Abstract
It is unknown whether intensive control of blood pressure (BP) and lipids can delay the progression of chronic kidney disease (CKD). This study examined the combined association of strict targets of systolic BP (SBP) and low-density lipoprotein cholesterol (LDL-C) levels with adverse kidney outcomes. In total, 2012 patients from the KoreaN Cohort Study for Outcomes in Patients With CKD (KNOW-CKD) were classified into four groups according to SBP of 120 mmHg and LDL-C of 70 mg/dl: group 1, <120 and <70; group 2, <120 and ≥70; group 3, ≥120 and <70; group 4, ≥120 and ≥70. We constructed time-varying models treating two variables as time-varying exposures. The primary outcome was the progression of CKD, defined as a ≥50% decrease in estimated glomerular filtration rate from the baseline or the onset of kidney failure requiring replacement therapy. The primary outcome events occurred in 27.9%, 26.7%, 40.3%, and 39.1% from groups 1 to 4. In the time-varying model, the hazard ratios (95% confidence intervals) for the primary outcome were 0.48 (0.33–0.69), 0.78 (0.63–0.96), and 0.96 (0.74–1.23) for groups 1 to 3, respectively, compared with group 4. When less stringent cut-offs of SBP of 130 mmHg and LDL-C of 100 mg/dl were used, this graded association was lost, while only SBP was associated with adverse kidney outcomes. In this study, the lower targets of SBP of <120 mmHg and LDL-C < 70 mg/dl were synergistically associated with a lower risk of adverse kidney outcomes.

Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is an important public health issue that affects ~10% of the adult population worldwide [1, 2]. CKD is defined as decreased kidney function based on glomerular filtration rate (GFR) less than 60 ml/min/1.73 m2, or presence of kidney damage such as structural abnormalities, abnormalities in urinary sediment, or increased urinary albumin excretion rates for 3 months or longer [3]. As CKD is characterized by irreversible and progressive nephron loss over the course of the disease, many patients require kidney replacement therapy (KRT) [1, 4]. In patients undergoing KRT, the cardiovascular risk is 20 times higher than in the general population [5]. Due to improved patient survival and increased access to KRT, the CKD management burden has grown.
Blood pressure (BP) control is important to attenuate the progression of CKD and prevent the occurrence of adverse cardiovascular events [6, 7]. Hyperlipidemia is also an independent risk factor for cardiovascular disease (CVD) in CKD patients [8, 9]. Because CVD is the leading cause of mortality in these patients, the optimal control of these two factors is a cornerstone of management. Recently, the 2021 Kidney Disease Improving Global Outcomes (KDIGO) BP guideline recommended a target systolic BP (SBP) of <120 mmHg for CKD patients [2]. This target was mainly driven by the results of the Systolic Blood Pressure Intervention Trial (SPRINT), which showed that lowering SBP < 120 mmHg resulted in a lower risk of cardiovascular events and all-cause mortality [10]. However, previous studies have shown conflicting results regarding the optimal BP target for preserving kidney function [11,12,13], and this issue remains an ongoing debate. Possible explanations for this discrepancy are differences in BP targets, BP measurement, study populations, and baseline kidney function among the studies.
As CKD and CVD share common risk factors, management of dyslipidemia is also expected to improve kidney outcomes. However, major trials investigating the efficacy of lipid-lowering therapy in the CKD population have focused on cardiovascular outcomes [14,15,16]. These studies demonstrated no meaningful cardiovascular benefits of lipid-lowering drugs in patients with kidney failure who underwent KRT [15, 16], but a significant reduction in cardiovascular events in those who did not [14]. Notably, the concept of a lower target of low-density lipoprotein cholesterol (LDL-C) level, the better the cardiovascular outcome, has been proven in several clinical trials [17,18,19]. Of these studies, two trials showed that a reduction in LDL-C < 70 mg/dl with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors on a background of statin therapy significantly reduced the risk of CVD in patients with atherosclerotic CVD. Thus, most guidelines have adopted a target LDL-C level of <70 mg/dl, particularly in patients at high risk of CVD [20, 21].
