Abstract
Bone microarchitecture can be studied noninvasively using high-resolution peripheral quantitative computed tomography (HR-pQCT). However, this technique is not widely available, so more simple techniques may be useful. BMA is a new 2D high-resolution digital X-ray device, allowing for bone texture analysis with a fractal parameter (Hmean). The aims of this study were (1) to evaluate the reproducibility of BMA at two novel sites (radius and tibia) in addition to the conventional site (calcaneus), (2) to compare the results obtained with BMA at all of those sites, and (3) to study the relationship between Hmean and trabecular microarchitecture measured with an in vivo 3D device (HR-pQCT) at the distal tibia and radius. BMA measurements were performed at three sites (calcaneus, distal tibia, and radius) in 14 healthy volunteers to measure the short-term reproducibility and in a group of 77 patients with chronic kidney disease to compare BMA results to HR-pQCT results. The coefficient of variation of Hmean was 1.2, 2.1, and 4.7% at the calcaneus, radius, and tibia, respectively. We found significant associations between trabecular volumetric bone mineral density and microarchitectural variables measured by HR-pQCT and Hmean at the three sites (e.g., Pearson correlation between radial trabecular number and radial Hmean r = 0.472, P < 0.001). This study demonstrated a significant but moderate relationship between 2D bone texture and 3D trabecular microarchitecture. BMA is a new reproducible technique with few technical constraints. Thus, it may represent an interesting tool for evaluating bone structure, in association with biological parameters and DXA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Areal measurement of bone mineral density (aBMD) by dual-energy X-ray absorptiometry (DXA) is the gold standard method to evaluate fracture risk. Nevertheless, up to 50% of fractures occur among individuals who would not be classified as osteoporotic based on DXA measurement [1, 2]. Thus, in 2000, the U.S. National Institute of Health defined new “quality” criteria for the diagnosis of osteoporosis in addition to a decreased bone mass. Bone strength is influenced not only by the amount of bone but also by its spatial distribution, cortical and trabecular microarchitecture, bone turnover, and material properties such as matrix mineralization, collagen polymorphisms, and microdamage. These quality criteria cannot be evaluated by DXA. Thus, new bone-imaging techniques have been developed to assess bone microarchitecture parameters which may improve fracture risk prediction, including 2D bone-analysis (e.g., an X-ray-based fractal analysis or 2D analysis projection based on DXA images) and 3D techniques (e.g., quantitative computed tomography or magnetic resonance imaging) [3–5].
Peripheral quantitative computed tomography (pQCT), an X-ray-based technique, has been developed to measure separately trabecular and cortical density at peripheral sites. It allows the assessment of parameters of bone strength, such as polar moment of inertia, section modulus, and cortical thickness but not trabecular microarchitecture at the shaft of the radius and tibia [6]. This technique is not widely available in clinical practice. Recently, high-resolution pQCT (HR-pQCT) has been developed, with an isotropic voxel size of 82 μm, thus leading to a better resolution in comparison to previous pQCT devices [7]. HR-pQCT allows the measurement of both volumetric bone mineral density (vBMD) and microarchitectural parameters in total, cortical, and trabecular bone at the ultradistal radius and tibia, with low radiation exposure (effective dose of 3 μSv per measurement) with an acquisition time of 3 min. It has excellent precision for both density (0.7–1.5%) and structural parameters (1.5–4.4%), with similar results at the two sites [8]. However, despite these qualities, HR-pQCT measurements are limited to peripheral skeletal sites, and currently fewer than 20 devices are available worldwide.
Fractal analysis on conventional radiographs has been developed to evaluate bone biomechanical properties [9]. This texture analysis can characterize trabecular bone microarchitecture via 2D projection images (gray-level projection) and the calculation of a fractal parameter (Hmean). In cross-sectional analyses, bone texture analysis was independent of BMD for determining the risk of fracture [10]. A 2D high-resolution digital X-ray device (BMA) was recently proposed as an improvement of this general concept [11]. BMA provides better precision of bone texture parameters than previously found on digitized films, with a low radiation exposure (effective dose of 3 μSv/measurement) [11]. Previous studies which have evaluated this technique at the calcaneus found a significant decrease of Hmean with age [11]. It would therefore be interesting to evaluate texture analysis at other peripheral sites, such as the radius and tibia, to try to improve fracture risk prediction since other bone-imaging techniques (e.g., HR-pQCT, magnetic resonance imaging) have also been described at these sites [8, 12].
