Abstract
Urinary tract infections (UTIs) after kidney transplantation are associated with significant morbidity. However, data on the impact of UTI on graft survival are controversial. We conducted a retrospective cohort study of 380 kidney transplant patients. Recipients with symptomatic UTIs during the first year after transplantation were categorized into three groups: early (< 3 episodes from months 1st to 6th), late (< 3 episodes during months 7th to 12th) and recurrent (≥ 3 episodes throughout the whole first year). Graft function at three years was considered the primary outcome. Symptomatic UTIs occurred in 184 (48.4%) kidney transplant recipients during the first year; 83 (21.8%) patients developed early UTIs, 50 (13.2%) late UTIs and 51 (13.4%) recurrent UTIs. We observed a significant improvement in graft function after three years in all patients (P < 0.001) except those who had recurrent UTIs. A Kaplan–Meier analysis showed that recipients with recurrent UTIs had worse graft outcome (eGFR value < 60 mL/min/1.73 m2) (P = 0.01). Recurrent UTIs was an independent predictor of graft function at three years in a model adjusted for DGF and episodes of acute rejection (Hazard Ratio, 2.2; 95% CI, 1.3 to 3.5; P = 0.001). Recurrent symptomatic UTIs during the first year after transplantation have negative impact on long-term graft function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infection (UTI) is the most important infection and major cause of morbidity and hospitalization after kidney transplantation. The incidence of UTIs ranges between 26 and 76%; this wide range is probably due to differences in definition, diagnostic criteria, study design and length of follow-up [1,2,3,4,5,6]. UTIs can occur at any time after transplantation, but a higher incidence is found in the first 6–12 months post-transplant [1].
The urinary tract is defended against UTIs by the innate immune response, which eliminates the infection through antibacterial effector mechanisms and, at the same time, preserves tissue homeostasis. It has been demonstrated that genes which regulate the innate immune response to infection influence susceptibility to UTI and genetic predisposition is a major factor for developing UTIs [7]. Although mortality rate due to infection has significantly declined from 50 to 5% within last 20 years, it is considered as a severe risk factor for worse outcomes in kidney transplantation [8]. Data on the impact of UTIs on graft outcome are controversial. Some studies have shown that UTIs contracted during the first 3–6 months post-transplantation have a negative impact on graft and patient survival [9] but others have not found any relation between UTIs and graft loss. It was long believed that late UTIs, developed at least 6 months after transplantation, could be considered “benign”. However, this assumption was supported only by few studies [10,11,12,13]. A recent analysis of a large dataset has shown that, on the contrary, these infections can have a negative impact on graft survival [14,15,16].
Here we studied the epidemiology of different types of urinary tract infections in a large cohort of kidney transplant recipients trying to identify their potential predisposing factors and impact on graft function in the long term.
Methods
Setting and study population
We performed a retrospective cohort study of 380 patients who received a kidney transplant at the Transplant Center, Azienda Ospedaliero-Universitaria Consorziale Policlinico, Bari, Italy, between January 2008 and September 2015. Kidney transplant recipients were periodically followed up at the Nephrology, Dialysis and Transplant Unit, Azienda Ospedaliero-Universitaria Consorziale Policlinico, Bari, Italy, depending on the graft function and transplant age. Clinical features (living or deceased donor, recipient age, gender, BMI, history of diabetes, hypertension, type of dialysis, immunosuppression) were collected at the time of transplantation. Data about renal function at several time points (serum creatinine and estimated glomerular filtration rate (eGFR)), episodes of delayed graft function (DGF), defined as the need of hemodialysis during the first post-transplant week, urological complications, Cytomegalovirus (CMV) infections, episodes of UTIs, histological evidence of acute rejection (AR), graft failure (End Stage Renal Disease, ESRD) were collected during the follow-up period (up to 36 months) at each visit and anonymized. The anonymized datasets were then stored separately from the original datasets and subsequently analyzed. Urine culture was performed weekly during the first month after transplantation and then monthly during the first year, or when clinically indicated, during the follow-up. If UTI occurred, a follow-up urine culture after treatment was performed to assess clearance. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism’.
Antibiotics prophylaxis
Antibiotic prophylaxis was used in all patients after surgery. Cephalosporins were administered after transplantation for 2 weeks during which the removal of surgical drains and urinary catheter occurred. Three days after transplantation prophylaxis against Pneumocystis Pneumonia (PCP) was started and continued for 6 months using trimethoprim/sulfamethoxazole. The removal of the ureteral stent occurred about 5 weeks after transplantation, in the absence of active UTIs; a 5-day course of fluoroquinolones was given before the removal.