Unfortunately, these two stricter goals of SBP < 120 mmHg and LDL-C of <70 mg/dl with respect to delayed CKD progression have never been tested to date. Observational studies have shown that lower SBP and LDL-C levels are associated with a slower progression of kidney function decline [22,23,24]. However, there have been concerns about adverse kidney outcomes caused by intensive BP control in previous randomized controlled trial studies (RCTs) [25, 26]. With this background, we examined the combined association of SBP and LDL-C with the risk of adverse kidney outcomes using the recently recommended lower targets for SBP and LDL-C in CKD patients.
Materials and methods
Study design and participants
The KoreaN Cohort Study for Outcome in Patients with CKD (KNOW-CKD) is a multicenter prospective observational cohort study. Between June 2011 and February 2016, patients aged 20–75 years with CKD G1–G5, who did not receive KRT were enrolled from nine tertiary-care hospitals. The detailed design and methods of KNOW-CKD have been previously published (NCT01630486; http://www.clinicaltrials.gov) [27].
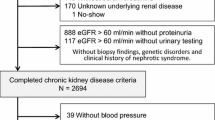
Among the 2238 patients in the KNOW-CKD cohort, we excluded 226 patients who had missing baseline SBP (n = 12), LDL-C (n = 57), and other covariates (n = 145). In addition, 12 patients with a follow-up duration of less than 1 year were excluded. Therefore, a total of 2012 patients were included in the analysis (Fig. 1). The study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Boards of participating centers. All participants were provided with informed consent.
Data collection and measurements
Socio-demographic characteristics including age, sex, economic status, education, alcohol intake, smoking status, comorbid disease and medication were recorded at enrollment. Education level was categorized as low, less than middle school; middle, middle school; and high, more than middle school. Economic status was divided into three groups by monthly income: low, <$1635 per month; middle, $1635–$4905 per month; and high, ≥$4905 per month. Body mass index (BMI) was calculated by initial body weight divided by height squared (kg/m2). BP and serum creatinine levels were measured at baseline, 6 months, and 12 months in the first year and annually thereafter. Lipid profiles were measured at baseline and first, third, and seventh years using the direct enzyme method. Detailed information regarding data collection and measurements is provided in the Supplementary Materials.
BP was measured at the clinic office using a calibrated oscillometer electronic sphygmomanometer after 5 min of rest in a sitting position. All measurements were performed by a trained nurse at each center following the standardized protocol as suggested by the American Heart Association [28]. The mean of 3 BP readings was used as the BP value for each visit. Hypertension was defined as a previous history of physician-diagnosed hypertension, SBP ≥ 140 mmHg, diastolic BP (DBP) ≥ 90 mmHg, or use of antihypertensive drugs.
Blood samples were collected and sent to the central laboratory (Lab Genomics, Seoul, Korea) for measurements of creatinine after overnight fast. Serum creatinine was measured by an isotope-dilution mass spectrometry-traceable method, and estimated glomerular filtration rate (eGFR) was calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation [29]. The second voided urine was collected and immediately sent to the central laboratory. Urine protein and albumin excretion was determined as urinary protein-to-creatinine ratio (UPCR) and urinary albumin-to-creatinine ratio. Other laboratory tests including hemoglobin, calcium, phosphate, albumin, high-sensitive C-reactive protein, and lipid profiles (total cholesterol, LDL-C, triglycerides, and high-density lipoprotein cholesterol [HDL-C]) were performed at the local laboratory of each participating center.
Exposure and outcome ascertainment
The main predictors in this study were time-varying SBP and LDL-C at each visit. Patients were classified in a 2 × 2 design according to the cut-off values of SBP 120 mmHg and LDL-C 70 mg/dl, referring to the strict targets recommended by recent guidelines [21, 30,31,32]: SBP < 120 mmHg and LDL-C < 70 mg/dl (group 1); SBP < 120 mmHg and LDL-C ≥ 70 mg/dl (group 2); SBP ≥ 120 mmHg and LDL-C < 70 mg/dl (group 3); and SBP ≥ 120 mmHg and LDL-C ≥ 70 mg/dl (group 4, reference).
