Abstract
Background
The role of testosterone (T) replacement therapy (TRT) in subjects with late onset hypogonadism is still the object of an intense debate.
Methods
All observational studies and placebo-controlled or -uncontrolled randomized trials (RCTs) comparing the effect of TRT on different bone parameters were considered.
Results
Out of 349 articles, 36 were considered, including 3103 individuals with a mean trial duration of 66.6 weeks. TRT improves areal bone mineral density (aBMD) at the spine and femoral neck levels in observational studies, whereas placebo-controlled RTCs showed a positive effect of TRT only at lumber spine and when trials included only hypogonadal patients at baseline (total testosterone < 12 nM). The effects on aBMD were more evident in subjects with lower T levels at baseline and increased as a function of trial duration and a higher prevalence of diabetic subjects. Either T or estradiol increase at endpoint contributed to aBMD improvement. TRT was associated with a significant reduction of bone resorption markers in observational but not in controlled studies.
Conclusion
TRT is able to inhibit bone resorption and increase bone mass, particularly at the lumbar spine level and when the duration is long enough to allow the anabolic effect of T and estrogens on bone metabolism to take place.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testosterone (T) is essential for bone health during all ages [1], by acting though the androgen receptor expressed on osteoblasts, osteoclasts, osteocytes and marrow stromal cells [2, 3]. Furthermore, these cells express the estrogen receptor, which responds to estradiol (E2) formed from T aromatization through the activity of the CYP19A1 enzyme (aromatase) [4]. During puberty, T together with E2, stimulate periosteal apposition and trabecular bone growth, participate in pubertal growth spurts and acquisition of peak bone mass [1, 5, 6]. As a sum, T action leads to the development of wider bones with a thicker cortex as compared to women, and the final result is a higher peak bone mass and bigger, though not denser, skeletons in men with respect to women [1, 6, 7]. Once the peak bone mass has been achieved, T helps to maintain bone density and strength, by slowing the bone remodeling rate and by maintaining a balance between resorption and formation [1, 6].
Therefore, reduced T levels occurring either before achieving peak bone mass or during adulthood and senescence, might seriously affect bone health, in terms of mass and strength [6]. In addition, it is important to emphasize that other functions of the Leydig cells are important in the testis–bone crosstalk: they produce the peptide hormone Insulin-Like Factor 3 (INSL3) and participate in the activation of vitamin D by converting the inactive cholecalciferol into 25OH-D3 (calcifediol) [6, 8].
As a consequence, the presence of hypogonadism, both in young men (e.g., Klinefelter syndrome) and in aging subjects (late onset hypogonadism—LOH), is associated with lower bone mineral density (BMD) and represents a major risk factor for osteoporosis [6, 8]. Accordingly, current guidelines suggest that hypogonadal patients, patients who need androgen deprivation therapy, and men with a well-documented history of hypogonadism should be screened for osteoporosis by Dual energy X-ray Absorptiometry (DXA) [9]. On the other hand, osteoporotic men should be screened for hypogonadism and eventually treated [9, 10].
According to available guidelines, testosterone replacement therapy (TRT) in the setting of male osteoporosis is particularly recommended in young adult hypogonadal men to prevent bone loss and help acquire peak bone mass [2, 9], and it should be associated with antiresorptive drugs when fracture risk is high [9, 11]. Actually, the effect of TRT alone on bone health in hypogonadal men is still not well defined [6, 12], and no studies with fractures as their primary endpoint have been performed. Indeed, it is assumed that TRT can improve BMD, particularly at the vertebral level and when T levels are very low [6, 9, 13, 14]. The effect is more evident in younger men with organic hypogonadism, as a meta-analysis showed in men with Klinefelter syndrome [15]. In the other groups of patients, mainly older men with functional hypogonadism [11, 16,17,18], or specific categories of patients, such as those with HIV [19], the benefits of TRT are not well established. The combination of TRT with antiosteoporotic drugs has not been investigated, whereas the combination of TRT with vitamin D and calcium seems more effective in increasing BMD than TRT alone, at least in men with Klinefelter syndrome [20]. In older men, data are limited and heterogeneous in terms of patient selection, definition of hypogonadism and type and duration of TRT.
Therefore, the effect of TRT alone on bone health in hypogonadal men is still not well defined [12, 21]. Here, we aimed at investigating and meta-analyzing the effect of T supplementation on different bone parameters either in observational or placebo controlled and uncontrolled randomized studies.
Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [see Supplementary file 1].