Thus, the aims of this study were (1) to evaluate the reproducibility of BMA at two novel sites (distal radius and tibia) in addition to the conventional site (calcaneus), (2) to compare the results obtained with BMA at all of those sites, and (3) to study the relationship between the texture analysis (fractal parameter, Hmean) obtained with BMA and trabecular microarchitecture measured with a 3D device (HR-pQCT) in a cross-sectional analysis of patients with chronic kidney disease (CKD) who were known to have sizeable architectural impairment.
Patients and Methods
Subjects
The study was approved by a local independent ethical committee (Comité de protection des personnes Lyon Sud-Est II), and all patients gave written informed consent before participation. To assess the short-term reproducibility of BMA analysis, 14 healthy volunteers (aged 20–37 years) underwent three separate scans of the distal radius, tibia, and calcaneus within a 1-month period. The first and second measurements were performed the same day after repositioning; the third measurement was performed 1–2 weeks after, as previously recommended by Gluer et al. [13].
To assess the correlation between 2D and 3D devices, we performed BMA and HR-pQCT measurements in a group of CKD patients as CKD is associated with important bone-quality impairment [14].
HR-pQCT
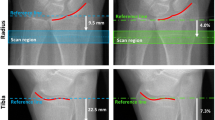
vBMD and trabecular microarchitecture were assessed at the ultradistal radius and tibia using an HR-pQCT system (XtremeCT; Scanco Medical, Brüttisellen, Switzerland). This system enables the simultaneous acquisition of a stack of 110 parallel slices with a nominal resolution (voxel size) of 82 μm [8], leading to a dimensional representation of approximately 9 mm in the axial direction. The following settings were used: effective energy of 60 kVp, X-ray tube current of 95 mA, and matrix size of 1,536 * 1,536 pixels. Positioning and detailed technical settings were previously described [8]. Briefly, the measurement region was manually defined on an anteroposterior scout view, with a reference line at the joint surface of the radius and tibia, as shown in Fig. 1. Then, the first slice was 9.5 and 22.5 mm proximal to the reference line for the distal radius and tibia, respectively. The outcome variables used in our analyses included vBMD (mg HA/cm3) for entire (Dtot) and trabecular (Dtrab) regions and trabecular thickness (Tb.Th, μm), number (Tb.N, mm−1), separation (Tb.Sp, μm), and intraindividual distribution of separation (Tb.SpSD, μm).
Positioning of the ROI at the tibia and the radius with HR-pQCT. Scout views demonstrating the reference line position (solid line) and the measurement site (between dotted lines). The first CT slice was 9.5 and 22.5 mm proximal to the reference line for the distal radius and distal tibia, respectively
BMA Analysis
As illustrated in Fig. 2, images were obtained at three sites: calcaneus, distal tibia, and distal radius. This system (D3A Medical Systems, Orléans, France) enables the acquisition of an image with a nominal resolution (pixel size) of 50 μm [11]. A first acquisition was performed in all subjects with the same technical settings (calcaneus 55 kV, 10 mAs; distal tibia 55 kV, 20 mAs; and distal radius 50 kV, 12 mAs). Then, for each site the region of interest (ROI) was positioned and the mean gray level was calculated within the ROI. If the mean gray level within the ROI was out of range, a second acquisition with an accordingly corrected intensity was then performed.
At the calcaneus, two anatomical landmarks were manually placed by the operator to position an ROI of 256 * 256 pixels [11]. At the distal tibia (radius), the anatomical landmarks were manually placed to obtain a tangent line to the middle of the tibia (radius) joint surface, and then a horizontal translation of 440 pixels/22 mm (180 pixels/9 mm) was applied to position the 256 * 180-pixel ROI so that we studied the same region as HR-pQCT measurement (Fig. 2).
The methods used to calculate the fractal parameter (Hmean) have been previously described in detail by Lespessailles et al. [11]. Briefly, the gray levels of the ROI were fitted to a fractal model for texture analysis, the fractional Brownian motion, which allows description of fractal shapes by a single parameter, H. For each ROI, Hmean was averaged based on the evaluation of H in eight different directions following a 22.5° step. The higher the Hmean, the smaller the roughness of the texture within the ROI. In texture analysis, roughness is a parameter that quantifies both the amplitudes and frequencies of the irregularities of a surface. In BMA application, the texture basis is given by the soft tissues and the irregularities are represented by trabecular elements. In clinical practice, the higher the Hmean, the better the trabecular status.
