Abstract
Background
Refractory exit-site infections (ESIs) and tunnel infections (TIs) are challenging complications for patients undergoing peritoneal dialysis (PD). This study compared the outcomes of surgical intervention, notably the cuff-shaving (CS) procedure coupled with negative-pressure wound therapy (NPWT), and conservative management strategies for patients with refractory ESI and TI.
Methods
We retrospectively reviewed patients who underwent PD at our center, focusing on the incidence and management of ESI and TI. We evaluated and compared treatment outcomes, including ESI scores, frequency of ESI and/or TI, identification of causative microorganisms, and duration of catheter survival or time until removal.
Results
We identified 97 episodes of catheter-related ESI and/or TI across 71 patients with an incidence rate of 0.15 episodes per patient-year. Of the 23 patients with refractory ESI and/or TI, surgical intervention was performed in 8, while 15 chose conservative management. In the one-month follow-up, patients who underwent CS combined with NPWT showed no complications such as leakage, and their local symptoms resolved completely. The mean PD catheter survival time was significantly longer in the surgical group (29.38 ± 7.25 months) than in the conservative group (7.86 ± 2.13 months). Surgical intervention demonstrated a significantly higher therapeutic efficacy and extended catheter survival.
Conclusions
The combination of CS and NPWT as a surgical approach is crucial for eradicating infectious foci and significantly improving the longevity of PD catheter function. This integrated surgical strategy offers a promising solution for the management of refractory ESI and TI in patients undergoing PD.
Similar content being viewed by others
Background
Peritoneal dialysis (PD) catheters are indispensable in patient’s treatment. PD catheter-related infections, including exit-site infection (ESI) and tunnel infections (TI) are significant risk factors for catheter loss and peritonitis. Although most ESI cases respond well to antibiotic treatment [1], infections involving the superficial cuff or tunnel are challenging to treat using medication alone. Surgical intervention is often necessary in such cases. The International Society for Peritoneal Dialysis (ISPD) guidelines recommend cuff-shaving (CS) or partial catheter reimplantation and catheter diversion procedures with exit-site renewal for ESI and TI without peritonitis or abscess involvement of the inner cuff [2, 3]. The current guidelines prioritize interventional therapy to maintain PD access and minimize the time gap before resuming PD in the event of catheter loss. However, this course of action may result in delayed PD restoration. Cuff shaving has the following advantages: it is less-invasive, requires a shorter hospital stay, causes less peritoneal damage, and requires no additional catheter materials [4,5,6,7]. Previous studies confirmed that CS is a feasible rescue therapy for refractory ESIs [8]. Whether there are methods to improve CS to accelerate the healing of ESIs and whether the survival time of peritoneal dialysis catheters is worth further exploration. Negative pressure wound therapy (NPWT), a technique that involves the application of negative pressure or suction to a wound through a specialized dressing and vacuum system, can also reduce inflammatory exudation from the incision site and promote wound healing [9]. NPWT may improve the prognosis of patients who are refractory to ESI and/or TI. This study aimed to explore the outcomes of surgical intervention, specifically CS combined with NPWT, and conservative treatment for refractory ESI and/or TI.
Methods
Patients and materials
From January 2016 to July 2022, 274 patients underwent the insertion of a straight double-cuff Tenckhoff catheter The demographic data collected included age, sex, primary disease, duration of dialysis, ESI and/or TI occurrence time, and secretory pathogen culture results. Laboratory findings were collected from patients with refractory ESI and/or TI, including measurements of haemoglobin, albumin, and C-reactive protein levels, as well as white blood cell count. Ultrasound assessments of the PD catheter exit site, superficial cuff, tunnel, and surrounding tissue were performed.
All patients were regularly followed-up in dialysis clinics, and observations included monitoring and recording of exit-site changes, ESI and/or TI scores, TI occurrence, and catheter survival time.
Patients’ informed consent for ESI and/or TI was obtained before the surgical intervention. This study complied with the principles of the Declaration of Helsinki. This study was approved by the China Ethics Committee of Peking University International Hospital (Ethics Committee No. 2021-KY-0044-02).
