Abstract
Background
Klinefelter syndrome (KS) frequently causes skeletal fragility characterized by profound alterations in bone microstructure with increased risk of fractures. Increased body fat mass associated with decreased body lean mass are frequent features of KS with possible detrimental effects on skeletal health. In this cross-sectional study, we evaluated the associations between body composition parameters, vertebral fractures (VFs) and trabecular bone score (TBS) in adult subjects with KS.
Methods
Seventy-one adult males (median age 41 years, range 18–64) with 47, XXY KS were consecutively enrolled by two Endocrinology and Andrology Units (IRCCS Humanitas Research Hospital in Milan and ASST Spedali Civili in Brescia). Dual-energy X-ray absorptiometry (DXA) was performed to assess bone mineral density (BMD) at lumbar spine, femoral neck and total hip, TBS and body composition. Prevalence of VFs was assessed by quantitative morphometry on lateral spine X-rays.
Results
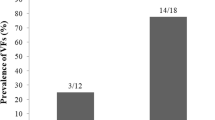
VFs were detected in 14 patients (19.7%), without significant association with low BMD (p = 0.912). In univariate logistic regression analysis, VFs were significantly associated with truncal/leg fat ratio (OR 2.32 per tertile; 95% CI 1.05–5.15; p = 0.038), whereas impaired TBS (detected in 23.4% of subjects) was associated with older age at study entry (p = 0.001) and at diagnosis of disease (p = 0.015), body mass index (BMI; p = 0.001), waist circumference (p = 0.007), fat mass index (FMI; p < 0.001), FMI/lean mass index (LMI) ratio (p = 0.001). Prevalence of VFs was not significantly different between subjects with impaired TBS as compared to those with normal TBS (26.7 vs. 18.4%; p = 0.485). Skeletal end-points were not significantly associated with duration of testosterone replacement therapy and serum testosterone and 25hydroxyvitamin D values.
Conclusion
Body composition might influence bone quality and risk of VFs in subjects with KS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Klinefelter syndrome (KS) is the most common sexual chromosome aneuploidy, with an estimated prevalence around 1–2/1000 male newborns, characterized by the presence of one or more supernumerary X-chromosomes [1]. Notwithstanding infertility and hypergonadotropic hypogonadism are the hallmarks of KS, subjects with KS frequently have wide and heterogeneous spectrum of comorbidities [2, 3], including altered body composition, obesity and impaired bone metabolism with higher incidence of fragility fracture as compared to the general population [1, 4, 5]. Interestingly, our group recently reported in subjects with KS a high prevalence of symptomatic vertebral fractures (VFs), which developed regardless of serum testosterone values [6]. Moreover, as previously observed for other forms of secondary osteoporosis, in KS fragility fractures might occur even in the context of normal BMD [6], confirming the low reliability of dual X-ray absorptiometry (DXA) in assessing bone health and predicting fractures in this clinical setting [7, 8]. Indeed, recent studies showed that subjects with KS can have altered bone microarchitecture, as assessed by high resolution computed tomography (HR-pQCT) [9, 10] and DXA-measured trabecular bone score (TBS) [11].
The relationship between body composition and skeletal health is an emerging area of research and clinical interest [12, 13]. As a matter of fact, recent studies provided evidence that increase in adiposity and decrease in lean body mass (LBM) may induce detrimental effects on bone microarchitecture in hypogonadal subjects with KS [10]. However, whether alterations in body composition may influence the risk of fracture in KS, such as demonstrated in other clinical settings [12], is still unknown.
In this cross-sectional study, we aimed to assess the relationship between body composition parameters, VFs and TBS in a relatively large population of individuals with KS.
Materials and methods
This is a multicenter, cross-sectional-observational study carried out in two referral centers of Northern Italy. Seventy-one patients (median age 41 years, range 18–64) with karyotype-confirmed diagnosis of 47,XXY KS were consecutively enrolled between 1st March 2021 and 31st September 2021 at two Endocrinology Units (IRCCS Humanitas Clinical and Research Hospital in Milan and ASST Spedali Civili Hospital in Brescia). Exclusion criteria were age < 18 years; (2) use of drugs causing osteoporosis; (3) previous history of traumatic injury or surgical intervention to spine; (4) treatment with bone-active medications, except calcium and vitamin D. The enrolled subjects had been already involved in a previous study [6] evaluating different endpoints from the present study.