Definitions
The presence of UTI was defined according to the guidelines of the Infectious Diseases Society of America and European Society of Clinical Microbiology and Infectious Disease [17, 18], as urine culture positivity with more than 105 colony-forming units (CFU) of bacteria per mL with UTIs symptoms. Based on the time of onset, UTIs were categorized into three groups: “Early” in patients who contracted less than three UTIs only in the first 6 months after transplantation; sporadic “Late” UTIs in patients who developed less than three UTIs starting from the sixth month post-transplantation; finally, “Recurrent” UTIs in patients who had three or more episodes throughout 12 months after transplantation, in particular defining “relapsing” the cases with recurrence of infection by the same species with an identical antibiogram after appropriate treatment.
Graft function was defined by changes in serum creatinine and eGFR (calculated using the MDRD formula) in patients with a complete 3-years follow-up. The eGFR at time 0 (T0) was defined as the highest value of eGFR during the first 2 months after transplantation. The primary outcome was graft function (eGFR ≤ 60 ml/min/1.73 m2) at 3 years from the date of kidney transplantation.
Statistical analysis
Parametric variables, expressed as mean values and standard deviation (SD) were compared using Student t test. Non-parametric variables, expressed as medians and interquartile range (IQR), were compared using the Mann–Whitney test. Categorical variables expressed in percentages were compared using Chi square test. Paired sample t tests were used to compare changes in eGFR. Cumulative event-free probabilities were calculated with the use of Kaplan–Meier analysis and tested with the use of the log-rank statistic based on a two-sided type I error rate of 0.05. The Cox model was used to estimate the hazard ratio for the primary outcome after adjustment for pre-specified baseline covariates. Statistical analyses were carried out using SPSS version 20 (IBM SPSS Statistics, IBM Corporation, Armonk, NY, United States), and statistical significance was considered if P values were < 0.05.
Results
UTIs occurred in 184 kidney transplant recipients (48.4%) during the follow-up period [median 64 (34–90) months], 83 patients (21.8%) developed early UTIs, 50 cases (13.2%) presented sporadic late UTIs and 51 patients (13.4%) had recurrent UTIs. The average number of episodes in patients with recurrent UTIs was 5.1 ± 2.5 and relapsing UTIs accounted for 45% of these cases.
The demographics and baseline characteristics of these patients are shown in Table 1.
Data on pathogens responsible of infections were available for 181 (98.4%) patients. Bacterial infections were more frequent compared to fungal ones (7.7%), and overall the most frequently isolated pathogen was Escherichia coli (68.5%), followed by Klebsiella pneumoniae (29.3%). The distribution of pathogens between the three groups of UTIs was not significantly different. Escherichia coli was the most prevalent in each group but there was a higher proportion of Proteus in late UTIs (p = 0.018) and Enterobacter spp in recurrent UTIs (P = 0.010) (Fig. 1). Escherichia coli (90%) was the most common frequent organism in relapsed episodes, followed by Klebsiella pneumonia (10%).
The comparison between the three groups of UTIs, based on recipient age, did not show any difference; nevertheless, the entire group of patients who developed UTIs was significantly older [median age, 50 (37–64) years] than the group of transplant recipients who did not have any episode of UTI during the first year post-transplant [median age, 47.5 (31–58) years UTIs, P = 0.02]. As expected, also the donor age was higher for patients who developed UTIs (P = 0.029).
Similarly to the general population, male kidney transplant recipients had a lower prevalence of UTIs (P < 0.001) and in both genders early UTIs was the predominant category. No significant differences were found between the groups in terms of body mass index (BMI), diabetes, dialysis vintage, type of dialysis, native kidney disease, type of donor, donor renal function, kidney donor risk index (KDRI) score, delayed graft function (DGF), history of acute rejection, panel reactive antibodies, donor specific antibodies (DSA) and occurrence of urological complications after transplantation. Furthermore, we did not find any significant association between the use of steroids, tacrolimus, cyclosporine, mycophenolate and mTOR inhibitors with the development of UTIs and between the different UTIs groups.
Impact of urinary tract infections on graft outcome
Next, we evaluated the impact of the three different categories of UTIs (early, late and recurrent) on graft function after three years (data available for 260 (68.5%) patients).