The primary outcome was the progression of CKD, defined as a composite of a ≥50% decrease estimated glomerular filtration rate (eGFR) from baseline values, or the onset of kidney failure with replacement therapy (KFRT). KFRT was defined as the initiation of KRT, including dialysis or kidney transplantation. The secondary outcomes included individual components of the composite kidney outcome and all-cause mortality. Patients were censored at the date of the last visit, for all events, or death. The observations were closed on March 31, 2020.
Statistical analysis
To evaluate the association of SBP and LDL-C with study outcomes, we primarily implemented time-varying models to account for time-dependent changes in the main exposures and other variables (body mass index (BMI), eGFR, lipid profile, hemoglobin, albumin, and medications) over time [33, 34]. Variables that were well-known risk factors for CKD progression or variables with p values <0.10 in univariable analyses were included in the model for adjustment. The adjusted covariates were age, sex, BMI, smoking status, Charlson comorbidity index (CCI), laboratory parameters, and medications. Laboratory parameters included eGFR, urinary protein-to-creatinine ratio (UPCR), hemoglobin, albumin, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglyceride levels. The medications included BP-lowering drugs (renin-angiotensin system blockers, beta-blockers, dihydropyridine calcium channel blockers, and diuretics) and lipid-lowering drugs (statins, fibrates, and ezetimibe). The results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). We additionally performed a conventional Cox proportional hazards regression analysis using baseline SBP and LDL-C levels. Furthermore, we performed two sensitivity analyses to validate our findings. First, we constructed additional Cox proportional hazards model after excluding patients with CKD G5 because eGFR <15 ml/min/1.73 m2 is considered comparable to KFRT. Second, based on less stringent cut-off values of SBP of 130 mmHg and LDL-C of 100 mg/dl, we classified them into four groups and then constructed time-varying models after adjustment for the same variables that were used in the primary analysis above. We also examined effect modification by several key subgroups stratified by age (<60 and ≥60 years), sex, BMI (<25 and ≥25 kg/m2), eGFR (<50 and ≥50 ml/min/1.73 m2), type 2 diabetes (T2DM; yes or no), and UPCR (<3.5 and ≥3.5 g/g). All statistical analyses were performed with Stata 14 statistical software (StataCorp, College Station, TX), with a p value of 0.05 considered significant.
Results
Baseline characteristics
The baseline characteristics of the participants according to SBP and LDL-C levels are described in Table 1. The mean age of the patients was 53.7 years, and 61.3% were male. The mean baseline eGFR was 53.2 ± 12.3 ml/min/1.73 m2 and the mean BMI was 24.6 ± 3.4 kg/m2. The mean SBP was 128.1 ± 16.2 mmHg and the mean LDL-C was 96.9 ± 31.5 mg/dl at baseline. Of these patients, 691 (34.3%) had T2DM and 314 (15.6%) had CVD. We observed several significant differences in baseline characteristics among the four groups. Of note, patients with SBP ≥ 120 mmHg had more T2DM and higher proteinuria, and were treated with more BP-lowering drugs than those with SBP < 120 mmHg. In addition, patients with LDL-C < 70 mg/l had a lower eGFR, experienced more CVD, and were more likely to be treated with statins than those with LDL-C ≥ 70 mg/dl (Table 1).
Association of SBP and LDL-C with adverse kidney outcome
During a median follow-up period of 4.88 years, 708 participants (35.2%) reached the primary outcome with an overall incidence rate of 75.5 (95% CI, 70.1–81.3) per 1000 person-years (Table 2). There were 39 (27.9%), 143 (26.7%), 112 (40.3%), and 414 (39.1%) events of the primary outcome with corresponding incidence rates of 57.6, 53.1, 93.8, and 86.0 per 1000 person-years in groups 1, 2, 3, and 4, respectively (Table 2). We then analyzed the association of SBP and LDL-C with the primary composite outcome using a time-varying model. The risk of the primary outcome was higher in the patients with higher SBP and LDL-C levels. Compared with a reference group 4, the adjusted HRs (95% CIs) for group 1, 2, and 3 were 0.48 (95% CI, 0.33–0.69), 0.78 (95% CI, 0.63–0.96), and 0.96 (95% CI, 0.74–1.23), respectively (Table 3). The p-for-interaction for this association was 0.648, suggesting that both factors were independently associated with the primary outcome. This graded association was also observed in the adjusted cumulative incidence curve (Fig. 2). A significantly graded association observed in the time-varying model was also observed in the Cox proportional hazards model using baseline SBP and LDL-C levels. Compared with group 4, the adjusted HRs (95% CIs) for groups 1, 2 and 3 were 0.64 (0.44–0.91), 0.74 (0.61–0.91) and 0.84 (0.65–1.08), respectively (Table 3).