For both BMA and HR-pQCT, examinations were performed at the nondominant radius and at the right tibia, except when there was a fracture history at these sites.
Quality Control
Calibration tests were carried out every day for the two techniques using an external phantom to detect any potential drift of the instrumentation. We did not observe any drift during the period of the protocol. Both examinations (BMA and HR-pQCT) were analyzed by the same observer (J. B.).
Statistical Analysis
For each subject in the reproducibility study, a coefficient of variation (CV) was calculated as the standard deviation of the three repeated measurements divided by the subject mean. Furthermore, the short-term precision errors were then calculated as root-mean-square (RMS) averages of the precision errors for each of the subjects. Relationships between the fractal parameter, vBMDs, and microarchitecture were estimated using Pearson or Spearman correlations, depending on the distribution of variables (i.e., Pearson for parametric distribution, Spearman for nonparametric distribution). Comparison of Hmean measured at the radius, tibia, and calcaneus was performed by repeated-measures ANOVA, followed by Tukey’s test for pairwise comparisons. The statistical level of significance was 0.05. All statistical analyses were performed with SPSS software (version 15.0; SPSS, Inc., Chicago, IL).
Results
Characteristics of CKD Patients
We performed BMA and HR-pQCT measurements in a group of 77 CKD patients (50 men and 27 women, mean age 67 ± 15 years, mean glomerular filtration rate [GFR] 33 ± 12 mL/min per 1.73 m2, body mass index 26.0 ± 4.6 kg/m2). Among these individuals, 13 had a history of fractures and 18 suffered from type 2 diabetes mellitus. Due to patient motion during the examinations, BMA measurements at the calcaneus and tibia could not be analyzed in four patients and in one patient at the radius; for the same reason, HR-pQCT measurements could not be analyzed in four patients at the radius and in two at the tibia. Thus, this results in 76, 73, and 73 BMA measurements (at the radius, tibia, and calcaneus, respectively) and 73 and 75 HR-pQCT scans (at the radius and tibia, respectively).
Biochemical assessments of phosphate/calcium parameters were as follows: serum calcium 2.31 ± 0.13 mmol/L, phosphate 1.18 ± 0.21 mmol/L, parathyroid hormone 99 ± 97 pg/L, 25 OH vitamin D 22 ± 14 ng/L, and total alkaline phosphatase 77 ± 26 IU/L.
Reproducibility of the Fractal Parameter with BMA
As summarized in Table 1, reproducibility was good at all sites, mainly at the calcaneus and radius (CV = 1.2 and 2.1%, respectively), with slightly less satisfying values at the tibia (4.7%). The CV and its 95% confidence interval (CI) for each site are also summarized in Table 1.
Comparison of the Fractal Parameter Obtained at Different Sites
Mean values of Hmean among our 77 CKD patients were 0.609 ± 0.038 at the radius, 0.562 ± 0.044 at the tibia, and 0.621 ± 0.024 at the calcaneus, as summarized in Fig. 3. When analyzing Hmean at the three sites, there was a significant association between the tibia and calcaneus (r = 0.437, P < 0.001, n = 69) and between the tibia and radius (r = 0.564, P < 0.001, n = 71) but not between the radius and calcaneus (n = 71).
Comparison Between BMA Analysis and HR-pQCT
As summarized in Table 2 and in Fig. 4, we found significant correlations between all trabecular parameters (density and microarchitecture) and the fractal value at the three sites except for tibial trabecular thickness, which did not correlate with Hmean at any site. The relationships observed between the two techniques were stronger at the same site, especially at the tibia (r > 0.5). The trend was similar when comparing HR-pQCT and BMA measurement at the radius. When comparing Hmean at the calcaneus with trabecular microarchitecture measured with HR-pQCT at the tibia and the radius, we also observed a relationship, though of lower significance.
Moreover, there were significant relationships between trabecular microarchitecture assessed with HR-pQCT at the two sites, ranging from 0.585 (for trabecular thickness) to 0.871(for trabecular density), with all P values <0.001 (n = 71).