We found that after 36 months, all patients, except those who developed recurrent UTIs, showed a significant improvement in eGFR compared to the levels at T0 (P < 0.001, Fig. 2).
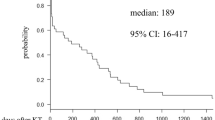
A Kaplan–Meier survival curve using a cut-off of < 60 ml/min for eGFR as end-point, revealed that kidney transplant patients with recurrent UTIs had the worst graft outcome compared to the other two groups (P = 0.01, Fig. 3).
A multivariate survival analysis using Cox’s regression showed that only recurrent UTIs were an independent predictor of graft function in a model adjusted for DGF and episodes of acute rejection (Hazard Ratio, 2.2; 95% CI, 1.3 to 3.5; P = 0.001).
Discussion
Urinary tract infections are a leading cause of hospitalization and comorbidity in kidney transplant recipients [19]. The reported incidence varies widely between 26% and 76%, probably due to differences in the populations studied, definitions, diagnostic criteria and duration of follow-up in the different cohorts [1, 2, 5, 20]. Although it was long believed that UTIs could be considered “benign”, in recent years, a better knowledge and management of transplant patients is highlighting the possibility that these infections can negatively impact on graft’s function, thus spurring many studies on the issue.
In our retrospective cohort study we found a prevalence of UTIs during the first post-transplant year of 48.4% and the majority presented early UTIs (less than 3 episodes during the first semester after transplantation), whereas late UTIs (less than 3 episodes after the first 6 months post-transplantation) and recurrent UTIs (3 or more episodes, persisting after the first semester post-transplantation) showed a similar frequency. Many authors have also reported that infections occurred mainly in the early post-transplant period, particularly in the first 3 to 6 months. This is probably due to the surgical trauma, the placement of urinary catheter and ureteral stent and the need for a higher level of immunosuppression [2, 21, 22].
As for the analysis of potential predisposing factors for UTIs, we took into account variables related to the donor, the recipient and the transplant itself. We compared the categories of UTIs according to various characteristics like recipient age and BMI at the time of the transplant, kidney function indices of the donor and KDRI score, without finding any statistically significant difference. Regarding the age of the recipient, several Authors have shown no correlation with UTIs [23,24,25]; by contrast, several studies have identified older age as an independent risk factor for UTIs in transplant recipient [3, 26, 27]. Kidney transplant in elderly subjects is often associated with a higher percentage of general infections than younger patients [28]. An ineffective cellular immunity and, probably, a lower tolerance to immunosuppression associated with comorbidities, such as diabetes, may contribute to the higher prevalence of bacterial infections found in older recipients. Our findings are in line with these previous reports, as we found that patients with UTIs during the first post-transplant year were significantly older compared to those who did not. Likewise, as expected, this result was mirrored when considering the donor age, given that usually the allocation process takes into account the age of the recipient.
Male recipients developed UTIs less frequently than women as found in other cohorts [2, 24, 29]. We took into account the influence of comorbidity, such as diabetes mellitus, polycystic kidney disease and uropathies, without finding any difference between patients who developed UTIs and those who did not and also between the three categories of UTIs. In the case of diabetes, a number of studies have shown conflicting results [2, 3, 14, 25, 30,31,32]. The different types of pre-transplant treatment (conservative therapy for preemptive kidney transplantation, hemodialysis and peritoneal dialysis) in our groups were not significant. Abbott et al. showed that hemodialysis vintage before transplantation represents a risk factor for the development of UTIs whereas in our cohort there was no association [14].
We did not find any influence of the type of donor (living or deceased), as well as for transplant complications (DGF, acute rejection, CMV infections) in the development of UTIs. In the literature, many studies have found an increased risk of developing UTIs with deceased donor graft [14, 30, 32,33,34,35,36,37], as opposed to Sqalli et al. [23]. The effect of acute rejection is still unclear; Rabkin et al. suggested that a correlation between UTIs and AR is most likely found in the need to treat graft rejection with more intensive immunosuppressive therapy [33]. In our cohort, we did not any association between various immunosuppressant such as prednisone, tacrolimus, cyclosporine, mycophenolate, mTORi, and the development of UTIs. Chuang et al. have found a causal link with the use of azathioprine [3], while Kamath et al. described the relationship with mycophenolate mofetil [25]. Munoz et al. have shown that late UTIs are associated with a corticosteroid dose of > 20 mg/day, baseline creatinine > 2 mg/dl, chronic viral diseases and multiple immunosuppression [38].