All included subjects underwent physical examination for anthropometric measures: body height was recorded to the nearest 0.5 cm and body weight to the nearest 0.1 kg; body mass index (BMI) was calculated as the ratio “weight (kg)/height (m2); waist circumference (WC) was measured as the midpoint between the lower border of the rib cage and the iliac crest using a flexible inch tape [14].
The primary end-point was the evaluation of body composition in KS subjects with VFs. As secondary end-points we explored the associations between body composition, TBS and BMD.
The study was approved by the Ethics Committees and all subjects gave informed consent to use their clinical data for research purposes.
Assessment of body composition
Body composition was evaluated in all enrolled subjects by total body DXA measuring fat body mass (FBM) (kg) and LBM (kg) in specific anatomical regions (limbs, trunk,). The fat amount was expressed as fat mass index (FMI), calculated as FBM/height2 (kg/m2) and trunk/leg fat mass ratio (TLR). Lean mass assessment was performed through lean mass index (LMI), calculated as lean mass/height2 (kg/m2). To have an integrated measure of FBM and LBM, we calculated the FMI/LMI ratio.
VF assessment
VFs were detected on lateral spine X-rays using a qualitative evaluation of vertebral shape and quantitative morphometric assessment. According to the quantitative morphometry method, the fractures were defined as mild, moderate, and severe based on height ratio decreases of 20–25%, 25–40%, and more than 40%, respectively [15]. For each vertebra, a grade of 0, 1, 2, or 3 was assigned for no fracture or mild, moderate, or severe fracture, respectively, and the spine deformity index (SDI) was calculated by summing the fracture grades of the 13 vertebrae from T4 to L4 [16]. The assessment of VFs was performed by two experienced clinicians in each center.
Measurement of BMD and TBS
BMD was measured in all enrolled subjects by DXA (Hologic Discover A) at lumbar spine, total hip and femoral neck. All scans were acquired and analyzed by two trained bone densitometrists adhering to protocols recommended by the International Society for Clinical Densitometry [17]. For patients aged ≥ 50 years, BMD was evaluated using the T score, comparing the results with those obtained in a sex-matched Caucasian population at the peak of bone mass [18]. Normal BMD was defined as all sites T score > − 1.0 SD, osteopenia was defined as a T score between − 1.0 SD and − 2.5 SD, and osteoporosis was defined as a T score equal or lower than − 2.5 SD. For patients younger than 50 years, BMD was evaluated using the Z score, comparing the results with those obtained in age- and sex-matched Caucasian population [18]. A Z score equal to or lower than − 2.0 SD was used to define a BMD “below the expected range for age.” For the study purposes, patients with osteopenia, osteoporosis or BMD “below the expected range for age” were classified as to have “low BMD.”
TBS was measured in 64 subjects using lumbar spine DXA images. Based on results of a meta-analysis, subjects were categorised as with normal TBS (values ≥ 1.310), partially degraded TBS (values between 1.230 and 1.310), and degraded TBS (values ≤ 1.230) [19]. For the study purposes, patients with partially degraded and degraded TBS were classified as to have “impaired TBS.”
Biochemical analyses
All blood samples were collected in the morning (08.00–11.00 a.m.) on a fasting state. Testosterone (T) was measured using Access Testosterone assay (Beckman Coulter Inc, Fullerton, CA) [20] and following appropriate timing in relation to testosterone replacement therapy (TRT) formulation: (a) at least 2 h after application of T gel (N = 29); (b) 14 days after injection of propionate T (N = 2); (c) a week before their repeat injections of undecanoate T, according to patient scheduling (N = 32). Vitamin D status was assessed by measuring serum 25hydroxyvitamin D [25(OH)D] and vitamin D sufficiency was defined by values ≥ 30 ng/ml [21]. Measurements of serum glucose and insulin were also obtained using standardized methods; HOMA-IR index was calculated according to the formula: HOMA-IR = [glucose] (mmol/l) × [insulin] (µU/ml)/22.5 [22].