Diagnostic criteria
ESI and TI were diagnosed in accordance with the definitions provided in the 2017 ISPD guidelines on PD catheter-related infections [10]. Exit-site infection is defined as the presence of purulent discharge with or without skin erythema at the catheter’s epidermal interface. Tunnel infection is defined as the presence of clinical inflammation (erythema, swelling, tenderness, or induration) with or without ultrasonographic evidence of fluid collection anywhere along the catheter tunnel. An exit site scoring system modified from Schaefer et al. [11] was used to monitor the exit sites.
Peritonitis was diagnosed when at least two of the following conditions were present: (1) clinical features consistent with peritonitis, such as abdominal pain and/or cloudy dialysis effluent; (2) dialysis effluent white blood cell count > 100/µ L or > 0.1 × 109/L (after a dwell time of at least 2 h), with > 50% polymorphonuclear cells; and (3) positive dialysis effluent culture [12]. Refractory ESI and/or TI was defined as a failure to respond to antibiotic therapy after 3 weeks of treatment [10].
Clinical management
Bacterial cultures were routinely obtained from catheter exit site secretions of patients diagnosed with ESI and/or TI (Fig. 1A). Patients with severe ESI underwent systemic treatment. Following the ISPD guidelines, empirical antibiotics targeting Gram-positive and Gram-negative microorganisms were initiated before obtaining swab results to guide ongoing therapy [10]. Antibiotic adjustments were made based on culture results; antibiotics can be administered orally or intraperitoneally for 2–3 weeks. Patients who did not respond to the antibiotic treatment underwent ultrasound assessment.
(A) The extent of the tunnel infection (outlined with a blue marker pen), the superficial cuff location (thick black arrow), and redness and swelling of the external orifice with purulent discharge (thin black arrow). (B) A skin incision of about 3–4 cm at the level of the exit-site in the direction of the superficial cuff. (C) Localization of the superficial cuff by blunt dissection and isolation of the superficial cuff from the adipose and tissue. (D) Careful shaving of the cuff using a scalpel and curved forceps until the cuff material was removed. (E) Placement of the high negative pressure drainage tube, and fixation of the high negative pressure drainage bottle with sutures. (F) The remaining shadow of the superficial cuff at the end of the procedure, and removal of the drainage tube through the outside of the incision
After more than 3 weeks of systemic antibiotic treatment, patients who showed no clinical response, presented with purulent discharge from the exit site, or exhibited ultrasound-confirmed effusion along the subcutaneous catheter tunnel were diagnosed with refractory ESI and/or TI [10].
The surgical procedures were performed in a sterile operating room under local anesthesia, with the patients in the supine position. This procedure involves an elliptical incision around the exit site. The incision was extended through the skin and the subcutaneous tissue along the course of the catheter until the superficial cuff was identified (Fig. 1B). Using sharp dissection, unhealthy granulation tissue and cellulitis were completely excised, resulting in an open wound (Fig. 1C). The surrounding tissue samples were obtained and sent for bacterial culture analyses. The cuff was removed by making parallel incisions along its surface using a scalpel blade and excising it using curved forceps in repeated slices (Fig. 1D). The local wound was irrigated with saline, and the catheter and shaved tubing segments were directed out of the medial aspect of the incision (Fig. 1E). A negative-pressure drainage tube (Drainobag, Germany) was inserted through the wound, based on the depth of the space, to provide continuous negative-pressure drainage. The skin was sutured and the drainage tube was fixed with sutures. The opposite end of the drainage tube was connected to a negative-pressure drainage bottle with a capacity of 150 mL to ensure uniform negative pressure (90 kPa) (Fig. 1F). The negative-pressure drainage tube was removed 2–4 days after surgery, depending on the wound and drainage conditions. Antibiotics were continued for 2–3 weeks post-surgery, and the dressing was changed daily.
Some patients with refractory ESI and/or TI opted for conservative treatment because they did not agree to undergo surgery.