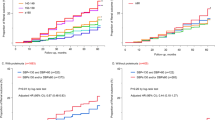
Adjusted cumulative incidence function for CKD progression according to strict targets of SBP and LDL-C. The model was adjusted for age, sex, body mass index, smoking status, and Charlson comorbidity index, estimated glomerular filtration rate, urinary protein-creatinine ratio, hemoglobin, albumin, total cholesterol, high-density lipoprotein cholesterol, triglyceride, and use of BP-lowering drugs (renin-angiotensin system blockers, beta-blockers, dihydropyridine calcium channel blockers and diuretics) and lipid-lowering drugs (statin, fibrate, and ezetimibe). CKD progression was defined as a decline of ≥50% in eGFR or the onset of KFRT
Secondary outcome analyses
For secondary analyses, we separately analyzed eGFR halving, KFRT, and all-cause mortality. During follow-up, events of eGFR halving occurred in 26 (18.6%), 103 (19.3%), 63 (22.7%), and 289 (27.3%) from groups 1 to 4 with corresponding incidence rates of 36.0, 36.6, 46.4, and 55.1 per 1000 person-years, respectively (Table 2). In analysis with time-varying SBP and LDL-C levels, compared with group 4, the adjusted HRs (95% CI) for groups 1, 2, and 3 were 0.46 (0.29–0.71), 0.83 (0.65–1.07), and 0.84 (0.61–1.15), respectively (Table S1). This association was similar in the analysis of baseline SBP and LDL-C levels. Additional analysis with an outcome of KFRT showed a similar association, but a p value was <0.05 only for SBP < 120 mmHg and LDL-C < 70 mg/dl in time-varying model (Table S2). For all-cause mortality, there was a trend for the association of lower SBP and LDL-C with lower risk of mortality in time-varying model, but it did not reach statistical significance (Table S3).
Sensitivity analysis
A sensitivity analysis after excluding 130 patients with CKD G5 (eGFR <15 ml/min/1.73 m2) showed consistent findings with the primary outcome. The adjusted HRs for groups 1, 2, and 3 were 0.51 (95% CI, 0.35–0.75), 0.80 (95% CI, 0.64–0.98), and 0.90 (95% CI, 0.68–1.19), compared with group 4 (Table S4). Additional analysis was performed using less stringent cut-off values of SBP of 130 mmHg and LDL-C of 100 mg/dl. Based on these cut-offs, patients were classified into four groups in the same manner as in the primary analysis. Among patients with SBP ≥ 130 mmHg, there was no difference in the risk of the primary outcome between LDL-C categories of <100 and ≥100 mg/dl. In contrast, among patients with SBP < 130 mmHg, the adjusted HRs for LDL-C categories of <100 mg/dl (HR, 0.73; 95% CI, 0.54–0.97) and ≥100 mg/dl (HR, 0.80; 95% CI, 0.65–0.99) were significantly lower than those with SBP ≥ 130 mmHg and LDL-C ≥ 100 mg/dl, but the magnitudes of HRs were similar between the two LDL-C categories (Table S5). These findings suggest that an LDL-C cut-off of 100 mg/dl did not significantly affect the association between SBP and adverse kidney outcomes.
Subgroup analysis
We further examined whether several subgroup factors might act as effect modifiers in the relationship of SBP and LDL-C with adverse kidney outcomes. All p-for-interactions were not significant, suggesting that a consistent association existed regardless of age, sex, BMI, T2DM, baseline kidney function, and urinary protein excretion (Fig. 3).