Statistical analyses
Data were presented as median and absolute range, unless otherwise stated. Since most of variables were non-normally distributed as assessed by Kolmogorov–Smirnov test, non-parametric tests were used. The comparisons between continuous variables were performed by Mann–Whitney’s test. Frequencies were compared by the Chi-squared test, with Fisher correction when appropriate. A univariate logistic regression analysis was performed and the odds ratio (OR) with 95% confidence interval (95% CI) were calculated to evaluate the determinants of impaired TBS and prevalent VFs. Body composition parameters were expressed in tertiles. P value < 0.05 was considered as significant.
Results
The study involved a total of 71 patients, with a median age at study entry of 41 years (range 18–64) and a median age at KS diagnosis of 25 years (range 1–57). Sixty-two patients were receiving TRT at study entry, and the median duration of therapy was 6.5 years (range 1–37). At study entry, 62 subjects (87.3%) were receiving vitamin D supplementation and 42 of them resulted to have vitamin D sufficiency.
Anthropometric, skeletal and body composition data of study population are summarized in Table 1. Low BMD at any skeletal site was found in 16 patients (22.5%; 8/57 subjects younger than 50 years, 8/14 older than 50 years), whereas 55 (77.5%) had normal BMD all skeletal sites. Of the 64 patients in whom TBS was measured, 15 (23.4%) showed an impaired TBS (10 with partially degraded and 5 with degraded TBS).
VFs were observed in 14 patients (19.7%) with median SDI 1 (range 1–8) and mean SDI 2.57. A total of 26 VFs were detected and according with Genant classification, 16 fractures were mild (66%), 10 were moderate (33%) whereas no severe fractures were detected. The prevalence of VFs was comparable between subjects with low BMD and those with normal BMD (18.8 vs. 20.0%; p = 0.912). Moreover, no significant difference in prevalence of VFs was observed between subjects with impaired TBS and those with normal TBS (26.7 vs. 18.4%; p = 0.485). Skeletal end-points were not significantly associated with duration of TRT, serum testosterone and 25(OH)D (Table 2).
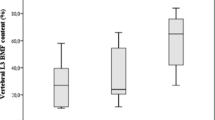
In the univariate logistic regression analysis, impaired TBS was found significantly associated with age of subjects at study entry (OR 1.14, 95% CI 1.04–1.19; p = 0.001), age of subjects at diagnosis of the disease (OR 1.07, 95% CI 1.01–1.12; p = 0.015), BMI (OR 1.28, 95% CI 1.10–1.49; p = 0.001), WC (OR 1.09, 95% CI 1.03–1.17; p = 0.007). FMI (OR 8.99 per tertile, 95% CI 2.75–29.42; p < 0.001), FMI/LMI ratio (OR 6.48 per tertile, 95% CI 2.24–18.73; p = 0.001) (Table 2). In fact, subjects with impaired TBS were older (Fig. 1a), received a later diagnosis of KS (Fig. 1b), had higher BMI (Fig. 1c), WC (Fig. 1d), FMI (Fig. 1e) and FMI/LMI ratio (Fig. 1f) as compared to subjects with normal TBS. VFs resulted to be associated only with TLR (OR 2.32 per tertile, 95% CI 1.05–5.15; p = 0.038; Table 2).
Discussion
We found that increased body fat, but more specifically the altered distribution of adipose tissue with an increase of visceral fat, has a significant negative association with bone quality as assessed by DXA-derived TBS in subjects with KS. Furthermore, significantly higher levels of abdominal fat were found in patients with VFs compared to non-fractured patients, without any significant difference in overall weight and T levels.
