the primary outcome occurred in 157 (14.7%) patients with a corresponding incidence rate of 31 (95% CI 27–37) per 1000 person-years. The adverse kidney outcome rates were greater in patients with higher baseline SBP than in those with lower SBP; 20 (15–28), 32 (23–43), 36 (26–50), and 50 (37–67) per 1000 person-years for SBP of < 120, 120–129, 130–139, and ≥ 140 mmHg, respectively (Table 2).
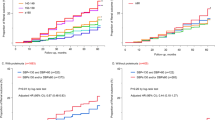
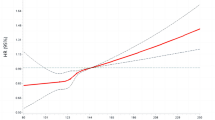
We then analyzed the association between time-updated SBP levels and risk of CKD progression using time-varying Cox model (Table 3). In the unadjusted model, there was a graded association between time-updated SBP and CKD progression (model 1). Higher SBP levels were associated with a significantly higher risk of the primary outcome. After adjustment of demographic factors, comorbidities, primary disease, laboratory parameters, medications and remission status, the HRs (95% CI) for SBP of 120–129, 130–139, and ≥ 140 mmHg were 1.48 (0.96–2.29), 2.07 (1.22–3.52), and 2.53 (1.13–5.65), respectively, as compared with SBP of < 120 mmHg (model 2). Statistical significance was observed only in SBP of 130–139 and ≥ 140 mmHg, while there was no significant difference in the risk of the primary outcome in SBP categories < 130 mmHg. In continuous SBP modeling with the same adjustment level, a 10 mmHg increase in time-updated SBP was associated with a 24% higher risk of the primary composite outcome (95% CI 1.02–1.52). The cumulative probability of the primary outcome was significantly higher in patients with SBP of 130–139 and ≥ 140 mmHg than in patients with other SBPs (Fig. 1b, Fig. S2B). The cubic spline curve analysis also showed a graded association between cumulative SBP and CKD progression (Fig. S3A). Besides SBP, the multivariable model revealed the history of cardiovascular disease, eGFR at baseline, UPCR at baseline, albumin, phosphate, remission status, RAAS blocker use, diuretics, and the number of anti-hypertensive agents as being significant determinants for CKD progression. In contrast, the baseline SBP did not associate with adverse kidney outcomes after adjustment (Fig. 1a, Fig. S2A; Table S1). During the follow-up, only four deaths occurred and no significant difference in death rates was observed among the SBP groups (Table 2).
Adjusted cumulative probability of composite kidney outcomes. a Was plotted according to baseline SBP categories, and b was plotted according to time-updated SBP categories. a Adjusted for age, sex, body mass index, smoking status, comorbid disease (hypertension, diabetes, cardiovascular disease, and cerebrovascular disease), pathology type, eGFR, total cholesterol level, serum albumin level, serum phosphorus level, random urinary potassium-to-creatinine ratio, RAAS blocker, statin, and diuretics usage. b Adjusted for age, sex, body mass index, baseline SBP, smoking status, comorbid disease (hypertension, diabetes, cardiovascular disease, and cerebrovascular disease), pathology type at baseline, eGFR at baseline, total cholesterol level, serum albumin level, serum phosphorus level, random urinary protein-to-creatinine ratio, RAAS blocker, statin, diuretics, immunosuppressant agent usage, and remission status based on the urinary protein-to-creatinine ratio. The systolic blood pressure was considered as a time-dependent variable and was the exposure of interest. Age, total cholesterol level, serum albumin level, remission status, and all medication history were considered as time-dependent variables. eGFR estimated glomerular filtration rate, RAAS renin–angiotensin–aldosterone-system, SBP systolic blood pressure
Subgroup analysis
In subgroup analysis, we found no effect modification in pre-specified subgroups by age, sex, BMI, kidney function, presence of hypertension and diabetes, and primary disease (Fig. 2). However, there was significant interaction between proteinuria and SBP group for developing the primary outcome, and the association of higher SBP with adverse kidney outcome was more pronounced in patients with high proteinuria (UPCR ≥ 1.0 g/g). Cubic spline curve analysis also showed different association between SBP and the primary outcome according to proteinuria level (Fig. S3B, S3C).