Subgroup analysis for the association of SBP and LDL-C with the CKD progression. Group 1, SBP <120 mmHg and LDL-C <70 mg/dl; group 2, SBP <120 mmHg and LDL-C ≥70 mg/dl; group 3, SBP ≥120 mmHg and LDL-C <70 mg/dl; group 4, SBP ≥120 mmHg and LDL-C ≥70 mg/dl. Group 4 was reference category. All models were adjusted for age, sex, BMI, smoking status, Charlson comorbidity index, laboratory parameters (eGFR, UPCR, hemoglobin, albumin, total cholesterol, HDL-C, and triglyceride), and use of BP-lowering drugs (RAS blockers, beta-blockers, dihydropyridine calcium channel blockers and diuretics) and lipid-lowering drugs (statin, fibrate, and ezetimibe). SBP systolic blood pressure, LDL-C low-density lipoprotein cholesterol, BMI body mass index, eGFR estimated glomerular filtration rate, UPCR urinary protein-creatinine ratio, HDL-C high-density lipoprotein cholesterol, RAS renin-angiotensin system, KRT kidney replacement therapy. CKD progression was defined as a decline of ≥50% in eGFR or initiation of KRFT
Discussion
We examined the association of the recently recommended lower targets of SBP and LDL-C with the risk of adverse kidney outcomes in CKD patients. We found that time-varying SBP < 120 mmHg and LDL-C < 70 mg/dl was associated with a significantly lower risk of the primary outcome of CKD progression. In both SBP categories (<120 and ≥120 mmHg), the magnitudes of the HRs for adverse kidney outcomes were numerically lower in patients with LDL-C < 70 mg/dl than in counterparts with LDL-C ≥ 70 mg/dl. However, this association was attenuated in the analysis with less stringent cut-offs of SBP 130 mmHg and LDL-C 100 mg/dl. Thus, lower SBP and LDL-C levels were synergistically associated with a decreased risk of adverse kidney outcomes when stricter SBP and LDL-C targets were used.
There have been paradigm shifts toward lowering the goals of BP and LDL-C in the management of atherosclerotic CVD. The KDIGO panel for BP guidelines agrees with the SPRINT study and recommends an SBP target of <120 mmHg as the cardiovascular benefit of this lower target was observed in CKD patients [2]. In addition, recent trials with lipid-lowering therapies have also demonstrated the clinical benefits of lowering LDL-C levels below 70 mg/dl [17,18,19]. As a result, the lower, the better concept has prevailed in cardiovascular research and clinical practice. However, the optimal goals for SBP and LDL-C levels in CKD patients are unknown. The KDIGO panel for BP guideline agrees with SPRINT study and recommends a SBP target <120 mmHg as cardiovascular benefits of this lower target was observed in patients with CKD. Moreover, lowering SBP to <120 mmHg resulted in more adverse kidney events, including acute kidney injury and CKD [26, 35]. In the Action to Control Cardiovascular Disease in Diabetes (ACCORD) study, similar SBP targets also caused more adverse kidney events [36]. These findings contrast with those of the Modification Diet in Renal Disease (MDRD) study, which showed that lowering the mean arterial BP to 92 mmHg significantly slowed the decline in kidney function in patients with proteinuria >1.0 g/day [37]. We previously showed a graded relationship between SBP and CKD progression, and lower SBP was associated with a significantly lower risk of adverse kidney outcomes, using time-updated SBP models [22]. The reasons for the discrepant findings among these studies are uncertain. Notably, participants in the SPRINT and ACCORD studies had a baseline eGFR >70 ml/min/1.73 m2, while the MDRD study and our cohort study included only CKD patients with a median eGFR <50 ml/min/1.73 m2 and most patients had overt proteinuria. Thus, it can be presumed that in CKD patients, a lower SBP target may be acceptable for improving the cardiovascular and kidney outcomes. However, we should also note that there has been a U-shaped association between BP and cardiovascular outcomes in many observational studies [38,39,40]. This association was also observed in our previous study that used the same cohort data [41]. Nevertheless, these observational studies cannot prove causality, and no RCTs have examined the effects of intensive BP control, particularly in patients with advanced CKD. Therefore, the optimal BP target to achieve the two important goals of cardio and renal protection remains inconclusive.