The role of hypogonadism in pathogenesis of skeletal fragility in KS is still a matter of uncertainty, since in several studies serum T levels resulted to be not directly associated with the entity of bone loss in this clinical setting [23,24,25,26]. Moreover, in a remarkable number of KS subjects exposed to TRT BMD are still low as compared to the general population [27]. Consistently, in our subjects with KS skeletal end-points did not correlate with duration of TRT and serum T values. One could argue that a single measurement of T values serum T is not able to reflect the real long-term compliance to TRT. However, besides low testosterone values other factors might affect skeletal heath in subjects with KS, such as increased FSH values and genetic factors [3, 8, 27,28,29,30,31,32].
Beyond bone mass loss, bone microarchitectural changes have been reported in KS men [9, 10]. Using HR-pQCT, lower trabecular density and number as well as reduced bone cortical area have been found in men with KS [9, 10]; however, pQCT is far from clinical practice application due to its high costs. In this concern, TBS has proved to be a sensitive tool to detect bone abnormalities in different conditions in which fracture susceptibility coexists with normal BMD [33], but only one study so far evaluated TBS in KS subjects [11]. Among our patients, impaired bone structure as assessed by TBS was found in 26%, which is noteworthy considering the relatively young age of the population and the absence of other known risk factors for bone disorders.
A novelty provided by this study was the analysis of association between skeletal end-points and body composition in KS. KS subjects harbour early-onset modification of body composition, with unfavorable metabolic profile, increased body fat and decreased lean mass [34,35,36]. Interestingly, recent evidence suggests a complex interaction between adipose tissue and bone, involving the role of visceral-fat derived proinflammatory cytokines and the chronic low-grade systemic inflammation, that can favour bone resorption by stimulating osteoclast activity [37]. Moreover, body composition can influence bone marrow adiposity that in turn has a role in regulation of bone remodelling and body energy metabolism [38]. Therefore, increased visceral adiposity could contribute to alterations of bone microstructure in KS [39]. In agreement with the working hypothesis, we found a significant association between impaired bone microarchitecture and body fat (BMI and FMI) as well as with parameters of central adiposity (WC, FMI and FMI/LMI). This is also consistent with what reported by Tahani et al. [11], who found a worse glycol-metabolic profile in subjects with lower TBS in a cohort of KS adults. Taken together, this evidence provides further support to the hypothesis that unfavourable body composition parameters negatively influence trabecular bone quality in KS men and highlights a possible new mechanism of skeletal damage, at least in part independent of T levels, as well as a potential therapeutic target.
VFs represent the most common and earliest complication of osteoporosis and are associated with disability, decreased quality of life, loss of independence and increased overall mortality [40, 41]. Indeed, data on fractures in KS are scanty and mostly limited to retrospective observations [42]. In a recent study, we reported radiological VFs in about 15% of young adults with KS and, in remarkable number of them, fractures resulted to be clinically relevant determining back pain [6]. In the present cohort, we found VFs in 19% of subjects, which is still lower than what observed in other forms of male hypogonadism [43,44,45], but significantly higher compared to the average male population of similar age (7.5%) and of the overall prevalence of VFs in all age groups (13.8%) [46]. Moreover, for the first time we investigated the impact of body composition parameters in the development of VFs. Noteworthy, KS subjects with vertebral fractures showed high values of TLR, that is a reliable marker of central truncal fat deposition associated with increased cardiometabolic risk [47] and diabetes mellitus [48] which in turn might contribute to determining skeletal fragility [49, 50].
This study has several limitations. First, the absence of an age-matched control group did not allow a comparison of skeletal and body composition parameters of KS subjects with healthy subjects. Second, since most of our patients were undergoing long-term TRT as well as calcium and vitamin D supplementation it was not possible to assess the potential impact of untreated hypogonadism and hypovitaminosis D on different skeletal end-points. The cross-sectional nature of the study did not allow drawing causal relationships and to assess the timing of VFs occurrence. Moreover, the size of study group and the low number of patients with VFs did not permit to perform a multivariate analysis of potential risk factors of fractures. Also, no functional tests (e.g. handgrip strength, gait speed test) were carried out, not allowing us to draw any conclusion in concern of possible relationship of skeletal and body composition parameters changes and impaired physical condition. Lastly, due to technical limitation linked to the poor/large amount of soft tissue overlying the measurement area, TBS assessment is recommended when BMI ranges from 15 to 35 kg/m2 and is not validated beyond this limit [51]. Nevertheless, in view of the body composition impairment of KS subjects, we need to acknowledge the possible underestimation of TBS measurement due to interposition of abdominal adiposity.
