Abstract
Background
Peritoneal dialysis (PD) related infections continue to be a major cause of morbidity and mortality in patients on PD. In the last ten years, in order to reduce cuff and exit-site infections, in continuous ambulatory peritoneal dialysis (CAPD) patients, we have positioned the superficial cuff subcutaneously 4 cm instead of 2 cm internal to the exit-site.
Methods
We analysed the infective episodes occurred in 123 CAPD patients (88 men and 35 women, mean age 62.4 ± 16.8) treated for 3337 months between 1st January 2011 and 31th December 2018 at Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico.
Results
31 of the 123 patients (25.2%) developed 52 episodes of exit site infection, with an incidence of 1 episode every 64.1 patient-months. The cumulative probability of remaining infection free was 80.7% at 12 months and 61.8% at 36 months. Gram-positive organism accounted for 78.7% of exit site infections. Forty-one episodes (87%) were successfully treated with medical therapy. Peritonitis incidence was 1 episode every 51.7 and 1 episode every 49.2 patient-months, in patients with or without a history of exit site infection respectively. The overall incidence of tunnel infection was 1 episode every 278.1 patient-months.
Conclusions
Positioning the superficial cuff subcutaneously at least 4 cm internal to the exit-site might prevent the bacterial cuff colonization and reduce ESIs, tunnel infections and peritonitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal dialysis (PD) related infections continue to be a major cause of morbidity and mortality in patients on renal replacement therapy with PD [1,2,3,4]. Over the three past decades great effort has been done in the preventing infectious complications in PD [5, 6]. Despite the improvements of the connection methods [7, 8], the optimization of the exit-site care [9] and the creation of specific schedules for PD training [10, 11], one third of technical failures are still caused by peritonitis [12, 13]. In addition, old and recent studies [14,15,16,17,18] support the hypothesis that exit-site infection (ESI) plays an important role in causing peritonitis trough the transmigration of the organisms from the exit-site along the tunnel of the PD catheter into the peritoneal cavity [19, 20]. Nevertheless, few cases of peritonitis associated to ESIs episodes without tunnel involvement have been reported, suggesting the possibility of an intraluminal transmission of the bacteria [16]. We suppose that the superficial cuff might represent a suitable site for microorganism colonisation causing recurring bacteria migration towards the exit-site. This process might induce peritonitis by increasing the likelihood of patient’s hand contamination and the subsequent migration of the microorganisms into the catheter lumen during the exchange manoeuvre.
According to this hypothesis, a longer subcutaneous tunnel with an increased distance between the superficial cuff and the exit-site, might decrease the incidence of ESIs and associated peritonitis.
Methods
Participants and study design
All incident, adult continuous ambulatory peritoneal dialysis (CAPD) patients between 1st January 2011 and 31th December 2018 at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico were included in the study. We did not include five patients treated with automated peritoneal dialysis. This was a retrospective cohort study undertaken through a case note review at a single nephrological center; ethical approval was therefore not required. Patients were followed until the end of the study period or until discontinuation of CAPD due to death, transplantation or technique failure.
We calculated the overall rates of infective episodes (ESIs, tunnel infections, peritonitis), the percentage of specific organisms and the percentage of ESI and peritonitis at determined months of follow-up. We reported the infective episode rates as an episode per number of patient-months of follow-up (relapsing episodes were counted once).
Definitions
According to the International Society for Peritoneal Dialysis [5, 6], ESI was defined as the presence of purulent discharge, with or without erythema of the skin at the catheter-epidermal interface. Tunnel infection was defined as the presence of clinical inflammation along the catheter tunnel. Peritonitis was diagnosed when at least 2 of these conditions were present: (1) clinical features consistent with peritonitis (e.g. abdominal pain and/or cloudy dialysis effluent); (2) dialysis effluent white cell count > 100/μL or > 0.1 × 109/L (after a dwell time of at least 2 h), with > 50% polymorphonuclear cells; and (3) positive dialysis effluent culture. Catheter-related peritonitis was defined as peritonitis in conjunction with a contemporary ESI or tunnel infection with the same organism. Death associated with peritonitis was defined as death with active peritonitis or within 4 weeks of a peritonitis episode.