Efficacy and outcome evaluation
After 1 month, the efficacy of the treatment was evaluated based on the exit-site and tunnel conditions using the following criteria: (1) Effective: Complete disappearance of local symptoms, with an ESI score of 0 (Exit-Site Scoring System [11]); (2) Improvement: Significant improvement in local symptoms compared to before, with a catheter ESI score < 4; (3) Ineffective: No improvement or worsening of local infection symptoms, with a catheter ESI score > 4.
The survival time of the PD catheter was recorded as the duration from its initial use at the time of the ESI diagnosis until the end of the follow-up period on July 1, 2022. Furthermore, we compared the identification of pathogenic bacteria and the treatment outcomes between patients who underwent surgery and those who did not. Additionally, instances of catheter loss due to infection were recorded.
Statistical analysis
Statistical analyses were performed using the SPSS software for Windows (version 27.0; IBM Corp., Armonk, NY, USA). Time-to-event data are presented as Kaplan–Meier survival curves. Comparative hypothesis testing was performed using chi-squared, one-way ANOVA, and log-rank tests. Data were analyzed at a 95% confidence interval, and statistical significance was set at p < 0.050.
Results
Clinical Data of PD patients with catheter-related infections
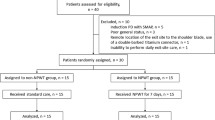
Among 274 PD patients, 71 patients (23.9%) experienced 97 episodes of catheter-related infections. During the study’s observation period, 274 PD patients collectively underwent PD treatment for a total of 615.4 patient-years. The incidence rate of ESI and/or TI was 0.15 episodes/patient-year. The average age of the 71 patients with ESI and/or TI was 57.54 ± 15.17 years. Among the 71 cases of ESI and/or TI patients, 60 were male (66.2%). The average duration of PD was 53.38 ± 31.83 months. The causes of ESRD were as follows: 30 patients (42.4%) had diabetic nephropathy, 26 (36.6%) had chronic glomerulonephritis, 10 (14.0%) had hypertensive renal impairment, 2 (2.8%) had tumor-related renal impairment, and 1 patient each had obstructive nephropathy, aristolochic acid nephropathy, and polycystic kidney disease, each accounting for 1.4%. The median time from PD catheter placement to the first ESI and/or TI was 10 months (interquartile range [IQR]: 3–25 months).
Catheter exit-site infection score and Pathogen distribution
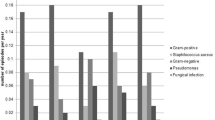
The median score for the exit-site of peritoneal dialysis catheters in 97 cases was 3 (4–6); 59 patients underwent secretion culture, 12 did not undergo bacterial culture, and 6 had negative culture results, resulting in a positive culture rate of 92.9% (79/85). Among the positive cultures, Gram-positive bacteria accounted for 43.5% (37 strains). Staphylococcus aureus was the most identified bacterium, accounting for 59.5% (22/37) of the Gram-positive infections. Among the Gram-negative bacteria, Pseudomonas aeruginosa accounted for 50.0% (8/16). The pathogenic bacteria cultured from the exit-site samples are listed in Table 1.
Refractory ESI/TI clinical data of patients
A total of 23 patients were diagnosed with refractory ESI and/or TI, with a mean age of 54.74 ± 13.66 years. Among them, 14 (60.9%) had diabetic nephropathy. Staphylococcus aureus was the most prevalent pathogen cultured from the secretions of 12 (52.2%) of the 23 patients, followed by P. aeruginosa in 6 (26.1%), Escherichia coli in 2 (8.7%), and other pathogen infections in 3 (13.0%). The median infection score was 5 (4–6).
Among the 23 patients, 15 chose conservative treatment with maintenance medication because they refused surgical treatment. In the conservative treatment group, five patients (33.3%) had Staphylococcus aureus cultured from their secretions, which was lower than the surgical treatment group (87.5%) (χ² = 6.135, p = 0.013) (Table 2).