The main aim of this study was to show the possible negative impact of UTIs on graft’s function. The analysis of data related to our transplant patients with a follow-up of at least 3 years, has demonstrated relevant clinical findings. Transplant recipient who did not develop UTIs and patients with early or late UTIs showed a significant improvement in graft functionality in terms of creatinine clearance, 36 months after nadir, whereas patients with recurrent infections during the first post-transplant year did not show any improvement. This latter group, in fact, had a worse graft survival curve in 3 years, considering a 60 ml/min cut-off for eGFR as endpoint. To confirm these results, we performed a multivariate Cox regression analysis, which showed that only recurrent UTIs are an independent factor of graft function deterioration, when adjusting the model to known risk factor, as gender, age and DGF.
Different monocentric studies have shown that acute pyelonephritis, especially in the first 3 months post-transplant, represents a risk factor for the worsening of graft function in the long term, but do not affect the loss of the organ in 5 years or the mortality [5, 9].
More controversial is the view on asymptomatic bacteriuria. According to several authors [14, 32, 34, 35, 39] it could be a risk factor for the development of a symptomatic UTI and acute pyelonephritis, and if associated with pyuria, can be detrimental on graft function causing urosepsis in 10–12%, which can be fatal in nearly half of the cases [3, 5]. Other studies have instead shown that asymptomatic bacteriuria may be associated with an insult on the graft, in particular, Ciszek et al. [40] have shown an increase in interleukin-8 levels in the urine of transplanted patients with asymptomatic bacteriuria compared to control, suggesting an inflammatory response that may affect graft function. For these reasons, the possible treatment of these episodes it is still very debated. There are conflicting data on UTIs that occur later in life after transplantation, or after at least 6 months. These have long been considered fairly benign. However, retrospective data from the United States Renal Data System from 28,942 patients show that late UTIs are independently associated with an increased risk (> 1.33 times) of recipient death and graft loss (> 2.35 times) [14]. Moreover, Dupont et al. [41] showed that cortical scarring, though not associated with reduced graft survival, was frequently found in patients with late UTIs, even in asymptomatic patients.
Our study has shown that recurrent UTIs during the first post-transplant year represent an independent predictor of graft’s functional deterioration; in particular patients with recurrent UTIs, unlike the others, did not recovered their graft function and presented a worse graft survival curve in a follow up of 3 years. This study has some limitations that have to be pointed out. It is a retrospective study and the follow up is limited. However, this work indicates that recurrent symptomatic UTIs are potentially dangerous for graft function and patient’s survival. Therefore there is the need for an individualized screening program in kidney transplant recipients in order to establish appropriate prevention and/or treatment, always taking into account the risk–benefit and the issue of antibiotic resistance.
In conclusion, recurrent UTI during the first year after kidney transplantation have negative impact on graft function at three years compared to other types of UTIs. Transplant recipients with recurrent UTIs should be closely monitored to establish an appropriate prevention and/or treatment.