Relapsing ESI was defined as an episode of ESI that occurred within 4 weeks after completion of therapy for a prior episode with the same organism. Repeated ESI was defined as two or more ESIs with the same organism within a 12-month period and an initial positive response to antibiotic therapy as recently proposed by Beckwith et al. [21].
Treatments of the ESI were classified as systemic (oral, intravenous, and/or intraperitoneal antibiotics) or non-systemic (topical antibiotics or other localized treatments).
Exit-site infection (ESI) was defined as mild in the presence of scarce purulent drainage, minor pain and no sign of superficial cuff involvement, while was classified as severe in the presence of vast erythema, purulent drainage, significant pain and/or sign of superficial cuff involvement.
Exit-site infection (ESI) was considered to be unresponsive to medical therapy (refractory ESI) when the infective episode was not healed after 3 weeks of appropriate treatment.
Clinical management
In every patient a straight Dacron double-cuffed Tenckhoff catheter was placed with a modified double purse-string technique around the inner-cuff either in semi-surgical or surgical procedure, as described elsewhere [22]. The placement of the subcutaneous part was created by using a piercing tunneller in a direction able to minimize shear forces with the superficial cuff located at least 4 cm from the exit of the skin. In all patients CAPD was started within 24 h after catheter placement. After the catheter was positioned, an occlusive dressing wetted with povidone iodine was applied and secured by tape. This dressing was changed every other day until healing was completed. After healing, all patients were instructed to apply hydrogen peroxide followed by 5% sodium hypoclorite solution to the skin surface 3–4 times per week. Patients covered their exit sites with sterile gauze. When ESI was diagnosed according to the definitions, microbiological examination including a combination of aerobic, anaerobic and fungal culture was performed. Subsequently, empiric treatment of ESI was started. The empiric treatment was not standardised, but it was decided on basis of the clinical features. In particular, when ESI was judged to be mild, it was initially treated with intensive local care, consisting of daily applications of hydrogen peroxide and 5% solution sodium hypochlorite and application of antibacterial cream (mupirocin or gentamicin according to clinical suspicion of Gram-positive or Gram-negative microorganism, respectively). In case of a severe ESI a systemic treatment was initiated. Generally, in the clinical suspicion of ESI caused by Gram-positive bacteria (copious purulent drainage and/or previous Gram-positive ESI), oral rifampicin (600 mg/die) plus intravenous vancomycin (1 g as loading dose and then the following dose being fixed according to the antibiotic serum level) were administered; otherwise monotherapy with an oral fluoroquinolone (either ciprofloxacin 500 mg/die o levofloxacin 250 mg/die) was given. Antibiotics were then adjusted according to the results of the culture. Whenever ESI was considered to be unresponsive to medical therapy, the catheter was surgically removed, and the antibiotic therapy maintained one more week after the removal.
Statistical analysis
Normally distributed variables are presented as mean ± standard deviation, while nonparametric data are presented as median with interquartile range. Categorical variables are expressed as frequency and percentage. The parametric continuous variables were compared by the Student t-test; otherwise the Mann–Whitney U-test was used. Fisher’s exact test for 2 × 2 contingency tables and Chi-square analysis for larger table were used to compare the nominal data. All probabilities were two tailed, and significance level was set at 0.05 to reject the null hypothesis. Life-table analysis (Kaplan–Meier method) was used to calculate the probability for a patient to be free from ESI, or peritonitis at a given time. Patients who were transplanted, shifted to haemodialysis or died were censored from the calculation. The life-table curves were statistically compared using the Logrank test and the Hazard Ratio was provided with a confidence interval of 95%. SPSS version 16.0 (SPSS, Inc, Chicago, IL, USA) software package was used for the statistical calculations.