Subgroup associations of SBP with CKD progression in patients with glomerular disease. Hazard ratios (HRs; 95% confidence intervals [95% CIs]) according to per 10-SBP (mmHg). HRs were adjusted for age, sex, body mass index, baseline SBP, smoking status, comorbid disease (hypertension, diabetes, cardiovascular disease, and cerebrovascular disease), pathology type at baseline, eGFR at baseline, total cholesterol level, serum albumin level, serum phosphorus level, random urinary protein-to-creatinine ratio, RAAS blocker, statin, diuretics, immunosuppressant agent usage, and remission status based on the urinary protein-to-creatinine ratio. Systolic blood pressure was considered as a time-dependent variable and was the exposure of interest. Age, total cholesterol level, serum albumin level, serum phosphorus level, remission status, and all medication history were considered as time-dependent variables. eGFR estimated glomerular filtration rate, HR hazard ratio, RAAS renin–angiotensin–aldosterone-system, SBP systolic blood pressure
Predictive performance of SBP
In glomerular disease, remission status is generally considered more important in determining future kidney outcomes than BP. During follow-up, the proportion and number of remissions increased up to one year (Fig. S4). In multivariable Cox model with the same adjustment level as that of the above model 4, the HR for the primary outcome was remarkably lower in patients with complete remission (HR, 0.13; 95% CI 0.06–0.30) than in patients with partial remission (HR, 0.35; 95% CI 0.21–0.57) (Table S2). To examine the association of SBP and remission status together with adverse kidney outcomes, we classified patients into 4 groups according to remission status (complete or partial) and the degree of BP control (well-controlled < 130 mmHg; less controlled ≥ 130 mmHg). The results showed that risk of adverse kidney outcome was lowest in patients with remission and well-controlled SBP. Interestingly, patients with remission and less controlled SBP had better kidney outcomes than those with non-remission and well-controlled SBP (Fig. 3). In addition, we examined whether SBP could affect remission status time-varying model, where primary outcome was the first achievement of complete or partial remission. The results showed no significant association of SBP with the probability of achieving remission (Table S3). We then created three prediction models (base, remission, and SBP models) and used c-statistics to compare their predictive performance for the primary outcome. The c-statistic of the base model was 0.77 (0.73–0.82). After adding remission status to the base model, c-statistic significantly increased from 0.77 to 0.84. However, c-statistic of the time-updated SBP model was slightly greater and significantly lower than that of base and remission models, respectively (Table S4). These findings suggest that the predictive performance of SBP was not greater than that of remission status, and that attainment of remission is more important than SBP in predicting adverse kidney outcomes.
Cumulative probability of composite kidney outcomes according to the combination of remission status and the degree of BP control. a Unadjusted. b Adjusted for age, sex, body mass index, baseline SBP, smoking status, comorbid disease (hypertension, diabetes, cardiovascular disease, and cerebrovascular disease), pathology type at baseline, eGFR at baseline, total cholesterol level, serum albumin level, serum phosphorus level, random urinary protein-to-creatinine ratio, RAAS blocker, statin, diuretics, and immunosuppressant agent usage. The systolic blood pressure and remission status based on the urinary protein-to-creatinine ratio were considered as a time-dependent variable and were the exposure of interest. Age, total cholesterol level, serum albumin level, serum phosphorus level, remission status, and all medication history were considered as time-dependent variables. BP blood pressure, eGFR estimated glomerular filtration rate, RAAS renin–angiotensin–aldosterone-system, SBP systolic blood pressure
DBP and risk of CKD progression
We further studied the association between diastolic blood pressure (DBP) and the risk of CKD progression. However, the association between DBP and the risk of CKD progression was not significant after adjustment (Table S5; Fig. S5).
Discussion
In this observational study, we found that higher SBP was associated with a higher risk of CKD progression in patients with primary glomerular disease. This association was more prominent for time-updated SBP but not evident for baseline SBP. The significant association between time-updated SBP and adverse kidney outcomes was particularly evident in patients with high proteinuria. However, the predictive performance of SBP was not greater than that of the remission status. These findings suggest that SBP is a significant predictor of loss of kidney function, but its clinical impact on glomerular disease may not be as strong as its impact on diabetic or hypertensive kidney diseases. Current guidelines do not provide a solid recommendation on the optimal target BP level to prevent loss of kidney function in CKD patients and evidence levels of these relevant guidelines are low [15]. Nevertheless, the KDIGO guideline suggests target BP levels < 130/80 mmHg and < 140/90 mmHg in patients with and without albuminuria, respectively [1]. However, there is lack of evidence that this BP goal can also be applied to patients with glomerular disease because most studies regarding this issue have been conducted in patients with diabetic and hypertensive kidney disease and there have been no randomized controlled studies to examine the effect of BP on kidney outcomes solely in primary glomerular disease. Interestingly, hypertension is common in primary proteinuric glomerulopathies [7,8,9,10, 16]. Possible causes for the elevated BP include activation of the RAAS, sodium retention, volume expansion, and the use of corticosteroids and calcineurin inhibitors [17, 18]. It should be noted that some circulating factors could play a more important role in damaging the filtration barrier than systemic BP in primary glomerulopathies. Therefore, it would be interesting to delineate the association between BP with CKD progression in such unique kidney diseases.