Comparison of the effects of surgical and conservative treatments
After 1 month of follow-up, none of the patients who underwent superficial CS combined with NPWT experienced complications such as leakage, and their local symptoms completely disappeared (Fig. 2). The ESI score of these patients was 0, and the efficacy rate was 100%. Conservative management was effective in two patients (13.3%), ineffective in nine patients (60.0%), and local symptoms improved in four patients (26.6%). The effectiveness rate of surgical treatment for infection was significantly higher than that of conservative management (χ² = 15.947, p < 0.01).
After 1 year follow-up, six patients in the surgical treatment group continued PD treatment, with a 1-year catheter survival rate of 75%. One patient died of peritonitis 11 months after CS combined with NPWT surgery, and one patient underwent catheter removal 5 months after surgery due to peritonitis. In the conservative management group, four patients continued PD treatment, resulting in a catheter survival rate of 26.7%. The catheter survival rate was significantly higher in the surgical treatment group than in the conservative management group (χ² = 4960, p = 0.026).
Patients were followed up for 62 months, with a median duration of 11 months (IQR: 2, 23). At the end of the follow-up period, two patients in the surgical treatment group continued PD treatment with the original catheter. Catheters were removed from five patients, including three who underwent new catheter insertion on the opposite side of the abdomen and two who required transfer to hemodialysis. Additionally, one patient died of catheter-related peritonitis. The catheter removal rate was 75% in the surgical treatment group, and the median time from surgery to peritonitis onset was 18 (IQR: 5.5, 24.8) months.
In the conservative management group, four patients continued to undergo PD treatment with the original catheter at the end of the follow-up period. The median time for achieving a complete response in cases of refractory ESI was 5.5 (IQR: 2.3, 9.5) months. Eleven patients underwent PD catheter removal, resulting in a catheter removal rate of 73.3%. Of these removals, eight were attributed to peritonitis and three were due to repeat infections.
The PD catheter survival time in the surgical treatment and conservative management group was 29.38 ± 7.25 and 7.86 ± 2.13 months, respectively. Therefore, the catheter survival time was longer in the surgical treatment group than in the conservative management group (log-rank test: χ2 = 6.017, p < 0.05). Figure 3.
Discussion
The incidence of PD-related infections has decreased with advancements in products and technologies. However, ESIs and TIs are regarded as major risk factors for PD-related peritonitis, accounting for nearly one-third of all technique failures [10, 13]. The relevance of catheter ESI/TI in catheter-related peritonitis has been reemphasized in the latest ISPD peritonitis prevention and control guidelines, which recommend active early infection control measures [12]. The 2023 update of the ISPD catheter-related infection guidelines recommends that the overall exit-site infection rate should be no more than 0.40 episodes per year at risk [3]. In the present study, the incidence rates of ESI and/or TI were 0.15 episodes/patient-year. Although our catheter infection rate was not high, ESI and/or TI remain the major causes of peritoneal dialysis technique failure.
Although TI usually occurs in the presence of ESI, it can also occur independently. Tunnel infections can progress to intra-tunnel abscesses, leading to peritonitis. Catheter removal has been required in considerable proportion (39–80%) of cases related to these complications [10, 12,13,14].
Several studies have since demonstrated the effectiveness of superficial cuff removal in treating drug-resistant ESI and/or TI [8, 15]. Soon et al. systematically reviewed 11 studies on the surgical treatment for refractory ESI and/or TI over the past 30 years and noted that improved surgical techniques resulted in fewer associated complications [16]. Treatment success depends on the degree of infection. Negative pressure wound therapy has been employed to optimize wound healing by applying sub-atmospheric pressure, which helps reduce inflammatory exudates and promotes granulation tissue formation. This is an effective approach for managing both acute and chronic wounds [8]. In our study, eight patients with resistance to ESI and/or TI underwent superficial CS combined with NPWT. The efficacy rate reached 100% after 1 month. The combination of CS and NPWT has proven helpful in removing infectious lesions and promoting wound healing, thereby prolonging catheter survival time compared with conservative management. Therefore, CS combined with NPWT can be considered as an early alternative treatment for refractory ESI and/or TI.