An extensive Medline, Embase and Cochrane search was performed including the following words ("testosterone"[MeSH Terms] OR "testosterone"[All Fields] OR "testosteron"[All Fields] OR "testosterones"[All Fields] OR "testosterone s"[All Fields]) AND ("bone and bones"[MeSH Terms] OR ("bone"[All Fields] AND "bones"[All Fields]) OR "bone and bones"[All Fields] OR "bone"[All Fields]) AND ("therapeutics"[MeSH Terms] OR "therapeutics"[All Fields] OR "treatments"[All Fields] OR "therapy"[MeSH Subheading] OR "therapy"[All Fields] OR "treatment"[All Fields] OR "treatment s"[All Fields]) AND ("hypogonad"[All Fields] OR "hypogonadal"[All Fields] OR "hypogonadic"[All Fields] OR "hypogonadism"[MeSH Terms] OR "hypogonadism"[All Fields] OR "hypogonadisms"[All Fields]) AND (("testosterone"[MeSH Terms] OR "testosterone"[All Fields] OR "testosteron"[All Fields] OR "testosterones"[All Fields] OR "testosterone s"[All Fields]) AND ("hormone replacement therapy"[MeSH Terms] OR ("hormone"[All Fields] AND "replacement"[All Fields] AND "therapy"[All Fields]) OR "hormone replacement therapy"[All Fields] OR ("replacement"[All Fields] AND "therapy"[All Fields]) OR "replacement therapy"[All Fields])). The search accrued data from January 1, 1969 up to April 15th, 2021. The identification of relevant studies was performed independently by two of the authors (V.G, W.V), and conflicts resolved by the third investigator (A.P). We did not employ search software. We hand-searched bibliographies of retrieved papers for additional references. The principal source of information was derived from published articles; if data were missing from a publication, an attempt at retrieval was made through clinicaltrial.gov website.
Study selection
We included all observational studies and placebo-controlled or -uncontrolled randomized trials (RCTs) comparing the effect of TRT on different endpoints [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] (see also Fig. 1, Table 1 and Supplementary Table 1). Only studies reporting data on areal bone mineral density (aBMD) expressed as g/cm2 and/or including results on bone remodeling markers were considered (see also Supplementary Table 1). Studies using androgens other than T, as well as studies with concomitant treatment with other hormones and drugs were also excluded, unless there was a clearly defined treatment arm that received only T treatment. Similarly, studies including only patients with genetic causes of male hypogonadism, such as Klinefelter Syndrome, were excluded from the analysis and revised elsewhere [15].
Outcomes
The principal outcome of this analysis was the effect of TRT, as compared with baseline, placebo or control groups, on aBMD at lumbar and femoral levels. Secondary outcomes included several other bone related parameters (Supplementary Table 1). In particular, bone resorption markers analyzed include cross-link, urinary deoxypyridinoline, serum C-terminal telopeptide and serum N-terminal telopeptide. Bone neoformation markers analyzed include bone alkaline phosphatase, propeptide collagen, and osteocalcin.
Quality assessment
The quality of trials was assessed using the Cochrane criteria [58] (see also Supplementary Table 2). In particular, for RCTs, the following criteria were evaluated: how the randomization sequence was generated, how allocation was concealed, whether there were important imbalances at baseline, which groups were blinded (patients, caregivers, data collectors, outcome assessors, data analysts), what the loss to follow-up rate was (in the intervention and the control arm), whether the analyses were by intention to treat, and how missing outcome data were dealt with. In observational studies we evaluated the following criteria: the weaknesses of the designs that have been used (such as noting their potential to ascertain causality), the execution of the studies through a careful assessment of their risk of bias, especially the potential for selection bias and confounding to which all observational studies are susceptible, and the potential for reporting biases, including selective reporting of outcomes For each study, we also assessed how the population was selected, the duration and route of TRT, and the adequacy of study follow-up [58].
Statistical analysis
Heterogeneity was assessed using I2 statistics. Even when a low heterogeneity was detected, a random-effects model was applied, because the validity of tests of heterogeneity can be limited with a small number of component studies. To estimate possible publication or disclosure bias we used funnel plots and the Begg adjusted rank correlation test [58, 59]. However, because these tests have low statistical power when the number of trials is small, undetected bias may still be present. In addition, since in some trials the significance of between group comparisons (p) was not reported, the analysis was performed evaluating the endpoint values of each parameter in different treatment groups, in a non-paired fashion (non-paired analysis). Considering that most of the studies, which did not describe p values, reported non-significant differences across groups, the mean (paired) analysis, which excludes those data is likely to overestimate the effect of treatments. On the other hand, the non-paired analysis is a very conservative approach, which could underestimate treatment effect. Since bone remodeling parameters were evaluated through different approaches and expressed in different ways, the mean difference for each study was divided by the pooled estimate of the SD, to express the effect size for each study in a common metric, namely, the standardized mean difference (SMD). According to Cohen [60], a small treatment-effect size is considered to be about 0.2, a medium effect size to be about 0.5, and a large effect size to be about 0.8. All other data were expressed as weight mean differences. Meta-regression analyses were performed to test the effect of different parameters on aBMD modification. In addition, a multivariate linear regression analysis model, weighting each study for the number of subjects enrolled, was performed to verify in controlled studies the effect of TRT on several parameters (see below). All analyses were performed using Comprehensive Meta-analysis Version 2, Biostat (Englewood, NJ, USA). Multivariate analyses were performed on SPSS (Statistical Package for the Social Sciences; Chicago, USA) for Windows 25.0.
Results
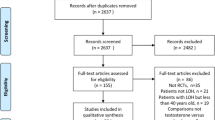
Out of 349 retrieved articles, 36 were included in the study (Fig. 1). In particular, 25 were controlled studies and 11 observational. Among the controlled studies, 19 were placebo-controlled RCTs. The characteristics of the retrieved trials (including parameters on trial quality) and type of outcomes considered are reported in Table 1 and Supplementary Tables 1 and 2. The retrieved studies included 1988 and 1115 individuals in TRT and control groups, respectively; mean trial duration was 66.6 weeks. TRT was administered in different doses, formulations and cohorts (Table 1). The vast majority of the studies included subjects with LOH or a mixed population of organic and functional hypogonadism, whereas only a limited number of the trials enrolled only hypogonadal subjects with an organic origin (Table 1).