A few studies have explored this issue. Recently, Sethna et al. [16] reported that the baseline hypertension status (> 140/90 mmHg) was associated with poor clinical outcomes in 433 participants in the NEPTUNE (Nephrotic Syndrome Study Network) study. In agreement with this finding, we showed that higher SBP was significantly associated with a higher risk of CKD progression. However, the NEPTUNE study focused primarily on BP variability and did not take time-dependent BP changes into account. Notably, in our study, time-updated SBP was more strongly associated with kidney outcomes than the baseline SBP. It is possible that BP is elevated over time as kidney function declines, and its clinical impact may not be clear in the early period after the occurrence of disease. Therefore, time-updated SBP could more robustly reveal the association of BP with adverse kidney outcomes. Similar findings were also reported by the Chronic Renal Insufficiency Cohort Study Investigators [19]. Presumably, systemic hypertension can worsen glomerular hemodynamics and hamper blood supply to nephrons over time, resulting in the acceleration of kidney disease progression.
In this study, the predictive performance of SBP was much lower than that of the remission status. As mentioned above, there are more important factors than SBP that could determine kidney outcomes in primary glomerulopathies. The potential circulating factors include under galactosylated IgA immune complex in IgA nephropathy [20,21,22], soluble urokinase receptor in FSGS [23], and anti-phospholipase A2 receptor antibody in MN [24]. Although there has been much debate on the role of these factors alone, they could trigger the downstream cascade and correlate well with clinical outcomes in each glomerulopathy. In particular, disappearance or reappearance of the anti-phospholipase A2 receptor antibody in the circulation could precede the detection of proteinuria in patients with MN [25, 26]. Many studies have shown that both achieving remission and residual proteinuria levels are very important factors in determining prognostic outcomes [27,28,29,30,31]. Even attaining partial remission can greatly improve clinical outcomes [30, 31]. In this study, we showed that the predictive performance of SBP did not surpass the remission status. This finding suggests that reducing proteinuria to the lowest possible level and achieving remission are fundamental in improving clinical outcomes, and target BP control is additionally required to maximize the therapeutic effects in patients with primary glomerulopathies.
The strengths of our study include the examination of three representative glomerulopathies, which are commonly encountered in clinical practice, the use of an analytical approach to reflect time-dependent effects, rigorous adjustment of many confounders, and detailed comparative analyses between SBP and other factors. In particular, we extensively analyzed the data and considered the relative importance of remission over SBP.
Our study has some limitations. First, all BP readings were obtained at the clinic office, which could not detect diverse patterns including white coat hypertension, BP variability, and reverse dipping pattern [32]. Nevertheless, the median number of BP measurements per patient was 11.0 (IQR 6.0–19.0) during the median 3.8 years of follow-up, which enabled us to take changes in BP trend into account for constructing the time-updated model. Second, our observational study cannot suggest an optimal target level of BP. However, as discussed above, the role of SBP is uncertain in primary glomerulopathy. A recent study involving 2047 Chinese patients with IgA nephropathy did not include BP as an important factor in their prediction model for adverse kidney outcomes [33]. In addition, in a Canadian cohort study with late-stage CKD patients, only extremely elevated BP was associated with risks of eGFR decline [34]. Therefore, BP may not play an important role in early glomerular disease until kidney function considerably declines and this issue should be further explored in future studies. Finally, patterns of BP control [35] and biopsy policy substantially differ among countries [36]. Indeed, there could be a wide range of BP depending on the CKD stage at the time of biopsy. Thus, our findings in only Korean patients cannot be generalized to other populations.
Conclusions
In patients with glomerular diseases, higher time-updated SBP was significantly associated with a higher risk of CKD progression. This association was more prominent in patients with high proteinuria. However, remission status predicted adverse kidney outcomes better than SBP. Our findings not only highlight the relationship between SBP and loss of kidney function, but also call for further studies to clarify the role of elevated BP in patients with glomerular diseases.
