Abstract
Chronic kidney disease (CKD) is associated with mineral and bone disorders (MBD) that are now considered as a syndrome. Bone fragility and a four to tenfold increased rate of skeletal fractures are often reported in CKD patients. The evaluation of the risk of these fractures in CKD patients should explore the same risk factors identified for the general population including low body weight, menopause, personal and familial history of osteoporosis, chronic inflammatory diseases, and corticosteroid therapy. The aim of this article is to provide a critical review of the tools used for the evaluation of bone loss and the risk of fracture in CKD patients, ranging from the measurement of bone mineral density (BMD), fracture risk assessment (Frax™), quantitative computed tomography (QCT), high-resolution peripheral quantitative computed tomography (HRpQTC), to circulating biomarkers of bone metabolism including vitamin D, parathyroid hormone (PTH), bone-specific alkaline phosphatase, osteocalcin, and some collagen type 1-related molecules indicators of bone remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
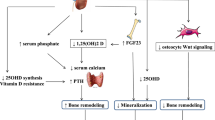
Chronic kidney disease (CKD) leads to mineral and bone disorders (MBD) that are now considered as a syndrome. This CKD-MBD syndrome associates three major domains: biochemical abnormalities (calcium, phosphate, parathyroid hormone (PTH), vitamin D, klotho, fibroblast growth factor 23 (FGF23) and sclerostin); cardiovascular calcifications; and bone abnormalities. The ancient histomorphometric concept of renal osteodystrophy (ROD) has been redefined and includes now skeletal fragility parameters such as bone volume and mineralization (KDIGO).
The abnormalities of bone and mineral biomarkers as well as of calciotropic hormones begin very early stages of CKD progression. For instance, an increased urinary fractional excretion of phosphate, subsequently to the decreased tubular phosphate reabsorption, is observed in CKD individuals with an estimated glomerular filtration rate (eGFR) as high as 110–119 ml/min/1.73 m2 and normal phosphatemia [1]. Simultaneously, alterations in circulating PTH, FGF23, sclerostin, klotho, 25OHD2, and 1,25OH2D3 are also evident at this normal range of eGFR [1, 2]. However, the skeletal impact of these alterations and the risk of bone demineralization and fractures appears only when the eGFR falls below 45 ml/min/1.73, at CKD stages 3b and lower. Compared with subjects with normal renal function in the general population, CKD stage 3b show 1.5 to threefold higher risk of fracture. Their 3-year cumulative incidence of peripheral fractures (humerus, forearm and hip) as well as axial fractures (pelvis) increased progressively with the decline in eGFR [3]. Strikingly, both genders, women and men with CKD stage 5 show an extremely high rate of fractures with 10% of women and 5% of men experiencing at least one fracture within 3-year period [3]. These findings are probably underestimated and the risk of hip fractures increases even in women with relatively preserved renal function if cystatin C is used as a surrogate marker for the renal function [4, 5]. Indeed, cystatin C, in the presence of normal creatinine-based eGFR, may identify preclinical states of renal dysfunction in older adults [4, 5].
In CKD-5D patients, several studies have reported an increased incidence of skeletal fractures. The second wave of DOPPS (Dialysis Outcomes and Practice Patterns), from 2002 to 2004, found that among the 12,782 prevalent dialysis patients, 2.6% had a history of having experienced a hip fracture before entering in the study, and an annual incidence of hip fracture of 0.89% and of 2.56% for any other new fractures [6]. Older age, female, hypoalbuminemia, history of kidney transplantation and cardiovascular diseases, and the prescription of psychotropic and/or anti-depressive medications such as inhibitors of serotonin reuptake, were all associated with the risk of new fractures [6]. Another analysis, now including the whole DOPPS cohort, 34,579 dialysis patients from 12 countries, and comprising four waves from 2002 to 2011 or 10 years of follow-up, showed that overall 3% of these patients have had a fracture. The annual incidence of fracture was variable from one country to another, being the lowest in Japan (1.2%) and the highest in Belgium (4.5%). The incidence of fracture did not vary over time with approximately 2–2.5% per year, but the fracture rate remained significantly higher than that reported for the general population [7]. The occurrence of fracture in this cohort revealed that CKD-5D patients had a 2–3 longer hospitalization stay and a 3–4 higher mortality rate compared to the overall DOPPS population [7].
In data extracted from the 2010 French National Hospital Database, including the 88,962 hospitalizations for hip fractures as a primary diagnosis per the ICD-10 codes, among these patients who suffered from hip fractures, 362 were CKD-5D. The incidence of hip fracture was fourfold higher in dialysis patients compared to non-dialysis patients. Women on dialysis experienced hip fractures at an earlier age than non-dialysis women. As observed in the DOPPS cohort, this study confirmed that older age, diabetes, cardiovascular disease and dementia were factors associated with increased risk of hip fractures [8]. Similarly, the length of hospitalization stay for hip fracture was 5 days longer, enhancing the financial burden of CKD and was also associated with an increased mortality rate in both genders in dialysis patients.