The mean age, baseline T and body mass index (BMI) of enrolled patients were 57.2 years, 8.8 nmol/L and 28.5 kg/m2. Subjects enrolled in controlled studies were older (63.3 ± 10.9 vs. 46.4 ± 10.3 years old; p < 0.0001) and had higher T levels (10.9 ± 3.2 vs. 5.4 ± 2.7 nmol/L; p < 0.0001), whereas the duration of follow-up (68.9 ± 56.3 vs. 62.5 ± 47.7 weeks; p = 0.703), and BMI (29.2 ± 3.6 vs. 27.2 ± 2.2 kg/m2; p = 0.09) was similar in comparison with observational studies. Similar results were observed when placebo-controlled RCTs were compared to observational studies (not shown).
Bone mineral density
Among studies reporting several outcomes, 35 out of 36 included information on aBMD at least in one site, whereas 30 studies analyzed aBMD in more than one section (Supplementary Table 1). When lumbar aBMD was considered, I2 for controlled and observational studies were 74.43, p < 0.0001 and 43.61, p = 0.036. Funnel plot and Begg-adjusted rank correlation test suggested no major publication bias in both types of studies (Kendall’s τ: 0.10; p = 0.53 and − 0.17; p = 0.45 for controlled and observational studies, respectively). TRT resulted in a significant improvement of aBMD at lumbar and femoral neck level both in controlled and in observational studies (Fig. 2, Supplementary Figs. 1A, B and 2A, B). Similar data were observed when those trials including only subjects with organic problems were excluded from the analysis (aBMD = 0.040[0.026; 0.054], p < 0.0001 and 0.041[0.013; 0.069], p = 0.004 for observational and controlled studies at endpoint, respectively). Since weighted baseline aBMD was 1.169 ± 0.185 g/cm2 and 0.870 ± 0.134 g/cm2 at lumbar and femoral neck, respectively, the relative observed increase of aBMD over controls at the endpoint was 3.08[0.86; 5.22]% and 2.07[0.23; 3.80]% at lumbar at femoral neck.
When aBMD at the lumbar level was considered and the analysis was limited to placebo-controlled RCTs, only a trend toward a significant effect was detected (Fig. 2 and Supplementary Fig. 1C). However, when the latter data were analyzed by including only those studies enrolling hypogonadal subjects (baseline total testosterone < 12 nmol/L), a significant effect of TRT was observed even in placebo-controlled RCTs (Fig. 2 and Supplementary Fig. 1D). Similar data were observed when the analysis was limited to those studies with a mean age of the population above 60 years (not shown). The relative lumbar aBMD increase over placebo at endpoint was 4.40[0.59; 8.20]% in RCTs on hypogonadal subjects. Conversely, no effect of TRT was observed at femoral neck when placebo-controlled RCTs were analyzed, even when the data were limited to those studies including only hypogonadal patients (Fig. 2 and Supplementary Fig. 2C, D). When other bone sections were analyzed, TRT resulted in a significant increase in aBMD at femoral trochanter level in observational but not in controlled trials (Table 2 and Supplementary Fig. 3A, B). No further differences were observed in aBMD evaluated at different sites—including femoral wards, hip, radial and total body—when either observational or controlled studies were considered (Table 2 and Supplementary Fig. 3C–L). No further sub-analyses were possible due to the limited data.
In controlled studies, meta-regression analysis showed that the effect of TRT on aBMD at lumbar level was higher with longer duration and when a higher proportion of diabetic subjects was included (Fig. 3A, B). In addition, an inverse relationship between baseline T levels and lumbar aBMD at follow-up was also observed (Fig. 3C). The association between lumbar aBMD differences at follow-up and Diabetes Mellitus (DM) or T levels at baseline were confirmed after alternative multivariate linear regression analyses, weighting each study for the number of subjects enrolled and adjusting for trial duration, age and BMI (β = 0.817 and − 0.339 for baseline TT and DM, respectively; all p < 0.0001). The association between lumbar aBMD differences at the endpoint and DM was confirmed even after the adjustment for baseline TT levels (β = 0.814, p < 0.0001). As expected, TRT resulted in a significant increase of circulating TT and E2 levels at endpoint when compared to controls (Supplementary Fig. 4A, B). Both E2 and TT differences significantly contributed to aBMD modifications at follow-up, even after the adjustment for trial duration, age and BMI (β = 0.312 and 0.127 for TT and E2, respectively; both p < 0.0001).
Remodelling bone markers
Information on bone remodeling markers was available in 20 studies. In particular, among them 12 were controlled and eight were observational studies. TRT was associated with a significant reduction of bone resorption markers in observational but not in controlled studies (Table 3 and Supplementary Fig. 5C, D). Conversely, no modification of bone neoformation markers either in observational or in controlled studies was observed (Table 3 and Supplementary Fig. 5A, B). No difference in both resorption markers in controlled studies were detected even when the data were limited to placebo-controlled RCTs including only hypogonadal patients (not shown).