Results
Population characteristics
The study population consisted of 123 CAPD patients. The demographic and baseline clinical characteristics of the study population are summarized in Table 1. Most patients were male (71.5%) with a mean age of 62.4 ± 16.9 years. Patients were on CAPD for a mean time of 24.6 ± 23.5 months and were followed up to catheter removal, kidney transplant or death for a total of 3337 patient-months.
Incidence, causative organisms and clinical outcomes
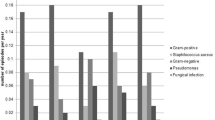
During the study period 31 patients (25.2%) out of 123 experienced 52 ESIs. Nineteen patients had 1 episode, two patients 3, two patients 4 and one patients 5 episodes. The overall ESI rate was 1 episode per 64.2 patient-months. ESIs were treated using systemic antibiotics in 46 and non-systemic treatment in 6 cases as summarized in Table 2. The microbiological causes of ESIs are reported in Table 3. In particular, Gram-positive and Gram-negative bacteria accounted for 78.7% and 21.3% of ESIs, respectively. The two most common Gram-positive microorganism were Staphylococcus aureus (53.8%) and Staphylococcus epidermidis (13.4%), while the most common Gram-negative was Pseudomonas aeruginosa (11.5%). In five cases results of the culture were missing, thus the causative organism remained unknown.
Twelve tunnel infections occurred in 11 patients. The overall tunnel infection rate was 1 episode per 278.1 patient-months. The two most common microbiological cause of tunnel infections were Staphylococcus aureus (33%) and Pseudomonas aeruginosa (25%) as summarized in Table 4.
Sixty-six peritonitis occurred in 48 patients and the global peritonitis incidence was 1 episode per 50.6 patient-months.
The peritonitis rate in patients without a history of ESI was 1 episode per 49.2 patient-months, while peritonitis incidence in patients with a history of ESI was 1 episode per 51.7 patient-months. Furthermore, the peritonitis rate during the period after the first ESI episode was 1 episode per 46.8 patient-months.
The overall probability of remaining free from ESI was 80.7% at 1 year and 61.8% at 3 years, while in patients who had experienced a previous episode of ESI the probability of remaining free from ESI after one episode of ESI was 55.9% at 1 year and 49.7% at 3 years. The two curves statistically differ each other [hazard ratio (HR) 0.51, 95% confidence interval (CI) 0.19–0.94, p = 0.04 (Fig. 1)].
The probability of remaining free from peritonitis for patients without a history of ESI was 78.2% at 1 year and 44.6% at 3 years; while the probability of remaining free from peritonitis for patients with a history of ESI was 73.5% at 1 year and 44% at 3 years. The two curves do not statistically differ each other [HR 0.9, 95% CI 0.49–1.64, p = 0.74 (Fig. 2)].
Probability of remaining free of peritonitis for patients with a history of ESI vs patients without ESI episodes. ESI exit site infection, NO H.ESI patients without a history of ESI, H.ESI patients with a history of ESI, No number, pt patients, m months, p p value: HR hazard ratio, CI confidence interval
The probability of remaining free from peritonitis for patients who experienced a previous episode of ESI was 74.1% at 1 year and 54% at three years. In comparison with patients without a history of ESI a significative statistical difference was not observed [HR 0.94, 95% CI 0.48–1.48, p = 0.85 (Fig. 3)].
Probability of remaining free of peritonitis for patients without ESI episodes vs patients after their first episode of ESI. ESI exit site infection, NO H.ESI patients without a history of ESI, ESI-1 patients after their first episode of ESI, No number, pt patients, m months, p p value, HR hazard ratio, CI confidence interval
On a global overview, 45 of 52 episodes of ESIs (86.5%) were responsive to medical therapy (Fig. 4). All six patients who received non-systemic treatment for mild ESI healed with topical treatment. Twelve events of tunnel infections secondary to ESI occurred and 3 catheter-related peritonitis. Five of 12 episodes of tunnel infections were responsive to systemic antibiotic therapy (42%), while in the 7 remaining cases (58%) the catheter was removed for tunnel infections unresponsive to medical therapy. In particular, 4 patients removed the catheter for S. aureus one of them with associated peritonitis, 1 for P. aeruginosa, and 2 for S. epidermidis one of them with associated peritonitis as showed in Table 4. The clinical outcomes of the patients who experienced removal of the catheter is detailed in Fig. 4. Specifically, one patient underwent simultaneous removal and reinsertion of Tenckhoff catheter with a new exit-site allowing him a prompt restart of PD, while in four patients, after a temporary shift to hemodialysis (HD) with a median period of 8 months, a second Tenckhoff catheter was inserted and PD was immediately restarted [22]. Only two patients were shifted permanently to HD. No catheter-related peritonitis deaths occurred (Fig. 4).