Evaluation of bone fragility in CKD
The assessment of bone fragility and the risk of fracture in CKD subjects should respect the same rules as for the people in the general population older than 50 years, including the identification of risk factors such as malnutrition or low body weight, menopause, familial history of osteoporosis, inflammation-related diseases (rheumatoid arthritis, inflammatory bowel disease, chronic obstructive pulmonary disease) and medications known to increase the fracture risk (glucocorticoids, selective serotonin reuptake inhibitors); identification of patients with low-kinetics fractures or after minimal trauma; bone mineral density (BMD) measurement is recommended in women from the age of 65 years and men of 70 years in the absence of known risk factors or earlier in case of significant fractures or additional risk factors; laboratory assessment is recommended essentially biochemical markers of mineral and bone metabolism, in particular if any medication to reduce the fracture risk is being considered. Finally, the assessment of the type of histological ROD should also be considered if the results will impact therapeutic decision Table 1. Three of these five topics will briefly be detailed in the following paragraphs, the use of BMD, high-resolution peripheral quantitative computed tomography (HRpQTC), and mineral and bone circulating biomarkers.
Bone mineral density
Bone mineral density (BMD) measurement using dual-energy X-ray absorptiometry (DEXA) as well as computerized tomography (CT) are useful tools for the assessment of bone fragility. However, several anatomical and histological particularities of the skeleton must be taken into consideration when interpreting the results, including that approximately 80% of the total skeleton is composed by cortical bone and the other 20% by trabecular bone; that bone alterations in CKD mainly affect cortical bones, which are often characterized by increased porosity and reduced cortical thickness, whereas trabecular bones appear to be spared; that DEXA cannot make the difference between cortical and trabecular bones; that DEXA cannot distinguish neither between high and low bone turnover; and finally that DEXA may overestimated BMD at the lumbar spine in CKD because of the high prevalence in these patients of arthritis, scoliosis, and abdominal aorta calcifications, as well as because the results reported from 13 cross-sectional studies are not homogenous and could be influenced by difference in age, gender, ethnicity, and dialysis vintage. For these reasons, the systematic use of BMD in the evaluation of fracture risk in CKD, especially in dialysis patients is still controversial, although recommended by the recent revised KDIGO.
Compared with the general population, low femoral BMD (total hip T-Score ≤2.5 standard deviation), is greater in patients with CKD stages 3–4 than in subjects with normal renal function [data from the third NHANES (National Health and Nutrition Examination Survey)]. Overall, 24% of women and 11% of men in this cohort had osteoporosis. Unsurprisingly, a significant number of the elderly subjects with osteoporosis also had a reduced eGFR or a CKD within stages 3–4 [9, 10]. The Rancho Bernardo study corroborated and extended these findings in the sense that they prospectively demonstrated a direct correlation between the decline in the renal function and the 4-year loss of BMD at the hip [11]. Among the 1713 subjects included in this study, 8% experienced new clinical fractures during the follow-up period [11]. Similarly, reduced renal function was associated with a higher rate of bone loss with aging in elderly Afro-Caribbean men [12].
In dialysis patients, the relation between BMD and the risk of fracture is not as strong as in the general population often leading to contradictory results. Indeed, five cross-sectional studies did not find any association between low BMD and the incidence of skeletal fractures [13–17], whereas eight other studies reported positive results [18–24]. Nevertheless, when collectively analyzed in a meta-analysis, a low BMD in pre-dialysis as well as in dialysis patients was associated with a high risk of fractures [25]. Despite these latest findings, the use of BMD measurement was not recommended in 2009 KDIGO, because of inconsistency in the results obtained from mostly cross-sectional designed studies as above described. Presently, the results of 4 prospective cohorts show a good predictive value of BMD for the risk of fracture in CKD stages 3 to 5D [26–29]. The first study carried out in 485 adult CKD-5D patients, lower femoral neck and total hip BMD was independently associated with increased risk of any type of incident fractures [26]. The second study was performed on 2754 older participants in the Health, Aging, and Body Composition (Health ABC) cohort and found that a low femoral neck BMD doubled the risk of any fracture in CKD patients as well as in participants without CKD [29]. The third study explored the community-based Canadian Multicentre Osteoporosis Study (CaMos) cohort, which included 1426 subjects and assessed bone fragility by DEXA and the evaluation of trabecular bone score (TBS) at L1-L4. Subjects with CKD, defined as eGFR < 60 ml/min/1.73 m2, and a TBS value below the median value had a significantly threefold higher 5-year fracture probability than those with a TBS above the median values [27]. Finally, the fourth study included 131 CKD patients (eGFR < 60 ml/min/1.73 m2) from three hospitals in Toronto, Canada, who were followed for 2 years [28]. At the baseline, mean BMD at the total hip, lumbar spine, and radius was significantly lower in patients with incident fracture compared with those without. They also found that for every 1 SD decrease in BMD at the total hip, there was a twofold increase in the risk of hip fracture. Likewise, for every 1 SD decrease of BMD at the lumbar spine, there was a 1.5-fold increase in the risk of incident fractures [28]. It should be emphasized that the prediction values of lumbar BMD in CKD patients are slightly lower than that observed in non-CKD subjects, in whom each reduction of 1 SD in the BMD at the lumbar spine doubles the fracture risk. This difference may be partially explained by the underdiagnosed of reduced lumbar BMD in these patients because of the presence of vascular calcifications and osteoarthritis.