Notwithstanding the aforementioned limitations, this cross-sectional study might provide clinical relevant information for management of individuals with KS. Since VFs are hallmark of skeletal fragility predisposing individuals to develop new fractures [52], it is reasonable to propose the use of bone-active drugs in subjects in whom VFs are discovered by a morphometric analysis, similarly to other forms of secondary osteoporosis in which fractures occur independent of BMD values [53]. In this context, assessment of body composition might provide more information on bone quality and fracture risk. As a matter of fact, our study highlights how in KS subjects, similarly to other forms of hypogonadism [12, 54], the holistic approach aimed at management of the “full blown syndrome” and complication associated (e.g. impairment of bone, lipid and glucose metabolism) should be embraced to improve general health and treatment outcomes [3, 55]. Indeed, future studies will clarify whether treatments oriented to improve body composition might favourably influence the effects of TRT and vitamin D replacement on skeletal end-points.
In conclusion, we report the first evidence of negative relationship between unfavourable body composition and bone microarchitecture in KS subjects, likely to enhance and stimulate research to clarify the role of adipose tissue, in particular visceral fat, in concern to bone quality and fracture risk in men with hypogonadism.
References
Zitzmann M, Aksglaede L, Corona G, Isidori AM, Juul A, T’Sjoen G et al (2021) European academy of andrology guidelines on Klinefelter Syndrome Endorsing Organization: European Society of Endocrinology. Andrology 9(1):145–167
Kanakis GA, Nieschlag E (2018) Klinefelter syndrome: more than hypogonadism. Metab Clin Exp 86:135–144
Bonomi M, Rochira V, Pasquali D, Balercia G, Jannini EA, Ferlin A (2017) Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest 40(2):123–134
Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA (2005) Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab 90(12):6516–6522
Bojesen A, Juul S, Birkebaek N, Gravholt CH (2004) Increased mortality in Klinefelter syndrome. J Clin Endocrinol Metab 89(8):3830–3834
Vena W, Pizzocaro A, Indirli R, Amer M, Maffezzoni F, Delbarba A et al (2020) Prevalence and determinants of radiological vertebral fractures in patients with Klinefelter syndrome. Andrology 8(6):1699–1704
Porcelli T, Maffezzoni F, Pezzaioli LC, Delbarba A, Cappelli C, Ferlin A (2020) Management of endocrine disease: male osteoporosis: diagnosis and management - should the treatment and the target be the same as for female osteoporosis? Eur J Endocrinol 183(3):R75-r93
Mazziotti G, Frara S, Giustina A (2018) Pituitary diseases and bone. Endocr Rev 39(4):440–488
Shanbhogue VV, Hansen S, Jørgensen NR, Brixen K, Gravholt CH (2014) Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in Klinefelter syndrome. J Bone Miner Res 29(11):2474–2482
Piot A, Plotton I, Boutroy S, Bacchetta J, Ailloud S, Lejeune H et al (2022) Klinefelter bone microarchitecture evolution with testosterone replacement therapy. Calcif Tissue Int 111(1):35–46
Tahani N, Nieddu L, Prossomariti G, Spaziani M, Granato S, Carlomagno F et al (2018) Long-term effect of testosterone replacement therapy on bone in hypogonadal men with Klinefelter Syndrome. Endocrine 61(2):327–335
Pedersini R, Amoroso V, Maffezzoni F, Gallo F, Turla A, Monteverdi S et al (2019) Association of fat body mass with vertebral fractures in postmenopausal women with early breast cancer undergoing adjuvant aromatase inhibitor therapy. JAMA Netw Open 2(9):e1911080
de Araújo IM, Parreiras ESLT, Carvalho AL, Elias J Jr, Salmon CEG, de Paula FJA (2020) Insulin resistance negatively affects bone quality not quantity: the relationship between bone and adipose tissue. Osteoporos Int 31(6):1125–1133