Figurative representation of patient outcomes. PD peritoneal dialysis, HD hemodialysis, ESI exit site infection, TI tunnel infection, P peritonitis, H with MT healed with medical therapy, n number, Sim RR simultaneous removal and reinsertion, RC + Sh to HD removal of catheter and temporary exchange to HD, R PD restart of PD, D Sh to HD Definite shifted to HD
Relapsing and repeated ESI
According to the definitions, during the study period three episodes of relapsing and seven repeated ESIs occurred. Six of 123 patients had repeated ESI (five patients had 1 episode of repeated ESIs and one patient had 2), representing a prevalence of 4.8% in an unselected PD population. Two cases of relapsing ESIs were caused by S. aureus and one by S. epidermidis. The latter episode did not respond to antibiotic therapy and caused tunnel infection and peritonitis which obligated to remove the catheter. Six cases of repeated ESIs were due to S. aureus and one due to P. aeruginosa. All the episodes responded to medical therapy and no peritonitis occurred within 60 days.
Discussion
A microbial biofilm is present on almost all surfaces of indwelling plastic and metal medical devices [23], as well as in PD catheter [24]. When a catheter is implanted, a film of ionic constituents, albumin, and fibrin, covers the surface in a short time [25, 26]. This coating promotes the bacterial adhesion to the device of bacteria coming from the skin and triggers the production of constituents for the biofilm matrix [25, 27]. Within few days, the matrix houses a densely populated colony of bacteria which aggregate to form biofilms in a sessile state [26], a condition that confers resistance to antimicrobial agents as demonstrated by studies on different medical devices [28, 29] and long-term PD catheters [30].
Sessile bacteria are also able to change from their “immediately attached” physiologic state to their original floating planktonic form. In this form, they are capable of seeding the surrounding area and transmigrate either towards the exit site or the deep cuff of the catheter representing a source of new and recurrent infections.
As a matter of fact, ultrasonography has demonstrated that during an episode of ESI with purulent discharge an involvement of proximal and deep cuff or tunnel occurred in up to 95% of the cases [31]. However, other papers reported lower percentages (45–80%) of positivity at the ultrasound (US) examinations [32,33,34]. This discrepancy may be explained by the fact the definition of ESI as based on the only presence of erythema around the exit site. Furthermore, Holley et al. [35] showed that when the US examination was performed in patient without clinically suspected tunnel infection more than 50% of the exams resulted positive. In addition, the persistence of the cuffs’ involvement at the US examination 2 weeks after the initiation of antibiotic therapy represented a negative prognostic factor for catheter removal and shift towards HD [33,34,35]. According to these evidences in the last 10 years we decided to place the superficial cuff at least 4 cm internal to the exit-site (instead of 2 cm) in order to decrease the ESIs and associated infectious complications.
In these records only about 1/4 of patients experienced at least one episode of ESI and the incidence was similar or lesser than that found by those studies that used the same strict definition of ESI [15, 36, 37].
Furthermore, considering the results obtained previously in our center, the cumulative incidence of ESI was lower after the modified tunnel creation than in the period before (1 episode per 64.2 patient/months vs 1 episode per 48.7 patient/months) as suggested by the survival curves (overall probability of remaining free from ESI was 80.7% and 61.8% vs 72% and 56% at 12 and 36 months, afterwards and prior the modified technique, respectively) [38]. Notably, these two populations are quite comparable since prevention and initial treatment of ESIs and peritonitis did not change in our center, and prophylactic antibiotic protocols were never employed. However, there was a mean age difference of almost 5 years less in the previous population (62.4 vs 57.4 years) [38].