Altogether, the results of these four studies were sufficient to convince the KDIGO experts to revise the 2009 3.2.1 guideline and to potentially recommend, in a future publication (KDIGO 2017, in press), the use of BMD measurement for the prediction of peripheral fractures in CKD stage 3a-5D, whereas there is no evidence that BMD could be predictive of vertebral fractures.
FRAX™ is a web-based fracture risk assessment (FRAX) tool developed by researchers at the Sheffield University, UK, to provide a way to calculate an individual’s risk of having an osteoporotic fracture within the next 10 years, based on specific risk factor, with and/or without having BMD results. The utility of the use of FRAX™ in CKD patients was for the first time verified in a cross-sectional Canadian study including 353 patients with CKD stage 4, with prevalent skeletal fractures in one-third of them [30]. In this group of patients, FRAX™ score significantly discriminated between those with clinical peripheral, vertebral and any fractures and those without. FRAX™ was even better than BMD for the prediction of the risk of non-spine fractures, however, the best performances were observed when considering together FRAX™ and BMD [30]. The results of the CaMos study (Canadian Multicentre Osteoporosis Study) also illustrated the ability of FRAX™ to predict osteoporotic fracture in 320 subjects with eGFR < 60, which was like that of 1787 subjects with normal renal function [27]. To the best of our knowledge, there is no study examining the use of FRAX™ in CKD-5 and dialysis patients, and this absence of study might be explained because at stages 4-5D of CKD, the disorders in bone metabolism are so dominant that WHO criteria for the diagnosis of osteoporosis and the use of FRAX™ may become invalid.
QCT and HR-pQCT measurement
Bone strength greatly depends on the quantity and quality of cortical bone, parameters which are highly altered in CKD. Data from bone biopsy studies in CKD revealed that most of these patients exhibit thin cortices with normal cortical porosity in case of low bone turnover, whereas they have high cancellous bone volume and normal cortical thickness in case of high bone turnover bone diseases [31]. Consequently, assessment of cortical bone is crucial in CKD as these patients loss principally cortical bone, mainly due to high cortical porosity or endo-cortical bone resorption, and because cortical bone loss is highly associated to peripheral fractures at both weight-bearing and non-weight-bearing sites [32].
As mentioned earlier, BMD as assessed by DEXA is not sufficient to assess the fracture risk because DEXA cannot discriminate between cortical and trabecular bone nor the type of ROD. Conventional quantitative computed tomography (QCT) can distinguish between both cortical and trabecular bone compartments, and has been used in several studies including CDK-5D to predict the risk of fractures. In one of the first studies, 21% of dialysis patients had tomographic signs of vertebral fractures. Lumbar QCT, quantifying separately BMD at cortical and trabecular, showed that cortical lumbar BMD was the best predictor of vertebral fractures [33]. In another study, peripheral (radial) QCT was used to assess the predictive value of cortical and trabecular BMD in a group of 52 CKD-5D patients. It was found that a decrease in cortical BMD increased by 16 folds the risk of fractures, decreased cortical area and thickness were also associated with increased risk of fractures [34]. Conventional QCT has also been shown to identify a higher number of patients experiencing bone loss at the hip when compared to DEXA (51.3 vs 38.5%) [35].
More recently, the measurement of BMD and microarchitecture by high-resolution peripheral quantitative computed tomography (HRpQTC) has raised some hope because this tool allows the measurement of cortical and trabecular bone parameters separately as well as the visualization of fine ultra-structural details, including trabecular thickness, number and separation [36, 37]. Using the pQCT device from Stratec, Hasegawa et al. showed that low cortical BMD, was a good and significant predictor of skeletal fractures in CKD patients [38]. In CKD stages 2–4, using the HRpQCT device from Scanco, it was showed that early alteration of trabecular bone, before the onset of severe SHPT, may partially explain the high risk of fractures at those earlier stages of CKD [39], but also across CKD evolution [36]. HRpQCT also confirms and extend the findings that bone loss in CKD stage 5D is mainly due to a reduction of cortical mineral density and thickness, but not of trabecular bone [32]. Moreover, these cortical features are strongly associated with fractures [36]. Although useful to predict fracture risk and identify the population who will benefit the most of therapeutic interventions, this equipment is not widely available and could not be used for routine assessment.
Biochemical parameters
PTH
In the absence of bone biopsy, PTH remains the more constant, oldest and best surrogate biomarker for bone histologic pattern in CKD [35]. Supporting this assumption, most CKD patients with adynamic bone disease show serum intact PTH levels below 150 pg/mL [40] and those with histological signs of SHPT a PTH above 600 pg/ml [41]. It should be emphasized that both high and low circulating PTH levels are associated with increased risk of fractures [6, 42, 43]. In most cross-sectional studies [18, 44] low values of PTH were associated with low BMD and a high incidence of fractures. Inversely, in the DOPPS cohort [45] the highest serum PTH levels (>900 pg/ml) were associated with the highest prevalence of fractures and a relative risk of new fracture increased by 76% [6, 7, 42].