Ma WY, Yang CY, Shih SR, Hsieh HJ, Hung CS, Chiu FC et al (2013) Measurement of waist circumference: midabdominal or iliac crest? Diabetes Care 36(6):1660–1666
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D et al (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J Bone Mineral Res 11(7):984–996
Crans GG, Genant HK, Krege JH (2005) Prognostic utility of a semiquantitative spinal deformity index. Bone 37(2):175–179
Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J (2013) The Official Positions of the International Society for Clinical Densitometry: acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom 16(4):520–536
Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom 16(4):455–466
McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H et al (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Mineral Res 31(5):940–948
Dittadi R, Matteucci M, Meneghetti E, Ndreu R (2018) Reassessment of the Access Testosterone chemiluminescence assay and comparison with LC-MS method. J Clin Lab Anal 32(3):e22286
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Bojesen A, Birkebæk N, Kristensen K, Heickendorff L, Mosekilde L, Christiansen JS et al (2011) Bone mineral density in Klinefelter syndrome is reduced and primarily determined by muscle strength and resorptive markers, but not directly by testosterone. Osteoporos Int 22(5):1441–1450
Foresta C, Ruzza G, Mioni R, Meneghello A, Baccichetti C (1983) Testosterone and bone loss in Klinefelter syndrome. Horm Metab Res 15(1):56–57
Ferlin A, Selice R, Di Mambro A, Ghezzi M, Di Nisio A, Caretta N et al (2015) Role of vitamin D levels and vitamin D supplementation on bone mineral density in Klinefelter syndrome. Osteoporosis Int 26(8):2193–2202
Ferlin A, Schipilliti M, Vinanzi C, Garolla A, Di Mambro A, Selice R et al (2011) Bone mass in subjects with Klinefelter syndrome: role of testosterone levels and androgen receptor gene CAG polymorphism. J Clin Endocrinol Metab 96(4):E739–E745
Pizzocaro A, Vena W, Condorelli R, Radicioni A, Rastrelli G, Pasquali D et al (2020) Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest 43(12):1675–1687
Aksglaede L, Andersson AM, Jørgensen N, Jensen TK, Carlsen E, McLachlan RI et al (2007) Primary testicular failure in Klinefelter’s syndrome: the use of bivariate luteinizing hormone-testosterone reference charts. Clin Endocrinol (Oxf) 66(2):276–281
Juel Mortensen L, Lorenzen M, Jørgensen N, Andersson AM, Nielsen JE, Petersen LI et al (2019) Possible link between FSH and RANKL release from adipocytes in men with impaired gonadal function including Klinefelter syndrome. Bone 123:103–114
Rochira V, Antonio L, Vanderschueren D (2018) EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology 6(2):272–285
Corona G, Vena W, Pizzocaro A, Giagulli VA, Francomano D, Rastrelli G et al (2022) Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J Endocrinol Invest 45(5):911–926
Ferlin A, Schipilliti M, Foresta C (2011) Bone density and risk of osteoporosis in Klinefelter syndrome. Acta paediatrica (Oslo, Norway: 1992) 100(6):878–884
Harvey NC, Glüer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C et al (2015) Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 78:216–224
Aksglaede L, Molgaard C, Skakkebaek NE, Juul A (2008) Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Arch Dis Child 93(1):30–34
Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P et al (2006) The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 29(7):1591–1598
Chang S, Skakkebæk A, Trolle C, Bojesen A, Hertz JM, Cohen A et al (2015) Anthropometry in Klinefelter syndrome–multifactorial influences due to CAG length, testosterone treatment and possibly intrauterine hypogonadism. J Clin Endocrinol Metab 100(3):E508–E517
Blüher M (2009) Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 117(6):241–250
Mazziotti G, Lania AG, Canalis E (2022) Skeletal disorders associated with the growth hormone–insulin- like growth factor 1 axis. Nat Rev Endocrinol 18(6):353–365