Single center observational studies from different parts of the world, as multinational registry studies have reported that the incidence of PD-related infections have steadily decreased over the last 10–20 years [40,41,42,43]. However, a wide variation on the incidence of PD related infections remains among different centers and countries. The results from multicenter studies conducted in the United States and Canada (1 episode every 32.7 and 27.6 patient-months respectively) [44], Hong Kong (1 episode every 37–45 patient-months) [45], Austria (1 episode every 51 patients-months) [46] and from a large single center in Korea (1 episode every 30 patient-months) [47] were better than those obtained in large national PD populations in the United Kingdom (1 episode every 18.8 patient-months) [13], Scotland (19.2 patient-months) [48] and Australia (1 episode every 20 patient-months) (12). In our centre the overall peritonitis rate was similar to that one reported by the Austrian study confirming the good results achieved.
Studies published before 2000 reported a relationship between ESIs and peritonitis when double-cuff catheters were inserted the superficial cuff located less than 4 cm from the exit site [14,15,16], but this data was not confirmed by Crabtree [36] in a prospective study of 63 incident PD patients who had received the insertion of a single deep cuff catheter. Recently, a post hoc analysis of a randomized controlled trial demonstrated a significantly higher risk of peritonitis 15 days post-ESI [18] and a time matched case–control study proved that the development of an ESI was associated with an increased risk of subsequent peritonitis [17].
In our study the life-table analysis did not show an increased risk of developing peritonitis in patients with a history of ESIs and even when was considered specifically the period of time subsequent to the ESI episodes, it was not observed a difference between patients with a history of ESI and those without. Conversely, in our previous experience, patients with a history of ESI had a higher incidence of peritonitis than patients without ESI (1 episode per 27 patient-months vs one episode per 35.5 patient-months, p < 0.01) and this relation was even more accentuated when considering the probability of remaining free of peritonitis for patients who had experienced a previous episode of ESI [38].
As described earlier, in our center most ESIs were treated with systemic therapy. Remarkably, 86.5% responded to medical therapy, while before the modified tunnel creation this percentage was below 65%. Moreover, prior to the tunnel modification more than 50% of ESIs caused by S. aureus were unresponsive to antibiotic therapy leading either to surgical revision and/or catheter removal [38, 39]. After tunnel modification, only 4 of the 28 S. aureus ESIs (14.2%) were refractory to medical therapy resulting eventually in catheter loss [38]. This data might suggest that a greater distance of the superficial cuff from the exit site could have decreased the probability of bacterial cuff colonisation and biofilm formation while increasing the ESI responsiveness to antibiotic treatment.
The existence of repeated ESIs has been reported more than 30 years ago. However, the incidence, prevalence and outcome of repeated ESIs has never been clearly reported. This was in part due to a missing definition of repeated ESI which was recently proposed by Beckwith et al. [21]. We further suggested a definition for relapsing ESI (“episode of ESI that occurred within 4 weeks of completion of therapy of a prior episode with the same organism”) in order to differentiate these entities. In our unselected population, nearly 10% of the patients experienced more than one episode of ESI and the overall prevalence of repeat ESI was 4.8%. Beckwith et al. reported that 65% of patients with repeated ESI developed subsequent peritonitis [21], while these results were not replicable in our cohort since we did not observe any episode of subsequent peritonitis in subjects with repeated ESI. Nevertheless, the life-table analysis showed that the development of an ESI was significantly associated with an increased risk of successive ESI episodes suggesting that a previous ESI might correlate with a higher probability of bacterial colonisation along the cuff and catheter wall. As observed by Beckwith [21], also in our study the 2 most common causative organisms for repeat ESI were S. aureus and P. aeruginosa which are both capable of forming biofilm layers [49, 50]. We speculate that the tunnel modification might also decrease the incidence of repeat ESIs and improve their outcomes when they occur.