The variation of PTH values over time rather than an isolate value seems to be much more relevant in the occurrence of these fractures. This assumption has been verified in the largest study examining the risk factors of hip fractures in CKD-5D, the Fresenius Medical Care North America cohort, which included 955,341 dialysis patients from 2000 to 2013. They found that the lowest quartiles of time-average PTH (181–272 pg/ml) were independently associated with the highest risk of hip fracture [46]. However, several studies have showed that serum PTH levels, just prior to a new fracture, are associated with a significantly greater risk of fracture, contrarily to baseline or time average PTH values. The upper and lower PTH values of the U shaped PTH curve are associated with a significantly higher risk of fracture compared with PTH values within the recommended NKF/K-DOQI target values [26]. It should be stressed that currently most of the decisions to treat CKD-MBD are taken with a single or two PTH value, and that the revised KDIGO have now included the term ‘persistently’ above the upper normal PTH level as well as ‘progressively rising’ PTH levels, rather than ‘above the upper normal limit.’ That is, treatment should not be based only on a single elevated PTH value (KDIGO 2016).
Various confounding factors may interfere with the interpretation of PTH values observed at the time when a new fracture occurs, such as previous surgical parathyroidectomy (PTX) that rapidly lowers serum PTH levels in most patients; and the use of calcimimetics that also may reduce serum PTH levels in patients with a long history of uncontrolled SHPT with high bone remodeling, and that at the time of the fracture, have normal or low PTH values. This long-term exposition to high serum PTH concentrations may had induced a preferential loss of cortical bone and the risk of fracture, which could be even more pronounced in CKD-5D women [47]. PTX reduces bone turnover, may improve bone mineral density and reduce long-term risk for fractures as observed in a national cohort of long-term CKD-5D patients who underwent PTX [48–50]. In the EVOLVE trial (Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events) a placebo-controlled trial that randomized 3883 hemodialysis patients with SHPT (PTH > 300 pg/ml) to receive cinacalcet or placebo for 5 years, 13.2% of the patients treated by cinacalcet had clinical fractures (median PTH of 694 pg/ml) compared with 12.2% in the placebo group. There was no difference between the two groups in an unadjusted intention-to-treat analysis. However, when accounting for differences in baseline characteristics, multiple fractures, and/or events prompting discontinuation of study drug, cinacalcet reduced the rate of clinical fracture by 16–29% [51].
Vitamin D
Vitamin D, both 25OHD and 125OH2D, plays a crucial role on bone metabolism, since optimal levels are necessary to keep normal intestinal and renal absorptions of calcium and phosphate, serum PTH concentration within normal ranges, and to maintain an appropriate bone mineralization and BMD. However, the optimal circulating 25OHD concentration in CKD that might protect against bone fractures is unknown and controversial [52]. Regarding 1,25OH2D, there is no evidence about the optimal 1,25OHD values in CKD. Despite of this, KDIGO guidelines recommend to maintain circulating 25OHD levels in CKD subjects above 30 ng/mL as for the general population [10]. In CKD-5D treated by dialysis and applying the same 30 ng/mL cut-off leads to an estimated prevalence of vitamin D insufficiency and/or deficiency that range from 50 to 98% with a combined mean prevalence of 82% on a total sample size of 3722 patients [53].
The unique study looking at the relation between circulating 25OHD concentration and bone histology in CKD was published by Coen et al. [54] In their retrospective study on CKD-5D patients, they found that serum 25OHD levels was associated with bone metabolism independently of serum PTH and calcitriol levels. Serum 25OHD levels <20 ng/mL positively correlated with bone formation rate (BFR), the velocity of osteoid synthesis, mineralization and all static histomorphometric parameters, inversely, values >40 ng/ml were associated with a downslope of dynamic parameters of bone turnover [54]. The authors also reported a progressive increase in osteoid thickness in patients with the lowest serum 25OHD levels and high PTH. However, the incidence of bone fracture was not reported in this study.