References
Becker GJ, Wheeler DC, De Zeeuw D, Fujita T, Furth SL, Holdaas H et al (2012) Kidney disease: improving global outcomes (KDIGO) blood pressure work group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2(5):337–414
Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI et al (2014) Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371(24):2255–2266
Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC (2005) Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol 16(4):1061–1068
Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S et al (1995) A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48(5):1600–1604
Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C et al (1989) A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 320(1):8–13
Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P et al (2004) Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol 15(1):157–163
Orofino L, Quereda C, Lamas S, Orte L, Gonzalo A, Mampaso F et al (1987) Hypertension in primary chronic glomerulonephritis: analysis of 288 biopsied patients. Nephron 45(1):22–26
Kheder MA, Maiz HB, Abderrahim E, El Younsi F, Moussa FB, Safar ME et al (1993) Hypertension in primary chronic glomerulonephritis analysis of 359 cases. Nephron 63(2):140–144
Johnston PA, Davison AM (1993) Hypertension in adults with idiopathic glomerulonephritis and normal serum creatinine. A report from the MRC glomerulonephritis registry. Nephrol Dial Transplant 8(1):20–24
Ridao N, Luño J, de Vinuesa SG, Gómez F, Tejedor A, Valderrábano F (2001) Prevalence of hypertension in renal disease. Nephrol Dial Transplant 16(Suppl 1):70–73
Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL (2010) Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol 31(5):426–434
Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW et al (2007) Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53(4):766–772
Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA (1982) Evaluating the yield of medical tests. JAMA J Am Med Assoc 247(18):2543–2546
Group KDIGO (KDIGO) GW (2012) KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2(2):139–274
Sethna CB, Meyers KEC, Mariani LH, Psoter KJ, Gadegbeku CA, Gibson KL et al (2017) Blood pressure and visit-to-visit blood pressure variability among individuals with primary proteinuric glomerulopathies. Hypertension 70(2):315–323
Ihm CG (2015) Hypertension in chronic glomerulonephritis. Electrolyte Blood Press 13(2):41–45
Ljutic D (2003) The role of arterial hypertension in the progression of non-diabetic glomerular diseases. Nephrol Dial Transplant 18(90005):28v–230
Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW et al (2015) Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med 162(4):258–265
Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H et al (2001) Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 59(3):1077–1085
Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J (1997) Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int 52(2):509–516
Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J (1999) Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 104(1):73–81
Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J et al (2011) Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17(8):952–960
Beck LH, Bonegio RGB, Lambeau G, Beck DM, Powell DW, Cummins TD et al (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361(1):11–21
Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RAK (2014) Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25(6):1357–1366
Ramachandran R, Yadav AK, Kumar V, Inamdar N, Nada R, Gupta KL et al (2018) Temporal association between PLA2R antibodies and clinical outcomes in primary membranous nephropathy. Kidney Int Reports 3(1):142–147
Nam KH, Kie JH, Lee MJ, Chang TI, Kang EW, Kim DW et al (2014) Optimal proteinuria target for renoprotection in patients with IgA nephropathy. PLoS ONE 9(7):e0101935. https://doi.org/10.1371/journal.pone.0101935
Reich HN, Troyanov S, Scholey JW, Cattran DC (2007) Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18(12):3177–3183
Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreñ OA, Vigil A et al (2010) Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21(4):697–704
Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC (2004) Idiopathic membranous nephropatliy: definition and relevance of a partial remission. Kidney Int 66(3):1199–1205
Chun MJ, Korbet SM, Schwartz MM, Lewis EJ (2004) Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol 15(8):2169–2177
Vischer AS, Burkard T (2017) Principles of blood pressure measurement—current techniques, office vs ambulatory blood pressure measurement. Adv Exp Med Biol 956:85–96
Chen T, Li X, Li Y, Xia E, Qin Y, Liang S et al (2019) Prediction and risk stratification of kidney outcomes in IgA nephropathy. Am J Kidney Dis 74(3):300–309
Sood MM, Akbari A, Manuel D, Ruzicka M, Hiremath S, Zimmerman D et al (2017) Time-varying association of individual BP components with eGFR in late-stage CKD. Clin J Am Soc Nephrol 12(6):904–911
Alencar de Pinho N, Levin A, Fukagawa M, Hoy WE, Pecoits-Filho R, Reichel H et al (2019) Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int 96(4):983–994
Fuiano G, Mazza G, Comi N, Caglioti A, De Nicola L, Iodice C et al (2000) Current indications for renal biopsy: a questionnaire-based survey. Am J Kidney Dis 35(3):448–457
Acknowledgements
This research was supported by the Research of Korea Centers for Disease Control and Prevention (2019ER690101).
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
SHH and HWK contributed to the research idea and study design; S-WK, T-HY, SHH, HJC, D-RR, EWK, TIC, JTP, and HWK were involved in data acquisition; SHH and HWK were responsible for data analysis/interpretation; SHH, YSJ, SCK, JYL, SL, and HWK contributed to statistical analysis; JTP, TIC, EWK, T-HY, HJC, S-WK, and SHH were responsible for data analysis/interpretation supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
The study was performed in accordance with the Declaration of Helsinki principles, and the institutional review board of Yonsei University Health System approved this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The Korean GlomeruloNEphritis Study (KoGNET) Group.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, H.W., Park, J.T., Joo, Y.S. et al. Systolic blood pressure and chronic kidney disease progression in patients with primary glomerular disease. J Nephrol 34, 1057–1067 (2021). https://doi.org/10.1007/s40620-020-00930-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00930-x