In conclusion, we believe that placing the superficial cuff subcutaneously at least 4 cm internal to the exit-site might avoid the bacterial cuff colonization resulting in decrease of the refractory ESI to medical therapy, ESI incidence and related peritonitis.
We are aware of the study limitations since it was a retrospective case note review from a single center without a contemporary control group. However, potential advantages may be represented by the possibility to compare the recent results with a historical population in who the aspects of exit-site care and clinical management of catheter-related infective episodes were not different. Clearly, prospective randomized controlled clinical trials are needed to confirm the advantage of a longer subcutaneous tunnel between the superficial cuff and the exit-site in terms of ESI and related peritonitis incidence and their responsiveness to medical therapy. Nevertheless, these preliminary results could suggest a possible role for the superficial cuff position in ESIs and tunnel infections in CAPD patients.
Further research should be focusing on the role of US in tunnel infection management [51]. The use of US should allow the clinician to identify the zone of tunnel involved in the infection, and to guide the appropriate therapy. In fact, when the infection is circumscribed to the superficial cuff, its removal and the subsequent creation of a new exit-site is sufficient to obtain a definite cure in 70% of the cases [52]. Instead, when the infection is localized either to the intercuff segment or deep cuff, the removal of the PD catheter prior to the development of peritonitis seems to be mandatory. Thus, the use of US could distinguish the refractory episodes of tunnel infections which might be healed with mini-invasive surgical approach from those inevitably needing removal of the catheter. Moreover, more data are necessary to firmly establish the role of the US in monitoring the patient’s response to antibiotic therapy in order to define either the length of the cure, or the appropriate timing of the catheter removal. Finally, the relation between relapsing/repeat peritonitis and concomitant asymptomatic tunnel infection need to be assessed.
References
Fried L, Bernardini J, Johnston J, Piraino B (1996) Peritonitis influences mortality in peritoneal dialysis patients. J Am Soc Nephrol 7:2176–2182
Bloembergen WE, Port FK (1996) Epidemiological perspective on infections in chronic dialysis patients. Adv Ren Replace Ther 3:201–207
Szeto C, Wong TY, Chow K, Leung C, Li PK (2003) Are peritoneal dialysis patients with and without residual renal function equivalent for survival study ? Insight from a retrospective review of the cause of death. Nephrol Dial Transpl 18:977–982
Fontan M, Rodriguez-Carmona A, Garcia-Naveiro R, Al E (2005) Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 25:274–284
Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE et al (2016) ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 36:481–508
Szeto C, Li PK, Johnson DW, Bernardini J, Dong J, Figueiredo AE et al (2017) ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 37:141–154
Scalamogna A, De Vecchi A, Castelnovo C, Guerra L, Ponticelli C (1990) Long-term incidence of peritonitis in CAPD patients treated by the Y set technique: experience in a single Center. Nephron 55:24–27
Strippoli GFM, Tong A, Johnson D, Schena FP, Craig JC (2004) Catheter-related interventions to prevent peritonitis in peritoneal dialysis: a systematic review of randomized, controlled trials. J Am Soc Nephrol 15:2735–2746
Flanigan M, Gokal R, City I, Infirmary MR, Kingdom U (2005) Peritoneal catheters and exit-site practices toward optimum peritoneal access: a review of current developments. Perit Dial Int 25:132–139
Chow KM, Szeto CC, Law MC, Suk J, Fung F, Li PK (2003) Influence of peritoneal dialysis training nurses’ experience on peritonitis rates. Clin J Am Soc Nephrol. 2:647–652