We face a similar unresolved issue with respect to lowering lipid levels in CKD patients. In the Study of Heart and Renal Protection (SHARP), the largest trial conducted for advanced CKD patients to date, statin and ezetimibe treatment significantly reduced the risk of major atherosclerotic CVD [14]. Based on these results, the 2013 KDIGO guidelines for lipid management in CKD recommended either statin or statin/ezetimibe treatment for CKD patients without KRT who are aged >50 years and with an eGFR of <60 [42]. However, there was no beneficial effect on kidney disease progression despite a significant reduction in LDL-C level [43,44,45]. Atherogenic lipoproteins can cause glomerular injury through the aggravation of atherosclerosis in the renal microcirculation and the deposition of lipoproteins in glomerular structures [46,47,48]. In addition, lipid accumulation can also induce infiltration of macrophage and formation of foam cell, and in turn, mesangial cells fail to contract and secrete extracellular matrix that promotes to glomerulosclerosis [49]. Although these findings may justify the use of lipid-lowering therapy for kidney protection, high-level clinical evidence on this issue is sparse. One possible explanation is the effect of decreased BP on intra-glomerular pressure. Lowered intra-glomerular pressure is a major mechanism responsible for beneficial effects of RAAS blockers and SGLT2 inhibitors [50,51,52]. Thus, the hemodynamic effect of BP may outweigh the effect of lowering LDL-C against kidney injury.
A notable finding in this study was that there was a synergistic association of SBP and LDL-C with adverse kidney outcomes when we analyzed the stricter targets of these two factors. This association was remarkably attenuated in the analysis with conventional targets, in which an LDL-C cut-off of 100 mg/dl contributed little to adverse kidney outcomes. Interestingly, the Japan Diabetes Optimal Integrated Treatment Study for three major risk factors of cardiovascular diseases (J-DOIT3) demonstrated that multifactorial interventions with BP < 120/75 mmHg, HbA1c < 6.2%, and LDL-C < 80 mg/dl significantly reduced the onset and progression of diabetic kidney disease compared to currently recommended care in patients with T2DM and baseline mean eGFR >80 ml/min/1.73 m2 [53]. In line with this study, intensive control of BP and lipids together also resulted in a greater reduction in adverse cardiovascular events than dual placebo in individuals with intermediate cardiovascular risk [54]. These findings can explain our data showing that lower targets of SBP and LDL-C levels were synergistically associated with a lower risk of adverse kidney outcomes.
This study had several limitations. First, owing to the observational design of this study, the causal relationship between exposures and kidney outcomes could not be ascertained, and potential confounding factors could not be completely controlled. However, we exclusively analyzed CKD patients and made efforts to reduce bias by adjusting for multiple variables that might affect kidney outcomes. Second, the lipid profiles including total cholesterol, LDL-C and HDL-C were measured at the local laboratory of each participating center. All centers employed the same direct enzymatic assay, which has minimized the bias derived from various methods. Third, we used annual BP readings for analysis, which might not precisely represent the BP control status throughout the observation period. To minimize this shortcoming, we used time-varying models with rigorous adjustments. The use of clinic office BP is another limitation although we used standardized office BP measurement as recommended by the AHA [28]. Fourth, achieving a target <120 mmHg is unrealistic in clinical practice. In fact, the Korean Hypertension Guidelines also suggest a SBP target <130 mmHg. However, achieved BPs in cohort studies and lowered BPs in RCTs may have different clinical implications with respect to CKD outcomes. Nevertheless, there was a concern on unintended adverse effects of excessive BP lowering particularly in frail elderly individuals. Unfortunately, our study is not an RCT looking at the effects of BP, hypotension-related side effects were not recorded as adverse events during follow-up. Finally, the lower targets of SBP and LDL-C in this study were based on recent clinical trials. In our cohort study, both pharmacologic and non-pharmacologic interventions were allowed for risk management, and we could not discern the relative contribution of these interventions to the achieved targets. Moreover, it is unknown whether the clinical implications of inherently low SBP and LDL-C levels are equivalent to those of the same levels reduced by specific interventions. Further well-designed RCTs are needed to explore this unresolved issue.