Discussion
This is the largest meta-analysis evaluating the effects of TRT in male patients on several bone-related outcomes. In addition, for the first time, both controlled and observational studies were analyzed. Our data indicate that TRT improves aBMD either at spine or femoral neck level both in uncontrolled and controlled trials. However, when the analysis was limited to placebo-controlled RCTs, the positive effects of TRT were limited to lumber spine and to those trials including only hypogonadal patients at baseline (TT < 12 nM). Interestingly, the results were more robust in subjects with lower T levels at baseline; in addition, the effect increased as a function of trial duration. Both TT and E2 increase at endpoint independently contributed to aBMD improvement at lumber level. Finally, an original finding of this study is that TRT resulted in a better aBMD increase at lumbar levels in those studies including a larger proportion of diabetic patients.
Diabetes mellitus (DM) and osteoporosis are chronic medical conditions commonly affecting aging people [61, 62]. The specific role of DM in the pathogenesis of reduced aBMD, osteoporosis and bone fracture risk is still an object of an intense debate [61, 62]. In 1927, Morrison and Bogan [63] reported, for the first time, a possible association between DM and bone loss. A decreased peak of bone mass- due to insulin and/or insulin-like growth factor defects, causing reduced osteoblast proliferation and poor collagen synthesis—has been considered a crucial factor in type 1 DM (T1DM) [64]. Conversely, the possible association between bone health and type 2 DM (T2DM) is more controversial. Accordingly, normal, reduced or even increased aBMD has been described in T2DM [65,66,67]. Despite the evidence related to aBMD, several studies have described an increased risk of hip, vertebral and non-vertebral fractures both in T1DM and T2DM [61, 68, 69]. Chronic hyperglycemia and increased advanced glycation end products (AGEs) may support the modification of local bone metabolism, resulting in structural abnormalities, including trabecular bone loss, decreased cortical BMD and increased cortical porosity, which eventually leads to a decreased bone strength [70]. In addition several antidiabetic drugs can contribute to the increased risk of fractures, by increasing the risk of hypoglycemic episodes and falls (e.g., sulfonylureas) [71, 72] or interacting with bone metabolism at several levels, such as thiazolidinediones [71, 73, 74]. The present study suggests that sex hormone alteration should be considered as another important factor in the pathogenesis of bone loss in DM subjects. A large body of evidence has documented that DM, and T2DM in particular, is associated with reduced T levels [75,76,77,78]. The pathogenetic mechanisms underlining the latter association are not completely understood and revised elsewhere [76, 77]. Several observational studies have documented that TRT can improve body composition and metabolic profile in T2DM [79, 80]. However, data derived from placebo-controlled RCTs are more conflicting [81]. The present meta-analysis suggests that TRT might improve aBMD at lumbar levels, particularly in the diabetic population. These data are in line to what recently reported by Collelouri et al. [57], in a single arm, open-label clinical trial involving 105 hypogonadal (total T < 10.4 nmol/L) with or without T2DM. After 18 month TRT resulted in greater BMD improvement at lumbar in diabetic subjects when compared to non-diabetic counterparts [57]. Whether or not the latter result might reduce the risk of fractures in either a diabetic or general population cannot be determined by the present data and should be investigated in further studies. In addition, it is important to recognize that the role of DM in T-induced aBMD improvement, at least at the spine level, was confirmed even after the adjustment for T levels, supporting the multifactorial origin of the osteopenia/osteoporosis in the diabetic population.
The association between mild-to-severe T deficiency, reduced BMD and an increased risk of osteopenia/osteoporosis in young adult men with organic hypogonadism is well documented [2, 3]. Conversely, the role of T and TRT on bone homeostasis in aging men with LOH or functional hypogonadism is more conflicting [9, 11]. Data from several population based studies, including the European Male Aging Study (EMAS) [82], the Rancho Bernardo Study [83, 84] and the Tromso Study [85], have reported an inverse association between bioavailable T levels and aBMD. However, data from the EMAS indicated that only individuals with overt hypogonadism (total T < 8 nmol/L) have reduced aBMD, when compared to eugonadal subjects [82]. In addition, the same studies have also disclosed a direct relationship between serum E2 levels, particularly the bioavailable fraction, and aBMD, supporting a role for the relative decline of E2, frequently observed in aging men, and bone health [3]. Similarly, Finkelstein et al. [86], in an elegant RCT including 198 healthy men, receiving goserelin acetate to suppresses endogenous gonadal steroid production, showed an association between worse BMD when T levels were below 7 nmol/L. These results have been replicated by the same group [87] and by others authors [88].
The present data are essentially in line with what has been previously reported. The effects of TRT at the endpoint were more defined in patients with a more severe hypogonadism at baseline and confirmed in placebo-controlled RCTs, when only those studies including hypogonadal subjects (total T < 12 nmol/L) were considered. Similar results were previously reported when other outcomes, such as sexual function and metabolic profile, were analyzed [89]. Interestingly, the present meta-analysis suggests that both a T or E2 increase at endpoint independently contribute to the increase of aBMD, at least at the lumbar level, supporting a possible role of both sex steroids in bone homeostasis regulation in aging males. Accordingly, these results were confirmed even when only studies considering patients with LOH were investigated. The role of circulating or locally produced estrogens on bone homeostasis is well known and revised elsewhere [5]. T has direct and indirect effects contributing to the maintenance of correct bone homeostasis [3, 8, 9]. In particular, besides the direct effects on osteoblast differentiation and proliferation, T indirectly regulates the activity of osteoclasts by the modulation of the receptor activator of nuclear factor k − B (RANK–ligand). In addition, other indirect effects include the positive action of T on several growth factors and cytokines, such as growth hormone and insulin-like-growth factor 1. Finally, the positive effects of T on muscle mass might positively contribute to bone heath and to a possible fracture risk reduction [8, 9].