Surgical interventions can effectively control ESI and/or TI, leading to an increased PD catheter survival rate of 22.7–81% [14]. In this study, the 1-year catheter survival rate was 75.0%, significantly higher than that of the conservative management group (26.7%). Patients willing to undergo conservative management with catheters have a poor prognosis, and active surgical intervention is recommended.
In recent years, several observational studies suggest a potential benefit of relocating the exit site in patients with exit site infection refractory to antibiotics [6, 17,18,19]. Another study demonstrated that removing the superficial CS combined with tunnel reconstruction effectively treated refractory TI, resulting in a catheter survival rate of up to 89.7% at 1 year [18]. These findings suggest that catheter replacement is a viable treatment option for refractory ESI and/or TI, with a higher PD catheter survival rate. However, it remains uncertain whether any technique is superior to the others [18].
ESI and/or TI are caused by various microorganisms. The most serious and common exit-site pathogens are S. aureus and P. aeruginosa, which frequently cause peritonitis [10]. Infections caused by S. aureus tend to recur, increasing the risk of peritonitis, and resulting in adverse outcomes for PD patients [15]. In this study, the catheter removal rate was 75% in the surgical treatment group, which was higher than that in the conservative management group (73.3%). Further analysis showed that patients who underwent PD catheter removal in the surgical treatment group had Staphylococcus aureus as the predominant pathogen. This higher removal rate is likely due to the increased risk of recurrence associated with S. aureus infections. Notably, the PD catheters were removed in 11 of the 12 patients with Staphylococcus aureus infection. Although superficial CS combined with NPWT can prolong the survival time of PD catheters in Staphylococcus aureus infections, the long-term prognosis remains poor. Therefore, aggressive treatment of these infections is necessary, emphasizing the importance of early intervention and treatment.
This study had several limitations, including its retrospective nature and small sample size. In the future, we plan to conduct a multicenter prospective study to further evaluate the therapeutic effects of superficial CS combined with NPWT on refractory ESI and/or TI. In addition, we will explore the effects and long-term prognosis of removing and replacing a new catheter within a specific timeframe after surgical treatment of the infection.
Conclusion
Peritoneal dialysis catheter-related infections are common complications in patients undergoing PD. Surgical removal of the superficial CS combined with NPWT is an effective approach for refractory ESI and/or TI as it helps remove inflammatory lesions and prolong catheter use. In cases of Staphylococcus aureus with ESI and/or TI, which have a poor prognosis and a high risk of peritonitis, early intervention is required to improve outcomes. Further studies are needed to investigate the optimal timing of surgery for excised external cuffs and evaluate the long-term impact of CS combined with NPWT and tunnel reconstruction on catheter survival.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ISPD:
-
International Society for Peritoneal Dialysis
- PD:
-
Peritoneal dialysis
- ESIs:
-
Refractory exit-site infections
- TIs:
-
Tunnel infections
- CS:
-
Cuff-shaving
- NPWT:
-
Negative pressure wound therapy
References
Tsai CC, Yang PS, Liu CL, et al. Comparison of topical mupirocin and gentamicin in the prevention of peritoneal dialysis-related infections: a systematic review and meta-analysis. Am J Surg. 2018;215:179–85. https://doi.org/10.1016/j.amjsurg.2017.03.005.
Crabtree JH, Shrestha BM, Chow KM, et al. Creating and maintaining optimal peritoneal Dialysis Access in the adult patient: 2019 update. Perit Dial Int. 2019;39:414–36. https://doi.org/10.3747/pdi.2018.00232.
Chow KM, Li PK, Cho Y, et al. ISPD catheter-related infection recommendations: 2023 update. Perit Dial Int. 2023;43:201–19. https://doi.org/10.1177/08968608231172740.
Fukasawa M, Matsushita K, Tanabe N, et al. A novel salvage technique that does not require catheter removal for exit-site infection. Perit Dial Int. 2002;22:618–21.
Muraoka K, Ishibashi Y, Yamaguchi J, et al. Early partial re-implantation of Tenckhoff catheters to treat intractable exit-site or tunnel infection. Perit Dial Int. 2011;31:350–3. https://doi.org/10.3747/pdi.2010.00181.