Few studies have looked at the correlation between serum vitamin D levels and BMD in the general as well as in CKD populations. In a homogeneous population of southern Europe postmenopausal women, BMD was lower in vitamin D deficient groups, which also had higher levels of markers of bone resorption and formation [55]. The relation between serum 25OHD levels and BMD was explored in an Indian pre-dialysis population, they found a stepwise decrease of serum 25OHD levels and lumbar BMD from CKD stage 3–5 as well as a positive correlation between lumbar spine BMD and 25OHD values [56]. In another study looking at the determinant factors of BMD and the risk factors of fractures in 70 CKD-5D patients, it was showed that BMD was significantly reduced only at the mid-radius but did not predict the risk of fracture, moreover, circulating 25OHD and 1,25OH2D levels did not correlate to BMD at any site. Nevertheless, bone fractures were associated with dialysis vintage and with low total body BMD, and rib fractures were frequent and associated with a poor nutritional state [23]. A small observational study in 130 CKD-5D patients [57] found that 16% of those patients experiencing a low-trauma fracture, and that serum 25OHD levels in these patients were twice lower than in the patients without fractures, 15 versus 30 nmol/L. In addition, low 25OHD, low BMD at the distal radius, low PTH, and prior bone fracture before starting dialysis were independent predictors of fractures [57]. Inversely, higher circulating 25OHD levels are associated with a decrease in trabecular network heterogeneity [32]. Likewise, another study, in 69 CKD-5D patients with a high prevalence of vitamin D deficiency (59%), showed a significant negative correlation between serum 25OHD and PTH levels, and a positive correlation between 25OHD and BMD at the radius. Low vitamin D was also independently associated with reduced BMD at the calcaneus as assessed by ultrasounds [58].
An Australian study carried out in 242 CKD-5D patients, who were on the waiting list for either kidney or kidney plus pancreas transplantation, found a significant lower serum 25OHD levels in patients treated by peritoneal dialysis (49 nmol/L) compared with patients on hemodialysis (77 nmol/L), as well as a negative correlation between serum 25OH levels and BMD (z score) at lumbar spine and wrist. Lower femoral neck BMD was also associated with an increased prevalence of vertebral fracture and fragility fracture at any site [19]. Another Japanese, cross-sectional observational study, enrolling 325 CKD 3–5 adult patients (210 men and 115 women), found that 80% of these patients had serum 25OHD lower than 30 ng/ml and that serum 25OHD and body mass index were independent positive determinants of lumbar spine and femoral neck BMD [59].
Of note, supplementation with calcidiol (25OHD) in CKD-5D patients improves bone mineralization, whereas has a limited effect on reducing serum PTH levels similarly to non-CKD patients [60, 61]. Regarding calcitriol, it efficiently lowers serum PTH levels, but seems to have little or no effect in bone mineralization. A meta-analysis of several randomized trials that reported skeletal outcomes in CKD patients pointed out that in terms of patient-level skeletal outcomes such as fractures, bone pain, requirement of surgical parathyroidectomy, there was no benefit from the administration of any active vitamin D analogs [62].
Other bone biomarkers in the prediction of low BMD and fractures
There are scarce studies looking at the use of old and more recent bone biomarkers for the estimation of BMD and the risk of fracture. In one of them, 137 CKD-5D patients with distal radius BMD reduction showed a significantly higher serum bone-specific alkaline phosphatase (BASP) levels BAP and a tendency toward higher N-terminal pro-peptide of type 1 collagen (PINP) and (pyridinoline) PYD than those without BMD reduction. However, the difference did not reach statistical significance in serum osteocalcin (Oc), collagen type 1 beta cross-linked peptide (b-CTX) and deoxypyridinoline (DPD) [63]. The same authors also reported in a group of 195 dialysis patients, that PINP negatively and significantly correlated with the annual loss in BMD at the distal third of the radius Moreover, when these patients were stratified into tertiles, per degree of bone loss, those with the greatest bone loss had higher PINP, BSAP, and OC [64].
In a cross-sectional study including 82 CKD 3–5 patients, showed that the 23 patients having experienced fractures had low BMD at the femoral neck and higher values of Oc, PINP, and tartrate-resistant acid phosphatase 5b (Trap5b) [65]. Another study looked at the correlation between Trap5b and NTX (cross-linked N-telopeptide of type I collagen) with BMD at the second metacarpal bone in 103 dialysis patients [66]. They showed that the increase in these two bone resorption biomarkers were independently associated with low BMD [66]. Likewise, the highest quartile of serum NTX, b-CTX and DPD concentrations were associated with the fastest rate of annual bone loss, at the distal third of the radius, in 113 dialysis patients [67]. At the same skeletal site, the highest quartiles of serum b-CTX levels, in 160 dialysis patients, were associates with a rapid reduction of BMD [68].
Abnormal serum phosphate level is undeniably one of the most important components of CKD-BMD. Circulating phosphate levels slowly rise as CKD progresses and directly and indirectly contribute to skeletal fragility found in CKD-MBD, particularly through the stimulation of PTH and FGF23 production [69]. FGF23 is mainly produced by osteocytes and osteoblasts and it exerts its major physiological actions in the kidney stimulating urinary phosphate excretion and inhibiting calcitriol synthesis after binding a complex formed by alpha-klotho and one of the four canonical FGF receptors. It has been suggested that FGF23 could also play an important role in the regulation of bone mineralization. In fact, the absence of FGF23, as in FGF23 knockout animals, and the excess of FGF23, as in klotho knockout animals, there is severe bone demineralization. Unfortunately, in CKD-5D patients, a study looking at the relation between serum FGF23 levels and BMD did not find any significant correlation either at the femoral neck or at the lumbar spine [70]. However, high FGF23 values have been associated with reduced osteoid thickness and osteoid maturation time, in children with normal renal function and in CKD dialysis children [71]. This paradox appears to be deciphered as illustrated by a recent publication where it has been demonstrated that FGF23 modulates bone mineralization by regulating the tissue non-specific alkaline phosphatase (TNAP) specifically through the FGFR3 and independently of klotho. FGF23 inhibits TNAP and by this way increased extracellular concentration of pyrophosphate, reduced the amount of inorganic (free) phosphate, and indirectly stimulates osteopontin gene expression, a known mineralization inhibitor [72]. Excessive FGF23 has also been shown to contribute to bone loss in CKD through another klotho-dependent mechanism and the stimulation of the osteoblast Wnt inhibitors Dkk1 [73]. Therefore, the inactivation of the Wnt/b-catenin signaling pathway by the altered phosphate/FGF23/Klotho axis may provide another autocrine/paracrine mechanism favoring bone loss in CKD-MBD.