Figueiredo AE, Bernardini J, Bowes E, Hiramatsu M, Price V, Su C et al (2016) ISPD guidelines/reccomendations: a syllabus for teaching peritoneal dialysis to patients and caregivers. Perit Dial Int 36:592–605
Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, Johnson DW et al (2011) Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int 31:651–662
Davenport A (2009) Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002–2003. Perit Dial Int 29:297–302
Piraino B, Bernardini J, Sorkin M (1986) The influence of peritoneal catheter exit-site infections on peritonitis, tunnel infections, and catheter loss in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 8:436–440
Abraham G, Savin E, Ayiomamitis A, Izatt S, Vas SI, Mathews RE et al (1988) Natural history of exit-site infection (ESI) in patients on Continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int. 8:211–216
Gupta B, Bernardini J, Piraino B (1996) Peritonitis associated with exit site and tunnel infections. Am J Kidney Dis 28:415–419
Lloyd A, Tangri N, Shafer LA, Rigatto C, Perl J, Komenda P et al (2013) The risk of peritonitis after an exit site infection: a time-matched, case – control study. Nephrol Dial Transpl 28:1915–1921
Van DATN, Tomlinson GA, Jassal SV (2012) The association between exit site infection and subsequent peritonitis among peritoneal dialysis patients. Clin J Am Soc Nephrol 7:1266–1271
Bender FH, Bernardini J, Piraino B (2006) Prevention of infectious complications in peritoneal dialysis: best demonstrated practices. Kidney Int Suppl 103:S44–54
Piraino B (2009) Insights on peritoneal dialysis-related infections. Contrib Nephrol 163:161–168
Beckwith H, Clemenger M, Mcgrory J, Hisole N, Chelapurath T, Newbury S et al (2019) Repeat peritoneal dialysis exit-site infection: definition and outcomes. Perit Dial Int 39:344–349
Scalamogna A, Nardelli L, Zanoni F, Messa P (2019) Double purse-string around the inner cuff of the peritoneal catheter: a novel technique for an immediate initiation of continuous peritoneal dialysis. Int J Artif Organs 20:365–371
Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta MMT (1987) Bacterial biofilms in nature and disease. Ann Rev Microbiol 41:435–464
Marrie TJ, Noble MA, Costerton JW (1983) Examination of the morphology of bacteria adhering to peritoneal dialysis catheters by scanning and transmission electron microscopy. J Clin Microbiol 18:1388–1398
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Mack D, Rohde H, Dobinsky S, Riedewald J, Nedelmann M, Knobloch JK, Elsner HAFH (2000) Identification of three essential regulatory gene loci governing expression of Staphylococcus epidermidis polysaccharide intercellular adhesin and biofilm formation. Infect Immun 68:3799–3807
Urm J, Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Sn S et al (1995) Microbial biofilms. Annu Rev Microbiol. 49:711–745
Nickel JC, Downey J, Costerton JW (1992) Movement of Pseudomonas aeruginosa along the catheter surfaces. Urology. 39(1):93–98
Passerini L, Lam K, Costerton JW, King EG (1992) Biofilms on indwelling vascular catheters. Crit Care Med. 20(5):665–673
Dasgupta M, Kowalewska-Grochowska K, Larabie M, Costerton J (1991) Catheter biofilms and recurrent CAPD peritonitis. Adv Perit Dial 7:165–168
Kwan T, Tong MK, Siu Y, Leung K (2004) Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology 9:348–352
Plum J, Sudkamp S, Grabensee B (1994) Results of ultrasound-assisted diagnosis of tunnel infections in continuous ambulatory peritoneal dialysis. Am J Kidney Dis 23(1):99–104
Korzets Z, Erdberg A, Golan E, Verner M, Rathaus V, Bernheim J (1996) Frequent involvement of the internal cuff segment in CAPD peritonitis and exit-site infection—an ultrasound study. Nephrol Dial Transpl 11:336–339
Vychytil A, Lorenz M, Schneider B, Hörl W, Haag-Weber M (1998) New criteria for management peritoneal dialysis patients of catheter infections using ultrasonography. J Am Soc Nephrol 9(2):290–296
Holley JL, Foulks CJ, Moss AH, Willard D (1989) Ultrasound as a tool in the diagnosis and management of exit-site infections in patients undergoing continuous ambulatory peritoneal dialysis. Am J Kidney Dis 14(3):211–216