Perspective of Asia
Several recent guidelines recommend lower targets of BP and LDL-C to reduce adverse cardiovascular outcomes, mostly based on clinical trials in Western countries showing clinical benefits of these lower targets [2, 20, 32, 42, 55, 56]. However, many Asian countries have not yet adopted such lower targets and these targets have never been tested in Asian patients. Moreover, in terms of kidney protection, there has been concern on untoward effects of intensive BP control on kidney function. Several studies have suggested that the association between high BP and CVD may be stronger in Asian countries than in Western countries [57, 58]. Notably, hypertension-related complications such as stroke and non-ischemic heart failure are more common in Asian countries. However, there is no customized strategy of BP and lipid control particularly for Asian patients. In this KNOW-CKD cohort comprising only Koreans, we examined the association of the recently recommended lower targets of SBP and LDL-C with the risk of adverse kidney outcomes. Among 2012 patients with CKD, SBP < 120 mmHg and LDL-C levels <70 mg/dl were synergistically associated with a lower risk of CKD progression. However, it is unknown whether such beneficial association of the lower targets of SBP and LDL-C could exist in other Asian patients. Thus, further studies are needed to clarify if there is ethnic difference in the effect of intensive BP and lipid control on diverse outcomes such as cardiovascular events, CKD progression, and mortality.
Conclusion
In conclusion, we found that SBP and LDL-C levels lower than the recently recommended lower targets were synergistically associated with a significantly lower risk of adverse kidney outcomes. This association was attenuated when the conventional targets of SBP and LDL-C. Whether the intensive control of these two targets is effective in advanced CKD remains a challenge.
References
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–33.
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:S1–s87.
Kidney Disease: Improving Global Outcomes (KDIGO). Chapter 1: definition and classification of CKD. Kidney Int Suppl (2011). 2013;3:19–62.
Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–52.
Gupta R, Woo K, Yi JA. Epidemiology of end-stage kidney disease. Semin Vasc Surg. 2021;34:71–8.
Hanratty R, Chonchol M, Havranek EP, Powers JD, Dickinson LM, Ho PM, et al. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin J Am Soc Nephrol. 2011;6:2605–11.
Tsuchida-Nishiwaki M, Uchida HA, Takeuchi H, Nishiwaki N, Maeshima Y, Saito C, et al. Association of blood pressure and renal outcome in patients with chronic kidney disease; a post hoc analysis of FROM-J study. Sci Rep. 2021;11:14990.
Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388:2665–712.
Wu CY, Hu HY, Chou YJ, Huang N, Chou YC, Li CP. High blood pressure and all-cause and cardiovascular disease mortalities in community-dwelling older adults. Medicine. 2015;94:e2160.
Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16.
Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31.
Collard D, Brouwer TF, Olde Engberink RHG, Zwinderman AH, Vogt L, van den Born BH. Initial estimated glomerular filtration rate decline and long-term renal function during intensive antihypertensive therapy: a post hoc analysis of the SPRINT and ACCORD-BP randomized controlled trials. Hypertension. 2020;75:1205–12.
Beddhu S, Greene T, Boucher R, Cushman WC, Wei G, Stoddard G, et al. Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol. 2018;6:555–63.
Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92.
Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48.
Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88.
Lee JY, Park JT, Joo YS, Lee C, Yun HR, Yoo TH, et al. Association of blood pressure with the progression of CKD: findings from KNOW-CKD study. Am J Kidney Dis. 2021;78:236–45.
Lee C, Park JT, Chang TI, Kang EW, Nam KH, Joo YS, et al. Low-density lipoprotein cholesterol levels and adverse clinical outcomes in chronic kidney disease: results from the KNOW-CKD. Nutr Metab Cardiovasc Dis. 2022;32:410–9.
Chen SC, Hung CC, Kuo MC, Lee JJ, Chiu YW, Chang JM, et al. Association of dyslipidemia with renal outcomes in chronic kidney disease. PLoS ONE. 2013;8:e55643.