Bone biopsy
Bone histomorphometry is the gold standard to evaluate CKD-MBD [74] although not routinely recommended except if the results will impact therapeutic decisions (KDIGO 2016). Unfortunately, there is no evidence showing an association between fractures and the histological type of ROD.
Conclusions
The etiology of the increased fracture risk in CKD population is multifactorial and involved most of the risk factors identified in the general population including low BMD, low body weight, early menopause, maternal history of osteoporosis and inflammation-related diseases. However, the distinction between fractures occurring because of severe osteoporosis or because of ROD related to CKD-MBD fractures is not clear-cut and does not often offer a clear view in terms of prognosis. Obviously, the prevention of skeletal fractures is the principal clinical objective in CKD-MBD subjects and yet is absent in the current CKD-MBD guidelines. There is neither established evidence-based data helpful in clinical practice to support clinicians ordering imaging tests to identify and manage CKD patients with skeletal fractures. The revised KDIGO guidelines will now recommend the use of BMD measurement for the prediction of peripheral fractures in CKD stage 3a-5D, whereas there is no evidence that BMD could be predictive of vertebral fractures, nor if MBD should be performed at any giving interval for the monitoring of bone loss. The combination of BMD with FRAX improves the risk prediction of bone fractures. Finally, the usefulness of circulating biomarkers of bone metabolism in predicting the risk of skeletal fractures and bone loss in CKD remains unreliable.
References
Dhayat NA, Ackermann D, Pruijm M et al (2016) Fibroblast growth factor 23 and markers of mineral metabolism in individuals with preserved renal function. Kidney Int 90(3):648–657
Urena-Torres P, Prie D, Keddad K et al (2014) Changes in fibroblast growth factor 23 levels in normophosphatemic patients with chronic kidney disease stage 3 treated with lanthanum carbonate: results of the PREFECT study, a phase 2a, double blind, randomized, placebo-controlled trial. BMC Nephrol 15:71
Naylor KL, McArthur E, Leslie WD et al (2014) The three-year incidence of fracture in chronic kidney disease. Kidney Int 86(4):810–818
Ensrud KE, Parimi N, Cauley JA et al (2013) Cystatin C and risk of hip fractures in older women. J Bone Miner Res 28(6):1275–1282
Ensrud KE, Parimi N, Fink HA et al (2014) Estimated GFR and risk of hip fracture in older men: comparison of associations using cystatin C and creatinine. Am J Kidney Dis 63(1):31–39
Jadoul M, Albert JM, Akiba T et al (2006) Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70(7):1358–1366
Tentori F, McCullough K, Kilpatrick RD et al (2014) High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 85(1):166–173
Maravic M, Ostertag A, Urena P, Cohen-Solal M (2016) Dementia is a major risk factor for hip fractures in patients with chronic kidney disease. Osteoporos Int 27(4):1665–1669
Klawansky S, Komaroff E, Cavanaugh PF Jr et al (2003) Relationship between age, renal function and bone mineral density in the US population. Osteoporos Int 14(7):570–576
KDIGO (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 113:S1–S130
Jassal SK, von Muhlen D, Barrett-Connor E (2007) Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res 22(2):203–210
Kuipers AL, Egwuogu H, Evans RW et al (2015) Renal function and bone loss in a Cohort of Afro-Caribbean Men. J Bone Miner Res 30(12):2215–2220
Ersoy FF, Passadakis SP, Tam P et al (2006) Bone mineral density and its correlation with clinical and laboratory factors in chronic peritoneal dialysis patients. J Bone Miner Metab 24(1):79–86
Gerakis A, Hadjidakis D, Kokkinakis E, Apostolou T, Raptis S, Billis A (2000) Correlation of bone mineral density with the histological findings of renal osteodystrophy in patients on hemodialysis. J Nephrol 13(6):437–443
Jamal SA, Chase C, Goh YI, Richardson R, Hawker GA (2002) Bone density and heel ultrasound testing do not identify patients with dialysis-dependent renal failure who have had fractures. Am J Kidney Dis 39(4):843–849
Negri AL, Barone R, Quiroga MA et al (2004) Bone mineral density: serum markers of bone turnover and their relationships in peritoneal dialysis. Perit Dial Int 24(2):163–168
Piraino B, Chen T, Cooperstein L, Segre G, Puschett J (1988) Fractures and vertebral bone mineral density in patients with renal osteodystrophy. Clin Nephrol 30(2):57–62