Crabtree J, Siddiqi R (1999) Dialysis catheter infection related peritonitis: incidence and time dependent risk. ASAIO J 45(6):574–580
Manani SM, Virzì GM, Giuliani A, Crepaldi C, Ronco C (2019) Catheter-related infections in peritoneal dialysis: comparison of a single center results and the literature data. J Nephrol 32:837–841
Scalamogna A, Castelnovo C, De Vecchi A (1991) Exit-site and tunnel infections in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis 18(6):674–677
Scalamogna A, de Vecchi A, Maccario M, Castelnovo C, Ponticelli C (1995) Cuff-shaving procedure. A rescue treatment for exit-site infection unresponsive to medical therapy. Nephrol Dial Transplant. 10:2325–2327
Huang J, Hung K, Yen C, Wu K, Tsai T (2001) Comparison of infectious complications in peritoneal dialysis patients using either a twin-bag system or automated peritoneal dialysis. Nephrol Dial Transpl 16:604–607
Moraes T, Pecoits-Filho R, Riberiro S, Rigo M, Silva M, Teixeira PS et al (2009) Peritoneal dialysis in Brazil: twenty-five years of experience in a single center. Perit Dial Int 29:492–498
Han S, Lee S, Ahn S, Lee J, Choi H, Kim B et al (2007) Improving outcome of CAPD: twenty-five years’ experience in a single Korean center. Perit Dial Int 27:432–440
Rocha A, Rodrigues A, Teixeira L, João M, Mendonca D, Cabrita A (2012) Temporal trends in peritonitis rates, microbiology and outcomes: the major clinical complication of peritoneal dialysis. Blood Purif 33:284–291
Mujais S (2006) Microbiology and outcomes of peritonitis in North America. Kidney Int 70:S55–62
Li P, Law M, Chow K, Chan WK, Szeto CC, Cheng YL et al (2002) Comparison of clinical outcome and ease of handling in two double-bag systems in continuous ambulatory peritoneal dialysis: a prospective, randomized, controlled, multicenter study. Am J Kidney Dis 40:373–380
Kopriva-Altfahrt G, Konig P, Mundle M, Prischl F, Roob J, Wiesholzer M et al (2009) Exit-site care in Austrian peritoneal dialysis centers—a nationwide survey. Perit Dial Int 29:330–339
Kim D, Yoo T, Ryu D, Xu Z, Kim H, Choi K (2004) Changes in causative organisms and their antimicrobial susceptibilities in CAPD peritonitis: a single center ’s experience over one decade. Perit Dial Int 24:424–432
Brown MC, Simpson K, Kerssens JJ, Robert AM (2011) Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post-millenium (2000–2007). Perit Dial Int. 31:639–650
Xu Z, Islam S, Wood TK, Huang Z (2015) An integrated modeling and experimental approach to study the influence of environmental nutrients on biofilm formation of Pseudomonas aeruginosa. Biomed Res Int 2015:506782 https://doi.org/10.1155/2015/506782
Otto M (2013) Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188
Nardelli L, Scalamogna A, Zeiler M, Messa P (2020) Use of ultrasounds in PD catheter related infections: indications and clinical implications. G Ital Nefrol (in press)
Scalamogna A, De Vecchi A (2000) Nuova tecnica di rimozione della cuffia superficiale nei pazienti con infezione dell’emergenza resistente alla terapia medica. G Ital di Nefrol 17:635–639
Funding
None.
Author information
Authors and Affiliations
Contributions
Research idea and study design: LN, AS; data acquisition: LN; data analysis/interpretation: LN, AS; statistical analysis: LN; supervision or mentorship: PM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
We have read and understood Journal of Nephrology’s policy on disclosing conflicts of interest and declare that we have none.
Ethical approval
No required (retrospective cohort study undertaken through a case note review).
Informed consent to participate
No required.
Informed consent to publish
No doubt that anonymity can be maintained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nardelli, L., Scalamogna, A. & Messa, P. The impact of the superficial cuff position on the exit site and tunnel infections in CAPD patients. J Nephrol 34, 493–501 (2021). https://doi.org/10.1007/s40620-020-00788-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00788-z