References
Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, West MS, Sillix DH, Chandrasekar PH, Haririan A (2006) Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant 20(4):401–409. https://doi.org/10.1111/j.1399-0012.2006.00519.x
Alangaden G (2007) Urinary tract infections in renal transplant recipients. Curr Infect Dis Rep 9(6):475–479
Chuang P, Parikh CR, Langone A (2005) Urinary tract infections after renal transplantation: a retrospective review at two US transplant centers. Clin Transplant 19(2):230–235. https://doi.org/10.1111/j.1399-0012.2005.00327.x
Ergin F, Arslan H, Yapar G, Karakayali H, Haberal M (2003) Urinary tract infections in renal transplant recipients. Transplant Proc 35(7):2685–2686
Pelle G, Vimont S, Levy PP, Hertig A, Ouali N, Chassin C, Arlet G, Rondeau E, Vandewalle A (2007) Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am J Transplant 7(4):899–907. https://doi.org/10.1111/j.1600-6143.2006.01700.x
Takai K, Aoki A, Suga A, Tollemar J, Wilczek HE, Naito K, Groth CG (1998) Urinary tract infections following renal transplantation. Transplant Proc 30(7):3140–3141
Godaly G, Ambite I, Svanborg C (2015) Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr Opin Infect Dis 28(1):88–96. https://doi.org/10.1097/QCO.0000000000000127
Ak O, Yildirim M, Kucuk HF, Gencer S, Demir T (2013) Infections in renal transplant patients: risk factors and infectious agents. Transplant Proc 45(3):944–948. https://doi.org/10.1016/j.transproceed.2013.02.080
Giral M, Pascuariello G, Karam G, Hourmant M, Cantarovich D, Dantal J, Blancho G, Coupel S, Josien R, Daguin P, Mechineau S, Soulillou JP (2002) Acute graft pyelonephritis and long-term kidney allograft outcome. Kidney Int 61(5):1880–1886. https://doi.org/10.1046/j.1523-1755.2002.00323.x
Schmaldienst S, Dittrich E, Horl WH (2002) Urinary tract infections after renal transplantation. Curr Opin Urol 12(2):125–130
Brown PD (2002) Urinary Tract Infections in Renal Transplant Recipients. Curr Infect Dis Rep 4(6):525–528
Rao KV, Andersen RC (1988) Long-term results and complications in renal transplant recipients. Observations in the second decade. Transplantation 45(1):45–52
Grekas D, Thanos V, Dioudis C, Alivanis P, Tourkantonis A (1993) Treatment of urinary tract infections with ciprofloxacin after renal transplantation. Int J Clin Pharmacol Ther Toxicol 31(6):309–311
Abbott KC, Swanson SJ, Richter ER, Bohen EM, Agodoa LY, Peters TG, Barbour G, Lipnick R, Cruess DF (2004) Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis 44(2):353–362
Oguz Y, Bulucu F, Oktenli C, Doganci L, Vural A (2002) Infectious complications in 135 Turkish renal transplant patients. Cent Eur J Public Health 10(4):153–156
Ghasemian SM, Guleria AS, Khawand NY, Light JA (1996) Diagnosis and management of the urologic complications of renal transplantation. Clin Transplant 10(2):218–223
Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE, Infectious Diseases Society of A (2010) Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50(5):625–663
Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE,, Infectious D, Infectious Diseases Society of A, European Society for M (2011) International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52(5):e103–e120. https://doi.org/10.1093/cid/ciq257
Fiorentino M, Pesce F, Schena A, Simone S, Castellano G, Gesualdo L (2019) Updates on Urinary Tract Infections in Kidney transplantation. J Nephrol (In press)
Karuthu S, Blumberg EA (2012) Common infections in kidney transplant recipients. Clin J Am Soc Nephrol 7(12):2058–2070. https://doi.org/10.2215/CJN.04410512
Saemann M, Horl WH (2008) Urinary tract infection in renal transplant recipients. Eur J Clin Invest 38 Suppl 2:58–65. https://doi.org/10.1111/j.1365-2362.2008.02014.x
Senger SS, Arslan H, Azap OK, Timurkaynak F, Cagir U, Haberal M (2007) Urinary tract infections in renal transplant recipients. Transplant Proc 39(4):1016–1017. https://doi.org/10.1016/j.transproceed.2007.02.060
Sqalli TH, Laboudi A, Arrayhani M, Benamar L, Amar Y, Ouzeddoun N, Bayahia R, Rhou H (2008) Urinary tract infections in renal allograft recipients from living related donors. Saudi J Kidney Dis Transpl 19(4):551–553
Memikoglu KO, Keven K, Sengul S, Soypacaci Z, Erturk S, Erbay B (2007) Urinary tract infections following renal transplantation: a single-center experience. Transplant Proc 39(10):3131–3134. https://doi.org/10.1016/j.transproceed.2007.10.005