BMA Results in CKD Patients According to the History of Fractures
When comparing the 13 patients with history of fractures to the other CKD patients, there were no significant differences regarding age (69 ± 9 vs. 67 ± 16 years, P = nonsignificant [NS]), BMI (24.7 ± 4.4 vs. 26.4 ± 4.6 kg/m2, P = NS), GFR (31 ± 11 vs. 32 ± 12 mL/min per 1.73 m2, P = NS), proportion of diabetes mellitus (23 vs. 24%, P = NS), and proportion of men (46 vs. 68%, P = NS). However, Hmean at the calcaneus (0.611 ± 0.014 vs. 0.623 ± 0.025, P = 0.024) was significantly lower in CKD patients with a history of fractures, with a similar trend at the radius (0.592 ± 0.038 vs. 0.613 ± 0.038, P = 0.068). By contrast, results were not significant at the tibia. Concerning HR-pQCT, we found significant decreased total BMD (257 ± 52 vs. 299 ± 71 mgHA/cm3, P = 0.05), trabecular BMD (137 ± 53 vs. 174 ± 44 mgHA/cm3, P = 0.015), and trabecular thickness (67 ± 10 vs. 79 ± 13 μm, P = 0.004) in the fractured CKD patients at the tibia; results were similar at the radius.
Discussion
In this study we applied the measurement of the fractal parameter Hmean with BMA at two novel sites (tibia and radius) and at the conventional site (calcaneus) and assessed their relationship with trabecular microarchitecture measured at the distal radius and tibia by HR-pQCT. Thus, the main findings of the present study are (1) the good reproducibility of Hmean assessment at the three sites; (2) a significant association between the results of bone texture analysis at the tibia and the calcaneus, on the one hand, and at the tibia and the radius, on the other hand; and (3) the association between trabecular microarchitecture assessed with HR-pQCT and Hmean at the three sites.
The reproducibility of Hmean at the calcaneus was similar to that reported by Lespessailles et al. [11], who found a CV of 1.2%. At the radius, the CV of BMA measurement was similar to that provided by DXA for the measurement of aBMD (1–2%) or HR-pQCT (0.7–1.5% for density and 0.9–4.4% for structural parameters). The results were slightly lower at the tibia (4.7%), comparable to the upper values found with HR-pQCT, likely due to a more difficult positioning of patients. Thus, the most reproducible site for measuring Hmean appears to be the calcaneus. Since the results of reproducibility are satisfying at the radius when compared to other imaging techniques, the use of both the radius and the calcaneus to assess Hmean could represent a valuable option to improve the evaluation of bone status with BMA.
As illustrated in Fig. 3, the Hmean values obtained at the three sites were significantly different. This apparent discrepancy could be explained by the differences in BMA and structure at these three sites, as previously described, e.g., with magnetic resonance imaging [15].
We found a moderate correlation between the BMA texture parameter and HR-pQCT microarchitectural variables, similar to Chappard et al. [16], who demonstrated a good relationship between 2D texture analysis on radiographs and trabecular histomorphometry. All of these results are consistent with the fact that Hmean was developed to be an index of the 3D trabecular microarchitecture, which is not addressed by DXA. We can therefore hypothesize that Hmean at all sites could be independent of total aBMD but correlated to trabecular BMD and microarchitecture, as illustrated in Table 2. Thus, since it has been well demonstrated that trabecular structure or texture parameters of the radius or the calcaneus could help to identify spine and hip fractures [17–19], BMA measurement could represent a new tool to improve the prediction of fracture risk; however further longitudinal studies are required to test this hypothesis.
A first limitation of this study is its focus on CKD patients, who usually experience bone damage as soon as their GFR falls below 60 mL/min per 1.73 m2 [14]. Indeed, the impact of CKD on bone and mineral status may be immediate (serum phosphocalcic dysequilibrium) or delayed (fractures, vascular calcifications); in hemodialysis patients there is a fourfold increased risk of hip fracture [20], whereas there is a twofold increased risk of hip fracture in predialysis patients [21]. Even if several studies have reported on DXA in CKD patients with contradictory results [14, 22–24], recent international guidelines have emphasized the fact that BMD assessment by DXA analysis should no longer be performed in CKD patients since it does not predict fracture risk in this population and does not predict the type of renal osteodystrophy [25].
Several studies have been performed in CKD patients with a pQCT device that measures separately trabecular and cortical BMD at peripheral sites but does not assess trabecular microarchitecture. Studies on dialysis patients usually have reported decreased cortical vBMD and thickness compared to controls, without significant changes in trabecular vBMD [26–29]. All of these studies confirmed a cortical impairment, probably due to secondary hyperparathyroidism, often seen during end-stage renal disease. In predialysis patients, Obatake et al. [30] reported a decrease in total, cortical, and trabecular BMD in 53 CKD patients after a 1-year follow-up. In 2005, Tsuchida et al. [31] reported a negative correlation between trabecular BMD and biomarkers of bone formation in 85 predialysis patients. To our knowledge, no studies have evaluated bone status with bone texture analysis in CKD patients and only two studies have focused on this topic with HR-pQCT. Nickolas et al. [32] recently reported a more important trabecular loss and decreased BMD in 32 predialysis CKD patients with a history of fractures in comparison to 59 CKD patients without fractures, similar to our present results that are, however, from cross-sectional data. The second study evaluating the interest of HR-pQCT during CKD was performed in the present cohort of CKD patients, studied in comparison to the epidemiological cohorts STRAMBO and OFELY: we demonstrated that these predialysis CKD patients experienced a moderate but significant trabecular impairment, positioning their results between those of normal and osteopenic controls [33].