Atsumi K, Kushida K, Yamazaki K, Shimizu S, Ohmura A, Inoue T (1999) Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis 33(2):287–293
Elder GJ, Mackun K (2006) 25-Hydroxyvitamin D deficiency and diabetes predict reduced BMD in patients with chronic kidney disease. J Bone Miner Res 21(11):1778–1784
Fontaine MA, Albert A, Dubois B, Saint-Remy A, Rorive G (2000) Fracture and bone mineral density in hemodialysis patients. Clin Nephrol 54(3):218–226
Jamal SA, Gilbert J, Gordon C, Bauer DC (2006) Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res 21(4):543–548
Kaji H, Suzuki M, Yano S et al (2002) Risk factors for hip fracture in hemodialysis patients. Am J Nephrol 22(4):325–331
Urena P, Bernard-Poenaru O, Ostertag A et al (2003) Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol Dial Transplant 18(11):2325–2331
Yamaguchi T, Kanno E, Tsubota J, Shiomi T, Nakai M, Hattori S (1996) Retrospective study on the usefulness of radius and lumbar bone density in the separation of hemodialysis patients with fractures from those without fractures. Bone 19(5):549–555
Bucur RC, Panjwani DD, Turner L, Rader T, West SL, Jamal SA (2015) Low bone mineral density and fractures in stages 3–5 CKD: an updated systematic review and meta-analysis. Osteoporos Int 26(2):449–458
Iimori S, Mori Y, Akita W et al (2012) Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients–a single-center cohort study. Nephrol Dial Transplant 27(1):345–351
Naylor KL, Garg AX, Zou G et al (2015) Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol 10(4):646–653
West SL, Lok CE, Langsetmo L et al (2015) Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res 30(5):913–919
Yenchek RH, Ix JH, Shlipak MG et al (2012) Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 7(7):1130–1136
Jamal SA, West SL, Nickolas TL (2014) The clinical utility of FRAX to discriminate fracture status in men and women with chronic kidney disease. Osteoporos Int 25(1):71–76
Malluche HH, Mawad HW, Monier-Faugere MC (2011) Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 26(6):1368–1376
Nickolas TL, Stein EM, Dworakowski E et al (2013) Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res 28(8):1811–1820
Mares J, Ohlidalova K, Opatrna S, Ferda J (2009) Determinants of prevalent vertebral fractures and progressive bone loss in long-term hemodialysis patients. J Bone Miner Metab 27(2):217–223
Jamal SA, Swan VJ, Brown JP et al (2010) Kidney function and rate of bone loss at the hip and spine: the Canadian Multicentre Osteoporosis Study. Am J Kidney Dis 55(2):291–299
Malluche HH, Davenport DL, Cantor T, Monier-Faugere MC (2014) Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin J Am Soc Nephrol 9(7):1254–1262
Cejka D, Patsch JM, Weber M et al (2011) Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin J Am Soc Nephrol 6(9):2264–2271
Nickolas TL, Jamal SA (2015) Bone kidney interactions. Rev Endocr Metab Disord 16(2):157–163
Hasegawa K, Hasegawa Y, Nagano A (2004) Estimation of bone mineral density and architectural parameters of the distal radius in hemodialysis patients using peripheral quantitative computed tomography. J Biomech 37(5):751–756
Bacchetta J, Boutroy S, Vilayphiou N et al (2010) Assessment of bone microarchitecture in chronic kidney disease: a comparison of 2D bone texture analysis and high-resolution peripheral quantitative computed tomography at the radius and tibia. Calcif Tissue Int 87(5):385–391
Heaf J (2001) Causes and consequences of adynamic bone disease. Nephron 88(2):97–106
Behets GJ, Spasovski G, Sterling LR et al (2015) Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 87(4):846–856
Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM (2006) PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 47(1):149–156
Lertdumrongluk P, Lau WL, Park J, Rhee CM, Kovesdy CP, Kalantar-Zadeh K (2013) Impact of age on survival predictability of bone turnover markers in hemodialysis patients. Nephrol Dial Transplant 28(10):2535–2545
Coco M, Rush H (2000) Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 36(6):1115–1121
Fuller DS, Pisoni RL, Bieber BA, Gillespie BW, Robinson BM (2013) The DOPPS Practice Monitor for US dialysis care: trends through December 2011. Am J Kidney Dis 61(2):342–346
Fishbane S, Hazzan AD, Jhaveri KD, Ma L, Lacson E Jr (2016) Bone Parameters and Risk of Hip and Femur Fractures in Patients on Hemodialysis. Clin J Am Soc Nephrol 11(6):1063–1072
Russo CR, Taccetti G, Caneva P, Mannarino A, Maranghi P, Ricca M (1998) Volumetric bone density and geometry assessed by peripheral quantitative computed tomography in uremic patients on maintenance hemodialysis. Osteoporos Int 8(5):443–448