Kamath NS, John GT, Neelakantan N, Kirubakaran MG, Jacob CK (2006) Acute graft pyelonephritis following renal transplantation. Transpl Infect Dis 8(3):140–147. https://doi.org/10.1111/j.1399-3062.2006.00148.x
Bonkat G, Rieken M, Siegel FP, Frei R, Steiger J, Groschl I, Gasser TC, Dell-Kuster S, Rosenthal R, Gurke L, Wyler S, Bachmann A, Widmer AF (2012) Microbial ureteral stent colonization in renal transplant recipients: frequency and influence on the short-time functional outcome. Transpl Infect Dis 14(1):57–63. https://doi.org/10.1111/j.1399-3062.2011.00671.x
Vidal E, Torre-Cisneros J, Blanes M, Montejo M, Cervera C, Aguado JM, Len O, Carratala J, Cordero E, Bou G, Munoz P, Ramos A, Gurgui M, Borrell N, Fortun J, Spanish Network for Research in Infectious D (2012) Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transpl Infect Dis 14(6):595–603. https://doi.org/10.1111/j.1399-3062.2012.00744.x
Meier-Kriesche HU, Ojo A, Hanson J, Cibrik D, Lake K, Agodoa LY, Leichtman A, Kaplan B (2000) Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation 69(5):885–889
Lee JR, Bang H, Dadhania D, Hartono C, Aull MJ, Satlin M, August P, Suthanthiran M, Muthukumar T (2013) Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: a single-center report of 1166 kidney allograft recipients. Transplantation 96(8):732–738. https://doi.org/10.1097/TP.0b013e3182a04997
Glazier DB, Jacobs MG, Lyman NW, Whang MI, Manor E, Mulgaonkar SP (1998) Urinary tract infection associated with ureteral stents in renal transplantation. Can J Urol 5(1):462–466
Almond PS, Matas A, Gillingham K, Dunn DL, Payne WD, Gores P, Gruessner R, Najarian JS (1993) Risk factors for chronic rejection in renal allograft recipients. Transplantation 55(4):752–756; (discussion 756–757)
Goya N, Tanabe K, Iguchi Y, Oshima T, Yagisawa T, Toma H, Agishi T, Ota K, Takahashi K (1997) Prevalence of urinary tract infection during outpatient follow-up after renal transplantation. Infection 25(2):101–105
Rabkin DG, Stifelman MD, Birkhoff J, Richardson KA, Cohen D, Nowygrod R, Benvenisty AI, Hardy MA (1998) Early catheter removal decreases incidence of urinary tract infections in renal transplant recipients. Transplant Proc 30(8):4314–4316
de Souza RM, Olsburgh J (2008) Urinary tract infection in the renal transplant patient. Nat Clin Pract Nephrol 4(5):252–264. https://doi.org/10.1038/ncpneph0781
Papasotiriou M, Savvidaki E, Kalliakmani P, Papachristou E, Marangos M, Fokaefs E, Maroulis I, Karavias D, Goumenos DS (2011) Predisposing factors to the development of urinary tract infections in renal transplant recipients and the impact on the long-term graft function. Ren Fail 33(4):405–410. https://doi.org/10.3109/0886022X.2011.568137
Dantas SR, Kuboyama RH, Mazzali M, Moretti ML (2006) Nosocomial infections in renal transplant patients: risk factors and treatment implications associated with urinary tract and surgical site infections. J Hosp Infect 63(2):117–123. https://doi.org/10.1016/j.jhin.2005.10.018
Rivera-Sanchez R, Delgado-Ochoa D, Flores-Paz RR, Garcia-Jimenez EE, Espinosa-Hernandez R, Bazan-Borges AA, Arriaga-Alba M (2010) Prospective study of urinary tract infection surveillance after kidney transplantation. BMC Infect Dis 10:245. https://doi.org/10.1186/1471-2334-10-245
Munoz P (2001) Management of urinary tract infections and lymphocele in renal transplant recipients. Clin Infect Dis 33(Suppl 1):S53–S57. https://doi.org/10.1086/320905
Lyerova L, Lacha J, Skibova J, Teplan V, Vitko S, Schuck O (2001) Urinary tract infection in patients with urological complications after renal transplantation with respect to long-term function and allograft survival. Ann Transplant 6(2):19–20
Ciszek M, Paczek L, Bartlomiejczyk I, Mucha K (2006) Urine cytokines profile in renal transplant patients with asymptomatic bacteriuria. Transplantation 81(12):1653–1657. https://doi.org/10.1097/01.tp.0000226072.20185.f8
Dupont PJ, Psimenou E, Lord R, Buscombe JR, Hilson AJ, Sweny P (2007) Late recurrent urinary tract infections may produce renal allograft scarring even in the absence of symptoms or vesicoureteric reflux. Transplantation 84(3):351–355. https://doi.org/10.1097/01.tp.0000275377.09660.fa
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pesce, F., Martino, M., Fiorentino, M. et al. Recurrent urinary tract infections in kidney transplant recipients during the first-year influence long-term graft function: a single-center retrospective cohort study. J Nephrol 32, 661–668 (2019). https://doi.org/10.1007/s40620-019-00591-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-019-00591-5