Moreover, when comparing our results obtained with BMA to results obtained in a case–control, multicenter study evaluating bone texture analysis in osteoporosis in 219 controls (mean age 69 ± 10 years) and 159 osteoporotic postmenopausal women [34], CKD patients had Hmean values between those of healthy controls and osteoporotic patients with fractures (0.609 ± 0.038 vs. 0.612 ± 0.031 vs. 0.600 ± 0.035, respectively).
The present study consisted of a preliminary approach to BMA measurement, to determine its reproducibility at three sites and to study its relationship with 3D bone evaluation by HR-pQCT in a specific population of patients. Even if the data on our CKD patients seem close to both HR-pQCT and BMA values of controls having similar age and gender, these results need to be replicated in a cohort of individuals with normal renal function. Unfortunately, BMA reference values were not available at our center to locally compare the results observed in our CKD patients to those of healthy controls.
Moreover, this study is also limited by its cross-sectional design, which prevented us from examining the predictive value for incident fracture of the texture parameter. The association we found between architectural variables assessed using HR-pQCT and Hmean, on the one hand, and the observation of a significant decrease of Hmean at the calcaneus and probably an almost significant decrease at the radius in CKD patients with a history of fractures, on the other hand, can justify prospective studies testing the ability of texture analysis to predict fracture better than aBMD alone as it is associated with bone microarchitecture.
In conclusion, this study demonstrated the feasibility of Hmean measurement with BMA at two novel sites. However, the precision error was the lowest at the calcaneus. We also found a significant association between Hmean measurement and 3D trabecular microarchitecture measured by HR-pQCT at the same site. Thus, BMA measurement might be a promising simple tool for evaluating trabecular bone, but its ability to predict the risk of fracture should be evaluated in further longitudinal prospective studies.
References
Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA et al (2005) Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 90:2787–2793
Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD (2005) Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res 20:1813–1819
Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD (2007) Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433
Majumdar S (2008) Magnetic resonance imaging for osteoporosis. Skeletal Radiol 37:95–97
Prouteau S, Ducher G, Nanyan P, Lemineur G, Benhamou L, Courteix D (2004) Fractal analysis of bone texture: a screening tool for stress fracture risk? Eur J Clin Invest 34:137–142
Bachrach LK (2006) Measuring bone mass in children: can we really do it? Horm Res 65(Suppl 2):11–16
Nickolas TL, Leonard MB, Shane E (2008) Chronic kidney disease and bone fracture: a growing concern. Kidney Int 74:721–731
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515
Link TM, Majumdar S, Konermann W, Meier N, Lin JC, Newitt D et al (1997) Texture analysis of direct magnification radiographs of vertebral specimens: correlation with bone mineral density and biomechanical properties. Acad Radiol 4:167–176
Benhamou CL, Poupon S, Lespessailles E, Loiseau S, Jennane R, Siroux V et al (2001) Fractal analysis of radiographic trabecular bone texture and bone mineral density: two complementary parameters related to osteoporotic fractures. J Bone Miner Res 16:697–704
Lespessailles E, Gadois C, Lemineur G, Do-Huu JP, Benhamou L (2007) Bone texture analysis on direct digital radiographic images: precision study and relationship with bone mineral density at the os calcis. Calcif Tissue Int 80:97–102
Kazakia GJ, Hyun B, Burghardt AJ, Krug R, Newitt DC, de Papp AE et al (2008) In vivo determination of bone structure in postmenopausal women: a comparison of HR-pQCT and high-field MR imaging. J Bone Miner Res 23:463–474
Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK (1995) Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 5:262–270
Bacchetta J, Boutroy S, Juillard L, Vilayphiou N, Guebre-Egziabher F, Pelletier S et al (2009) Bone imaging and chronic kidney disease: will high-resolution peripheral tomography improve bone evaluation and therapeutic management? J Ren Nutr 19:44–49