Beddhu S, Shen J, Cheung AK, Kimmel PL, Chertow GM, Wei G, et al. Implications of early decline in eGFR due to intensive BP control for cardiovascular outcomes in SPRINT. J Am Soc Nephrol. 2019;30:1523–33.
Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, et al. Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis. 2018;71:352–61.
Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): design and methods. BMC Nephrol. 2014;15:80.
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH, et al. Executive summary of the KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:559–69.
Tonelli M, Wanner C, Cass A, Garg A, Holdaas H, Jardine A, et al. Kidney disease: improving global outcomes (KDIGO) lipid work group. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:1–315.
Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pr. 2017;23:1–87.
Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6:121.
Xie D, Yang W, Jepson C, Roy J, Hsu JY, Shou H, et al. Statistical methods for modeling time-updated exposures in cohort studies of chronic kidney disease. Clin J Am Soc Nephrol. 2017;12:1892–9.
Beddhu S, Rocco MV, Toto R, Craven TE, Greene T, Bhatt U, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med. 2017;167:375–83.
Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85.
Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–62.
Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16:2170–9.
Kovesdy CP, Lu JL, Molnar MZ, Ma JZ, Canada RB, Streja E, et al. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA Intern Med. 2014;174:1442–9.
Navaneethan SD, Schold JD, Jolly SE, Arrigain S, Blum MF, Winkelmayer WC, et al. Blood pressure parameters are associated with all-cause and cause-specific mortality in chronic kidney disease. Kidney Int. 2017;92:1272–81.
Lee JY, Park JT, Joo YS, Lee C, Yun HR, Chang TI, et al. Association of blood pressure with cardiovascular outcome and mortality: results from the KNOW-CKD study. Nephrol Dial Transplant. 2021. https://doi.org/10.1093/ndt/gfab257.
Wanner C, Tonelli M. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303–9.
Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17:2006–16.
Tonelli M, Isles C, Craven T, Tonkin A, Pfeffer MA, Shepherd J, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112:171–8.
Esmeijer K, Dekkers OM, de Fijter JW, Dekker FW, Hoogeveen EK. Effect of different types of statins on kidney function decline and proteinuria: a network meta-analysis. Sci Rep. 2019;9:16632.
Kamanna VS, Roh DD, Kirschenbaum MA. Atherogenic lipoproteins: mediators of glomerular injury. Am J Nephrol. 1993;13:1–5.
Kasiske BL. Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int. 1987;31:1153–9.
Diamond JR, Karnovsky MJ. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988;33:917–24.
Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol. 2004;24:46–53.
Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786–802.
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72.
Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39.
Ueki K, Sasako T, Okazaki Y, Miyake K, Nangaku M, Ohashi Y, et al. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int. 2021;99:256–66.
Yusuf S, Lonn E, Pais P, Bosch J, López-Jaramillo P, Zhu J, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. 2016;374:2032–43.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–115.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041.
Lawes CM, Bennett DA, Parag V, Woodward M, Whitlock G, Lam TH, et al. Blood pressure indices and cardiovascular disease in the Asia Pacific region: a pooled analysis. Hypertension. 2003;42:69–75.
Arima H, Murakami Y, Lam TH, Kim HC, Ueshima H, Woo J, et al. Effects of prehypertension and hypertension subtype on cardiovascular disease in the Asia-Pacific Region. Hypertension. 2012;59:1118–23.
Acknowledgements
We thank the clinical research coordinators of each participating center for their dedication during patient recruitment and data acquisition.
Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (grants 2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, 2019E320101, 2019E320102, 2022-11-007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, K.W., Koh, H.B., Kim, H.W. et al. Systolic blood pressure, low-density lipoprotein cholesterol levels, and adverse kidney outcome: results from KNOW-CKD. Hypertens Res 46, 1395–1406 (2023). https://doi.org/10.1038/s41440-023-01230-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01230-0
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Behavior of albuminuria in patients with a more intense control of their blood pressure
Hypertension Research (2023)
-
Association of strict targets of systolic blood pressure, low-density lipoprotein cholesterol level with albuminuria in patients with chronic kidney disease
Hypertension Research (2023)