Oki R, Hamasaki Y, Komaru Y, et al. Catheter diversion procedure with exit-site renewal promotes peritoneal dialysis catheter survival. Kidney Int Rep. 2020;6(2):325–32. https://doi.org/10.1016/j.ekir.2020.11.03.
Nichols WK, Nolph KD. A technique for managing exit site and cuff infection in Tenckhoff catheters. Perit Dial Int. 1983;3:4–5. https://doi.org/10.1177/089686088300304S02.
Meng C, Beco A, Oliveira A, et al. Peritoneal dialysis cuff shaving-a salvage therapy for refractory exit-site infections. Perit Dial Int. 2019;39(3):276–81. https://doi.org/10.3747/pdi.2018.
Norman G, Goh EL, Dumville JC, et al. Negative pressure wound therapy for surgical wounds healing by primary closure[J]. Cochrane Database Syst Rev. 2020;6(6):19–27. https://doi.org/10.1002/14651858.CD009261.
Szeto CC, Li PK, Johnson DW, et al. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 2017;37:141–54. https://doi.org/10.3747/pdi.2016.00120.
Schaefer F, Klaus G, Müller-Wiefel DE, et al. Intermittent versus continuous intraperitoneal glycopeptide/ceftazidime treatment in children with peritoneal dialysis-associated peritonitis. J Am Soc Nephrol. 1999;10:136–45.
Li PK, Chow KM, Cho Y, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110–53. https://doi.org/10.1177/08968608221080586.
Tian X, Xu G. Clinical characteristics and treatment of catheter-related tunnel infection in peritoneal dialysis patients: the results from a single centered study. Chin J Blood Purif. 2021;20:469–72.
Suh H, Wadhwa NK, Cabralda T, et al. Persistent exit-site/tunnel infection and subcutaneous cuff removal in PD patients. Adv Perit Dial. 1997;13:233–6.
Debowski JA, Wærp C, Kjellevold SA, et al. Cuff extrusion in peritoneal dialysis: single-centre experience with the cuff-shaving procedure in five patients over a 4-year period. Clin Kidney J. 2017;10:131–4. https://doi.org/10.1093/ckj/sfw089.
Soon JJ, Yi, et al. Are salvage techniques safe and effective in the treatment of peritoneal dialysis catheter-related exit-site and tunnel infections? A systematic review and description of the authors’ preferred technique. Perit Dial Int. 2022;42:591–501. https://doi.org/10.1177/08968608221116689.
Kang SH, Cho KH, Kim AY, et al. Catheter salvage using revision for a peritoneal dialysis catheter with intractable exit site and/or tunnel infections. Semin Dial. 2023;36:53–6. https://doi.org/10.1111/sdi.13094.
Cho KH, DO JY, Park JW, et al. Catheter revision for the treatment of intractable exit site infection/tunnel infection in peritoneal dialysis patients: a single centre experience. Nephrol (Carlton). 2012;17:760–6.
Kirmizis D, Bowes E, Ansari B, et al. Exit-Site Relocation: a novel, straightforward technique for exit-site infections. Perit Dial Int. 2019;39:350–5.
Funding
This study was supported by Peking University International Hospital Research Funds (NO. YN2021ZD01 and NO. YN2023QN05), Beijing, China.
Author information
Authors and Affiliations
Contributions
Conceptualization: Jiaxiang Ding. Data curation: Qinghua Yang, Xiaoying Ren, Xiaowan Fang.Supervision: Jiaxiang Ding.Writing – review & editing: Qinghua Yang, Jiaxiang Ding.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Peking University International Hospital Biomedical Ethics Committee (No.2021-KY-0044-02), who waived the requirement for informed consent because of the retrospective nature of the study. This study complied with the principles of the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Consent to publish
Not Applicable (NA).
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Q., Ren, X., Fang, X. et al. The efficacy of cuff-shaving combined with negative pressure wound therapy in refractory exit-site and tunnel infections: a single center experience. BMC Nephrol 25, 273 (2024). https://doi.org/10.1186/s12882-024-03714-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03714-8