Data derived from the present meta-analysis are essentially in line with what was reported by Isidori et al. [90] more than 15 years ago. In fact, TRT resulted in about a 3% and 2% increase in aBMD at lumbar and femoral neck, respectively, when compared to placebo. The data were even more impressive when hypogonadal subjects were considered (up to 8%). Interestingly, antiresorptive drug therapy produces in 12–24 months an effect size ranging from 0.3 to 3.8% using alendronate [91, 92] denosumab [91] or zolendronic acid [93]. Data derived from other available meta-analyses were either supporting [14] or not [16,17,18], the positive effects of TRT on aBMD. The differences in the study selection and the lack of sub-analysis according to baseline T levels and hypogonadal status can explain, at least partially, the conflicting results [16,17,18]. In addition, it is important to recognize that the vast majority of the available RCTs have used dual energy absorptiometry (DXA), measuring areal BMD (aBMD). More recently, the quantitative computed tomography (QCT) method, measuring volumetric BMD (vBMD), has been introduced. The latter can distinguish cortical from trabecular structures and predicts fracture risk, independently from aBMD and FRAX score [94]. Using QTC a recent large, placebo-controlled RTC showed that TRT increased vBMD particularly in cortical bone at both tibia and radius, with an effects size ranging from 2.9 to 3.1% after 2 years of treatment [56]. In addition, data from the T-trials study, including 211 patients older than 60 years, found that, after 1 year, TRT resulted in a vBMD increase, particularly at trabecular spine [53]. Data derived from longitudinal studies using QCT have documented that the age-related decline of sex steroids is associated with an accelerated bone loss, particularly at the cortical site [6]. Uncontrolled small studies have shown that TRT can improve both cortical and trabecular bone [24, 37, 91,92,93, 95]. Hence, available data support the hypothesis that TRT in hypogonadal men can improve both BMD and bone structure.
Another original finding of the present meta-analysis is the evaluation of TRT in observational studies. Overall, the data support what has been derived from RCTs, with an improvement of aBMD allocated with TRT. In addition, a positive effect on bone resorption markers was detected in observational but not in controlled studies. Observational studies included younger and more severe hypogonadal patients; this could explain, at least partly, the observed differences from RCTs with respect to bone resorption markers. However, it is important to recognize that data derived from observational studies should be interpreted with caution, due to the risk of selection bias related to the non-random assignment of T exposure. Accordingly, physicians frequently select to treat healthier individuals, and healthier individuals more often seek medical care for their hypogonadism-related problems. In addition, other limitations include the lack of information regarding the level of T before and during TRT, as well as the limited data regarding the type of T preparation used and the follow-up performed during treatment.
Other important limitations should be considered for correctly interpreting the data derived from the present study. First, the presence of osteoporosis was not required for trial eligibility. The vast majority of the included studies lasted less than 2 years, whereas conventional osteoporosis studies usually extend over a 3-year period. We here report that the effects of TRT increased as a function of trial duration. Hence, it is possible that TRT effect on bone would be even more evident if conducted for a longer time, in particular when enrolling patients with osteopenia/osteoporosis. Therefore, large placebo-controlled RCTs conducted with a follow-up longer than 2 years are advisable. Significant heterogeneity among studies was detected, which reflects the differences observed in population characteristics and type of T preparation used. Available data were insufficient for investigating the effect of different T preparations on aBMD outcome in hypogonadal subjects or to perform other subgroup analyses to explain reasons for heterogeneity in results. No information regarding the type of DM and the influence of hypoglycemic drugs was available. Considering the limited numbers of available studies, even a few outliers could produce relevant deviations of estimates; for example, the effect of TRT on femoral BMD in RCTs was largely driven by three trials. Finally, several life-style behaviors such as smoking, alcohol consumption, type of diet and level of physical activity can modulate BMD as well as TRT outcomes. Unfortunately, no sufficient information on the latter parameters was available. Similarly, the use of exercise, as well as the concomitant use of phosphodiesterase type 5 inhibitor (PDE5i) and other drugs, can modulate the aromatase activity, [96] resulting in a further possible source of bias.
Although this meta-analysis showed positive effects of TRT on bone health, several aspects should be considered in further studies dealing with osteoporosis in hypogonadal men: estimation of fractures and fracture risk, combined effect of TRT and antiresorptive drugs and vitamin D and calcium supplementation, skeletal muscle mass and strength, type and duration of TRT [6].
Conclusions
Taken together, these data showed that TRT in hypogonadal patients could inhibit bone resorption and increase bone mass. This is particularly evident at the lumbar spine level and when the duration of TRT lasts long enough to allow the anabolic effect of testosterone and estrogens on bone metabolism to take place. However, whether or not TRT is associated with a decreased risk of bone fractures remains to be established. This is particularly relevant in the diabetic population, where there is the need to decrease the risk factors for fragility fractures, because of reduced skeletal strength and increased cortical porosity. Considering that positive effects of TRT are particularly evident in the diabetic population, the present study offers new arguments in support of screening for hypogonadism in diabetes and, if detected, for an appropriate androgen replacement.