Lu KC, Ma WY, Yu JC, Wu CC, Chu P (2012) Bone turnover markers predict changes in bone mineral density after parathyroidectomy in patients with renal hyperparathyroidism. Clin Endocrinol (Oxf) 76(5):634–642
Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B (2007) Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol 18(8):2401–2407
Yajima A, Tanaka K, Tominaga Y et al (2001) Early changes of bone histology and circulating markers of bone turnover after parathyroidectomy in hemodialysis patients with severe hyperparathyroidism. Clin Nephrol 56(1):27–34
Moe SM, Abdalla S, Chertow GM et al (2015) Effects of Cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE trial. J Am Soc Nephrol 26(6):1466–1475
Urena-Torres P, Metzger M, Haymann JP et al (2011) Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis 58(4):544–553
Singer RF (2013) Vitamin D in dialysis: defining deficiency and rationale for supplementation. Semin Dial 26(1):40–46
Coen G, Mantella D, Manni M et al (2005) 25-hydroxyvitamin D levels and bone histomorphometry in hemodialysis renal osteodystrophy. Kidney Int 68(4):1840–1848
Napoli N, Strollo R, Sprini D, Maddaloni E, Rini GB, Carmina E (2014) Serum 25-OH vitamin D in relation to bone mineral density and bone turnover. Int J Endocrinol 2014:487463
Aggarwal HK, Jain D, Yadav S, Kaverappa V (2013) Bone mineral density in patients with predialysis chronic kidney disease. Ren Fail 35(8):1105–1111
Ambrus C, Almasi C, Berta K et al (2011) Vitamin D insufficiency and bone fractures in patients on maintenance hemodialysis. Int Urol Nephrol 43(2):475–482
Mucsi I, Almasi C, Deak G et al (2005) Serum 25(OH)-vitamin D levels and bone metabolism in patients on maintenance hemodialysis. Clin Nephrol 64(4):288–294
Tomida K, Hamano T, Mikami S et al (2009) Serum 25-hydroxyvitamin D as an independent determinant of 1–84 PTH and bone mineral density in non-diabetic predialysis CKD patients. Bone 44(4):678–683
Bischoff-Ferrari HA, Dawson-Hughes B, Whiting SJ (2011) Vitamin D supplementation and fracture risk. Arch Intern Med 171(3):265 (author reply—6)
Reid IR, Gallagher DJ, Bosworth J (1986) Prophylaxis against vitamin D deficiency in the elderly by regular sunlight exposure. Age Ageing 15(1):35–40
Nigwekar SU, Bhan I, Thadhani R (2012) Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis 60(1):139–156
Ueda M, Inaba M, Okuno S et al (2005) Serum BAP as the clinically useful marker for predicting BMD reduction in diabetic hemodialysis patients with low PTH. Life Sci 77(10):1130–1139
Ueda M, Inaba M, Okuno S et al (2002) Clinical usefulness of the serum N-terminal propeptide of type I collagen as a marker of bone formation in hemodialysis patients. Am J Kidney Dis 40(4):802–809
Nickolas TL, Cremers S, Zhang A et al (2011) Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol 22(8):1560–1572
Hamano T, Tomida K, Mikami S et al (2009) Usefulness of bone resorption markers in hemodialysis patients. Bone 45(Suppl 1):S19–S25
Maeno Y, Inaba M, Okuno S, Yamakawa T, Ishimura E, Nishizawa Y (2005) Serum concentrations of cross-linked N-telopeptides of type I collagen: new marker for bone resorption in hemodialysis patients. Clin Chem 51(12):2312–2317
Okuno S, Inaba M, Kitatani K, Ishimura E, Yamakawa T, Nishizawa Y (2005) Serum levels of C-terminal telopeptide of type I collagen: a useful new marker of cortical bone loss in hemodialysis patients. Osteoporos Int 16(5):501–509
Prie D, Beck L, Urena P, Friedlander G (2005) Recent findings in phosphate homeostasis. Curr Opin Nephrol Hypertens 14(4):318–324
Urena Torres P, Friedlander G, de Vernejoul MC, Silve C, Prie D (2008) Bone mass does not correlate with the serum fibroblast growth factor 23 in hemodialysis patients. Kidney Int 73(1):102–107
Wesseling-Perry K, Pereira RC, Tseng CH et al (2012) Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol 7(1):146–152
Murali SK, Roschger P, Zeitz U, Klaushofer K, Andrukhova O, Erben RG (2016) FGF23 Regulates Bone Mineralization in a 1,25(OH)2 D3 and Klotho-Independent Manner. J Bone Miner Res 31(1):129–142
Carrillo-Lopez N, Panizo S, Alonso-Montes C et al (2016) Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int 90(1):77–89
Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69(11):1945–1953
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Torres, P.A.U., Cohen-Solal, M. Evaluation of fracture risk in chronic kidney disease. J Nephrol 30, 653–661 (2017). https://doi.org/10.1007/s40620-017-0398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-017-0398-6