Lv S, Zhang A, Di W, Sheng Y, Cheng P, Qi H et al (2016) Assessment of Fat distribution and Bone quality with Trabecular Bone Score (TBS) in Healthy Chinese Men. Sci Rep 6:24935
Melton LJ 3rd (2003) Adverse outcomes of osteoporotic fractures in the general population. J Bone Mineral Res 18(6):1139–1141
Kendler DL, Bauer DC, Davison KS, Dian L, Hanley DA, Harris ST et al (2016) Vertebral fractures: clinical importance and management. Am J Med 129(2):221.e1–10
Bojesen A, Juul S, Birkebaek NH, Gravholt CH (2006) Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab 91(4):1254–1260
Mazziotti G, Porcelli T, Mormando M, De Menis E, Bianchi A, Mejia C et al (2011) Vertebral fractures in males with prolactinoma. Endocrine 39(3):288–293
Mazziotti G, Bianchi A, Bonadonna S, Cimino V, Patelli I, Fusco A et al (2008) Prevalence of vertebral fractures in men with acromegaly. J Clin Endocrinol Metab 93(12):4649–4655
Mazziotti G, Bianchi A, Cimino V, Bonadonna S, Martini P, Fusco A et al (2008) Effect of gonadal status on bone mineral density and radiological spinal deformities in adult patients with growth hormone deficiency. Pituitary 11(1):55–61
Waterloo S, Ahmed LA, Center JR, Eisman JA, Morseth B, Nguyen ND et al (2012) Prevalence of vertebral fractures in women and men in the population-based Tromsø Study. BMC Musculoskelet Disord 13:3
Cioffi CE, Alvarez JA, Welsh JA, Vos MB (2019) Truncal-to-leg fat ratio and cardiometabolic disease risk factors in US adolescents: NHANES 2003–2006. Pediatr Obes 14(7):e12509
Choi SI, Chung D, Lim JS, Lee MY, Shin JY, Chung CH et al (2017) Relationship between regional body fat distribution and diabetes mellitus: 2008 to 2010 Korean National Health and Nutrition Examination Surveys. Diabetes Metab J 41(1):51–59
Mazziotti G, Tupputi U, Ferrante G, Guglielmi G (2020) Abdominal aortic calcification as a marker of relationship between atherosclerosis and skeletal fragility. J Clin Densitom 23(4):539–542
Crepaldi G, Maggi S (2009) Epidemiologic link between osteoporosis and cardiovascular disease. J Endocrinol Invest 32(4 Suppl):2–5
Hans D, Šteňová E, Lamy O (2017) The trabecular bone score (TBS) complements DXA and the FRAX as a fracture risk assessment tool in routine clinical practice. Curr Osteoporos Rep 15(6):521–531
Lindsay R, Pack S, Li Z (2005) Longitudinal progression of fracture prevalence through a population of postmenopausal women with osteoporosis. Osteoporosis Int 16(3):306–312
Mazziotti G, Pedersini R, Vena W, Cosentini D, Carrone F, Pigni S et al (2022) Real-world effectiveness of denosumab and bisphosphonates on risk of vertebral fractures in women with breast cancer undergoing treatment with aromatase inhibitors. Calcif Tissue Int. https://doi.org/10.1007/s00223-022-01011-w
Mazziotti G, Vena W, Pedersini R, Piccini S, Morenghi E, Cosentini D et al (2022) Prediction of vertebral fractures in cancer patients undergoing hormone deprivation therapies: reliability of who fracture risk assessment tool (frax) and bone mineral density in real-life clinical practice. J Bone Oncol 33:100421
Foresta C, Ferlin A, Lenzi A, Montorsi P (2017) The great opportunity of the andrological patient: cardiovascular and metabolic risk assessment and prevention. Andrology 5(3):408–413
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they do not have conflict of interest that is relevant to the subject matter or materials included in this work.
Research involving human participants and/or animals
The study was approved by the Ethics Committees.
Informed consent
All subjects gave informed consent to use their clinical data for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vena, W., Carrone, F., Delbarba, A. et al. Body composition, trabecular bone score and vertebral fractures in subjects with Klinefelter syndrome. J Endocrinol Invest 46, 297–304 (2023). https://doi.org/10.1007/s40618-022-01901-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01901-8