References
Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R et al (2017) Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 97(1):135–187
Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM et al (2018) Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 103(5):1715–1744
Rochira V (2020) Late-onset hypogonadism: bone health. Andrology 8(6):1539–1550
Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA (2017) Estrogens in male physiology. Physiol Rev 97(3):995–1043
Rochira V, Kara E, Carani C (2015) The endocrine role of estrogens on human male skeleton. Int J Endocrinol 2015:165215
Porcelli T, Maffezzoni F, Pezzaioli LC, Delbarba A, Cappelli C, Ferlin A (2020) Management of endocrine disease: male osteoporosis: diagnosis and management—should the treatment and the target be the same as for female osteoporosis? Eur J Endocrinol 183(3):R75-r93
Farr JN, Khosla S (2015) Skeletal changes through the lifespan—from growth to senescence. Nat Rev Endocrinol 11(9):513–521
Ferlin A, Selice R, Carraro U, Foresta C (2013) Testicular function and bone metabolism—beyond testosterone. Nat Rev Endocrinol 9(9):548–554
Rochira V, Antonio L, Vanderschueren D (2018) EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology 6(2):272–285
Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES et al (2012) Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97(6):1802–1822
Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G et al (2020) European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology 8(5):970–987
Vidal M, Thibodaux RJ, Neira LFV, Messina OD (2019) Osteoporosis: a clinical and pharmacological update. Clin Rheumatol 38(2):385–395
Isidori AM, Balercia G, Calogero AE, Corona G, Ferlin A, Francavilla S et al (2015) Outcomes of androgen replacement therapy in adult male hypogonadism: recommendations from the Italian society of endocrinology. J Endocrinol Investig 38(1):103–112
Tracz MJ, Sideras K, Boloña ER, Haddad RM, Kennedy CC, Uraga MV et al (2006) Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab 91(6):2011–2016
Pizzocaro A, Vena W, Condorelli R, Radicioni A, Rastrelli G, Pasquali D et al (2020) Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Investig 43:1675–1687
Junjie W, Dongsheng H, Lei S, Hongzhuo L, Changying S (2019) Testosterone replacement therapy has limited effect on increasing bone mass density in older men: a meta-analysis. Curr Pharm Des 25(1):73–84
Zhang Z, Kang D, Li H (2020) The effects of testosterone on bone health in males with testosterone deficiency: a systematic review and meta-analysis. BMC Endocr Disord 20(1):33
Guo C, Gu W, Liu M, Peng BO, Yao X, Yang B et al (2016) Efficacy and safety of testosterone replacement therapy in men with hypogonadism: a meta-analysis study of placebo-controlled trials. Exp Ther Med 11(3):853–863
Maffezzoni F, Porcelli T, Delbarba A, Pezzaioli LC, Properzi M, Cappelli C et al (2020) Hypogonadism and bone health in men with HIV. Lancet HIV 7(11):e782–e790
Ferlin A, Selice R, Di Mambro A, Ghezzi M, Di Nisio A, Caretta N et al (2015) Role of vitamin D levels and vitamin D supplementation on bone mineral density in Klinefelter syndrome. Osteoporos Int 26(8):2193–2202
Russell N, Grossmann M (2019) Mechanisms in endocrinology: estradiol as a male hormone. Eur J Endocrinol 181(1):R23-r43
Morley JE, Perry HM 3rd, Kaiser FE, Kraenzle D, Jensen J, Houston K et al (1993) Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc 41(2):149–152
Young NR, Baker HW, Liu G, Seeman E (1993) Body composition and muscle strength in healthy men receiving testosterone enanthate for contraception. J Clin Endocrinol Metab 77(4):1028–1032
Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A (1996) Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 81(12):4358–4365
Hall GM, Larbre JP, Spector TD, Perry LA, Da Silva JA (1996) A randomized trial of testosterone therapy in males with rheumatoid arthritis. Br J Rheumatol 35(6):568–573
Reid IR, Wattie DJ, Evans MC, Stapleton JP (1996) Testosterone therapy in glucocorticoid-treated men. Arch Intern Med 156(11):1173–1177
Wang C, Eyre DR, Clark R, Kleinberg D, Newman C, Iranmanesh A et al (1996) Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab 81(10):3654–3662
Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH et al (1999) Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 84(6):1966–1972
Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A et al (2000) Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 85(8):2670–2677
Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG (2001) Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 56(5):M266–M272
Howell SJ, Radford JA, Adams JE, Smets EM, Warburton R, Shalet SM (2001) Randomized placebo-controlled trial of testosterone replacement in men with mild Leydig cell insufficiency following cytotoxic chemotherapy. Clin Endocrinol (Oxf) 55(3):315–324
Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G et al (2001) Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin Endocrinol (Oxf) 54(6):739–750
Crawford BA, Liu PY, Kean MT, Bleasel JF, Handelsman DJ (2003) Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab 88(7):3167–3176
Schubert M, Bullmann C, Minnemann T, Reiners C, Krone W, Jockenhövel F (2003) Osteoporosis in male hypogonadism: responses to androgen substitution differ among men with primary and secondary hypogonadism. Horm Res 60(1):21–28
Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM et al (2004) Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 89(2):503–510
Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ et al (2004) Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 89(5):2085–2098
Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B et al (2005) Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res 20(10):1785–1791
Merza Z, Blumsohn A, Mah PM, Meads DM, McKenna SP, Wylie K et al (2006) Double-blind placebo-controlled study of testosterone patch therapy on bone turnover in men with borderline hypogonadism. Int J Androl 29(3):381–391
Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL et al (2008) Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 299(1):39–52
Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R (2008) Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res 20(4):378–387
Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO et al (2010) Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc 58(6):1134–1143
Aversa A, Bruzziches R, Francomano D, Greco EA, Fornari R, Di Luigi L et al (2012) Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male 15(2):96–102
Behre HM, Tammela TL, Arver S, Tolrá JR, Bonifacio V, Lamche M et al (2012) A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male 15(4):198–207
Deb P, Gupta SK, Godbole MM (2012) Effects of short-term testosterone replacement on areal bone mineral density and bone turnover in young hypogonadal males. Indian J Endocrinol Metab 16(6):947–951
Borst SE, Yarrow JF, Conover CF, Nseyo U, Meuleman JR, Lipinska JA et al (2014) Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am J Physiol Endocrinol Metab 306(4):E433–E442
Bouloux PM, Legros JJ, Elbers JM, Geurts TB, Kaspers MJ, Meehan AG et al (2013) Effects of oral testosterone undecanoate therapy on bone mineral density and body composition in 322 aging men with symptomatic testosterone deficiency: a 1-year, randomized, placebo-controlled, dose-ranging study. Aging Male 16(2):38–47
Lee MJ, Ryu HK, An SY, Jeon JY, Lee JI, Chung YS (2014) Testosterone replacement and bone mineral density in male pituitary tumor patients. Endocrinol Metab (Seoul) 29(1):48–53
Corona G, Vignozzi L, Sforza A, Maggi M (2013) Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health 31(2):103–125
Wang YJ, Zhan JK, Huang W, Wang Y, Liu Y, Wang S et al (2013) Effects of low-dose testosterone undecanoate treatment on bone mineral density and bone turnover markers in elderly male osteoporosis with low serum testosterone. Int J Endocrinol 2013:570413
Francomano D, Bruzziches R, Barbaro G, Lenzi A, Aversa A (2014) Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Investig 37(4):401–411
Tirabassi G, delli Muti N, Gioia A, Biagioli A, Lenzi A, Balercia G (2014) Effects of testosterone replacement therapy on bone metabolism in male post-surgical hypogonadotropic hypogonadism: focus on the role of androgen receptor CAG polymorphism. J Endocrinol Investig 37(4):393–400
Dias JP, Melvin D, Simonsick EM, Carlson O, Shardell MD, Ferrucci L et al (2016) Effects of aromatase inhibition vs. testosterone in older men with low testosterone: randomized-controlled trial. Andrology 4(1):33–40
Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE et al (2017) Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med 177(4):471–479
Ng Tang Fui M, Hoermann R, Nolan B, Clarke M, Zajac JD, Grossmann M (2018) Effect of testosterone treatment on bone remodelling markers and mineral density in obese dieting men in a randomized clinical trial. Sci Rep 8(1):9099
Barnouin Y, Armamento-Villareal R, Celli A, Jiang B, Paudyal A, Nambi V et al (2021) Testosterone replacement therapy added to intensive lifestyle intervention in older men with obesity and hypogonadism. J Clin Endocrinol Metab 106(3):e1096–e1110
Ng Tang Fui M, Hoermann R, Bracken K, Handelsman DJ, Inder WJ, Stuckey BGA et al (2021) Effect of testosterone treatment on bone microarchitecture and bone mineral density in men: a two-year RCT. J Clin Endocrinol Metab 106:e3143–e3158
Colleluori G, Aguirre L, Napoli N, Qualls C, Villareal DT, Armamento-Villareal R (2021) Testosterone therapy effects on bone mass and turnover in hypogonadal men with type 2 diabetes. J Clin Endocrinol Metab 106(8):e3058–e3068
Higgins JPTSJ, Savovi J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S (2016) A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev 10:29–31
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Cohen J (2013) Statistical power analysis for the behavioral sciences. Academic Press, Cambridge
Vilaca T, Schini M, Harnan S, Sutton A, Poku E, Allen IE et al (2020) The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: a systematic review and meta-analysis update. Bone 137:115457
Qiu J, Li C, Dong Z, Wang J (2021) Is diabetes mellitus a risk factor for low bone density: a systematic review and meta-analysis. BMC Endocr Disord 21(1):65
Morrison L, Bogan I (1927) Bone development in diabetic children: a roentgen study. Am J Med Sci 174:313–319
van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A et al (1995) Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study. Ann Intern Med 122(6):409–414
Leidig-Bruckner G, Ziegler R (2001) Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes 109(Suppl 2):S493-514
Schwartz AV (2003) Diabetes mellitus: does it affect bone? Calcif Tissue Int 73(6):515–519
Thrailkill KM, Lumpkin CK Jr, Bunn RC, Kemp SF, Fowlkes JL (2005) Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab 289(5):E735–E745
Fan Y, Wei F, Lang Y, Liu Y (2016) Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos Int 27(1):219–228