Link TM, Bauer J, Kollstedt A, Stumpf I, Hudelmaier M, Settles M et al (2004) Trabecular bone structure of the distal radius, the calcaneus, and the spine: which site predicts fracture status of the spine best? Invest Radiol 39:487–497
Chappard D, Guggenbuhl P, Legrand E, Basle MF, Audran M (2005) Texture analysis of X-ray radiographs is correlated with bone histomorphometry. J Bone Miner Metab 23:24–29
Majumdar S, Genant HK, Grampp S, Newitt DC, Truong VH, Lin JC et al (1997) Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res 12:111–118
Link TM, Majumdar S, Augat P, Lin JC, Newitt D, Lu Y et al (1998) In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Miner Res 13:1175–1182
Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD (2009) Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res 24:737–743
Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR et al (2000) Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58:396–399
Nickolas TL, McMahon DJ, Shane E (2006) Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17:3223–3232
Urena P, Bernard-Poenaru O, Ostertag A, Baudoin C, Cohen-Solal M, Cantor T et al (2003) Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol Dial Transplant 18:2325–2331
Jamal SA, Hayden JA, Beyene J (2007) Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis. Am J Kidney Dis 49:674–681
Yamaguchi T, Kanno E, Tsubota J, Shiomi T, Nakai M, Hattori S (1996) Retrospective study on the usefulness of radius and lumbar bone density in the separation of hemodialysis patients with fractures from those without fractures. Bone 19:549–555
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl S1–S130
Russo CR, Taccetti G, Caneva P, Mannarino A, Maranghi P, Ricca M (1998) Volumetric bone density and geometry assessed by peripheral quantitative computed tomography in uremic patients on maintenance hemodialysis. Osteoporos Int 8:443–448
Hasegawa K, Hasegawa Y, Nagano A (2004) Estimation of bone mineral density and architectural parameters of the distal radius in hemodialysis patients using peripheral quantitative computed tomography. J Biomech 37:751–756
Negri AL, Barone R, Lombas C, Bogado CE, Zanchetta JR (2006) Evaluation of cortical bone by peripheral quantitative computed tomography in continuous ambulatory peritoneal dialysis patients. Hemodial Int 10:351–355
Jamal SA, Gilbert J, Gordon C, Bauer DC (2006) Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res 21:543–548
Obatake N, Ishimura E, Tsuchida T, Hirowatari K, Naka H, Imanishi Y et al (2007) Annual change in bone mineral density in predialysis patients with chronic renal failure: significance of a decrease in serum 1,25-dihydroxyvitamin D. J Bone Miner Metab 25:74–79
Tsuchida T, Ishimura E, Miki T, Matsumoto N, Naka H, Jono S et al (2005) The clinical significance of serum osteocalcin and N-terminal propeptide of type I collagen in predialysis patients with chronic renal failure. Osteoporos Int 16:172–179
Nickolas TL, Stein E, Cohen A, Thomas V, Staron RB, McMahon DJ et al (2010) Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol 21:1371–1380
Bacchetta J, Boutroy S, Vilayphiou N, Juillard L, Guebre-Egziabher F, Rognant N et al (2010) Early impairment of trabecular microarchitecture assessed with HR-pQCT in patients with stage II–IV chronic kidney disease. J Bone Miner Res 25:849–857
Lespessailles E, Gadois C, Kousignian I, Neveu JP, Fardellone P, Kolta S et al (2008) Clinical interest of bone texture analysis in osteoporosis: a case control multicenter study. Osteoporos Int 19:1019–1028
Acknowledgments
We thank Pr. Maurice Laville and Drs. Laurent Juillard, Fitsum Guebre-Egziabher, Nicolas Rognant, Catherine Chaubo, and Alexandre Klein (Department of Nephrology, Hôpital Edouard Herriot, Réseau TIRCEL, Lyon, France) for help in patient recruitment. We also thank Dr. Clotilde Gadois (D3A Medical Systems) for her scientific support. This work was partly supported by a 2007 Société Française de Pédiatrie/Archives de Pédiatrie educational grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bacchetta, J., Boutroy, S., Vilayphiou, N. et al. Assessment of Bone Microarchitecture in Chronic Kidney Disease: A Comparison of 2D Bone Texture Analysis and High-Resolution Peripheral Quantitative Computed Tomography at the Radius and Tibia. Calcif Tissue Int 87, 385–391 (2010). https://doi.org/10.1007/s00223-010-9402-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9402-z