Wang J, You W, Jing Z, Wang R, Fu Z, Wang Y (2016) Increased risk of vertebral fracture in patients with diabetes: a meta-analysis of cohort studies. Int Orthop 40(6):1299–1307
Ho-Pham LT, Chau PMN, Do AT, Nguyen HC, Nguyen TV (2018) Type 2 diabetes is associated with higher trabecular bone density but lower cortical bone density: the Vietnam Osteoporosis Study. Osteoporos Int 29(9):2059–2067
Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, Eastell R et al (2018) Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 29(12):2585–2596
Monami M, Cresci B, Colombini A, Pala L, Balzi D, Gori F et al (2008) Bone fractures and hypoglycemic treatment in type 2 diabetic patients: a case-control study. Diabetes Care 31(2):199–203
Benvenuti S, Cellai I, Luciani P, Deledda C, Baglioni S, Giuliani C et al (2007) Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J Endocrinol Investig 30(9):Rc26-30
Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR et al (2008) Rosiglitazone-associated fractures in type 2 diabetes: an analysis from a diabetes outcome progression trial (ADOPT). Diabetes Care 31(5):845–851
Maseroli E, Corona G, Rastrelli G, Lotti F, Cipriani S, Forti G et al (2015) Prevalence of endocrine and metabolic disorders in subjects with erectile dysfunction: a comparative study. J Sex Med 12(4):956–965
Grossmann M, Ng Tang Fui M, Cheung AS (2020) Late-onset hypogonadism: metabolic impact. Andrology 8(6):1519–1529
Corona G, Mannucci E, Forti G, Maggi M (2009) Following the common association between testosterone deficiency and diabetes mellitus, can testosterone be regarded as a new therapy for diabetes? Int J Androl 32(5):431–441
Corona G, Giorda CB, Cucinotta D, Guida P, Nada E (2014) Sexual dysfunction at the onset of type 2 diabetes: the interplay of depression, hormonal and cardiovascular factors. J Sex Med 11(8):2065–2073
Haider KS, Haider A, Saad F, Doros G, Hanefeld M, Dhindsa S et al (2020) Remission of type 2 diabetes following long-term treatment with injectable testosterone undecanoate in patients with hypogonadism and type 2 diabetes: 11-year data from a real-world registry study. Diabetes Obes Metab 22(11):2055–2068
Yassin A, Haider A, Haider KS, Caliber M, Doros G, Saad F et al (2019) Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: eight-year data from a registry study. Diabetes Care 42(6):1104–1111
Corona G, Rastrelli G, Vignozzi L, Barbonetti A, Sforza A, Mannucci E et al (2021) The role of testosterone treatment in patients with metabolic disorders. Expert Rev Clin Pharmacol 41:1091–1103
Vanderschueren D, Pye SR, Venken K, Borghs H, Gaytant J, Huhtaniemi IT et al (2010) Gonadal sex steroid status and bone health in middle-aged and elderly European men. Osteoporos Int 21(8):1331–1339
Greendale GA, Edelstein S, Barrett-Connor E (1997) Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 12(11):1833–1843
Bjørnerem A, Ahmed LA, Joakimsen RM, Berntsen GK, Fønnebø V, Jørgensen L et al (2007) A prospective study of sex steroids, sex hormone-binding globulin, and non-vertebral fractures in women and men: the Tromso Study. Eur J Endocrinol 157(1):119–125
Bjørnerem A, Emaus N, Berntsen GK, Joakimsen RM, Fønnebø V, Wilsgaard T et al (2007) Circulating sex steroids, sex hormone-binding globulin, and longitudinal changes in forearm bone mineral density in postmenopausal women and men: the Tromsø study. Calcif Tissue Int 81(2):65–72
Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, Hahn CW et al (2016) Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Investig 126(3):1114–1125
Finkelstein JS, Lee H, Burnett-Bowie SM, Darakananda K, Gentile EC, Goldstein DW et al (2020) Dose-response relationships between gonadal steroids and bone, body composition, and sexual function in aging men. J Clin Endocrinol Metab 105(8):2779–2788
Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA et al (2006) Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 91(10):3908–3915
Corona G, Torres LO, Maggi M (2020) Testosterone therapy: what we have learned from trials. J Sex Med 17(3):447–460
Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A et al (2005) Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 63(3):280–293
Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E et al (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25(8):1886–1894
Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S (2010) A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res 25(12):2558–2571
Hansen S, Hauge EM, Beck Jensen JE, Brixen K (2013) Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res 28(4):736–745
Samelson EJ, Broe KE, Xu H, Yang L, Boyd S, Biver E et al (2019) Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol 7(1):34–43
Al Mukaddam M, Rajapakse CS, Bhagat YA, Wehrli FW, Guo W, Peachey H et al (2014) Effects of testosterone and growth hormone on the structural and mechanical properties of bone by micro-MRI in the distal tibia of men with hypopituitarism. J Clin Endocrinol Metab 99(4):1236–1244
Aversa A, Caprio M, Antelmi A, Armani A, Brama M, Greco EA et al (2011) Exposure to phosphodiesterase type 5 inhibitors stimulates aromatase expression in human adipocytes in vitro. J Sex Med 8(3):696–704
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any study with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Corona, G., Vena, W., Pizzocaro, A. et al. Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J Endocrinol Invest 45, 911–926 (2022). https://doi.org/10.1007/s40618-021-01702-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01702-5