Abstract
Purpose
Patients with hypoparathyroidism are at risk of vertebral fractures (VFs) despite high bone mineral density (BMD). We investigated this paradox by assessing trabecular bone score (TBS) and hip structural analysis (HSA) in non-surgical chronic hypoparathyroidism (cHypoPT) with and without VFs.
Methods
152 cHypoPT patients (age 40.2 ± 13.4 years, M: F = 81:71) with a median follow-up of 8 (2–13) years were assessed for BMD, VFs, TBS, and HSA and compared with 152 healthy controls. VFs at T7–L4 were assessed by Genant’s method. Average serum total calcium and phosphorus during follow-up were assessed.
Results
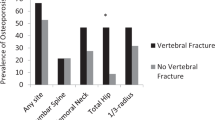
The lumbar spine and hip BMD were higher by 25.4 and 13.4% in cHypoPT than controls (P < 0.001). Paradoxically, VFs (30.9 vs.7.9%), including multiple (12.5 vs. 2.6%) were higher in cHypoPT (P < 0.001). Though overall average TBS (1.411 ± 0.091) was normal in cHypoPT, 25.4% of the females had subnormal TBS, more in post than pre-menopausal women (52.3 vs. 14%, P = 0.002) and as compared to males (6.1%, P = 0.001). TBS correlated with menopausal status and follow-up serum calcium–phosphorus product. For every gm/cm2 rise in BMD, TBS increase was only 0.227 in cHypoPT compared to 0.513 in controls. Frequency of VFs increased with declining TBS (P = 0.004). HSA was comparable between cHypoPT with and without VFs. 23.4% of cHypoPT with VFs had subnormal TBS.
Conclusion
31% of cHypoPT patients had VFs. TBS indicated degraded bone microarchitecture in 50% of the post-menopausal cHypoPT women. However, TBS has limitations to detect abnormal bone microarchitecture in cHypoPT as only one-fourth of patients with VFs showed low TBS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with hypoparathyroidism have a high bone mineral density (BMD), which is further increased during long-term follow-up [1,2,3]. Paradoxically, these patients are at increased risk for vertebral fracture (VFs), especially post-menopausal women or those on antiepileptic medications [3, 4]. Interestingly BMD is not a significant predictor for VFs in hypoparathyroidism [3, 4]. A parallel situation observed in type 2 diabetes, Cushing’s disease, and acromegaly has been explained by their poor bone microarchitecture [5,6,7]. Yamamoto et al. showed that poor spinal microarchitecture, as indicated by ‘trabecular bone score’ (TBS), was a more dominant factor for predicting VFs in type 2 diabetes than the decreased BMD [5]. TBS is a non-invasive tool assessing bone microarchitecture and predicts fragility separate from BMD [8,9,10].
There are only two studies to date assessing the relationship of TBS with VFs in hypoparathyroidism [4, 11]. Sakane et al. observed overall a high TBS in hypoparathyroidism, but a low TBS in six patients with a fragility fracture [11]. In contrast, Cipriani et al. observed low TBS in post-menopausal hypoparathyroid women with no difference in patients with and without VFs [4]. A variable relationship between TBS and VFs could be due to a limited number of patients assessed. We have been investigating a large cohort of patients with non-surgical chronic hypoparathyroidism (cHypoPT) for their etio-pathogenesis [12,13,14,15,16,17,18,19]. Their unique skeletal features including the occurrence of parathyroid spondyloarthropathy, higher BMD, and increased risk of VFs were described earlier [2, 3, 17]. Here, we assessed the relationship between TBS and VFs in a cohort of 152 male and female patients with cHypoPT. We also assessed hip structure analysis (HSA) for bone strength and its relation to VFs.
Materials and methods
The study participants were 152 patients with cHypoPT attending endocrine clinics of the All India Institute of Medical Sciences (Delhi, India) from 2017 to 2020. These patients were part of a larger cohort of 250 patients with cHypoPT being enrolled and managed since 1998. The diagnosis of hypoparathyroidism was based on hypocalcemia with low or inappropriately normal serum intact parathyroid hormone (iPTH) [20, 21]. Various biochemical parameters, including serum total calcium, phosphate, creatinine, presence of cataract, intracranial calcification, and seizures, were recorded for each patient at their initial presentation [16]. Patients were prescribed 0.5 to 1.0 µg of alfacalcidol/day, along with 1.0 to 2.0 gm of elemental calcium. The daily dose of alfacalcidol was increased to a maximum of 3.0 µg to achieve optimal calcemic control [22]. None of them were on phosphate binders, magnesium supplements, or thiazides. They were followed up at three-month intervals to monitor serum total calcium and phosphorus and urine calcium excretion. Patients with postsurgical hypoparathyroidism were not part of the study cohort. None of the patients included had clinical features of autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. Morning fasting serum cortisol and plasma ACTH were normal in all.
Pregnant or lactating women, patients aged < 20 years, and those with BMI > 37 kg/m2 were not included. Several patients included in this study had participated in our earlier studies related to BMD and VFs in cHypoPT [2, 3]. Average serum total calcium, phosphate, and their product and presence of intracranial calcification, cataract, and coexistent autoimmune illnesses were noted from their clinical records. Patients were called on a pre-scheduled date for measurement of BMD, TBS, VF assessment (VFA), and HSA [10, 23,24,25]. Biochemical parameters were measured on the same day.
Bone mineral density
BMD was measured using dual-energy X-ray absorptiometry (DXA) (Discovery A 84,023; Hologic Inc., MA, USA) at the lumbar spine (L1–L4), left hip, and non-dominant forearm, following guidelines of the International Society for Clinical Densitometry (ISCD) [10]. The precision was measured by testing BMD twice in a set of 30 healthy controls using the ISCD precision tool (www.iscd.org). The coefficient of variation (CV) of the precision error at the lumbar spine, hip, neck of the femur, and total forearm was 0.69, 1.73, 1.40, and 1.12%, respectively. The CV at 95% confidence for the least significant change at the corresponding sites was 1.9, 4.8, 3.9, and 3.1% respectively. Lumbar vertebrae showing fractures were excluded from the BMD analysis. Z scores for lumbar BMD were analyzed for cHypoPT patients < 50 years of age.
Trabecular bone score
TBS was measured using iNsight software (version 3.0.2.0, Med-Imaps, Bordeaux, France) installed on a DXA machine and calibrated with a TBS-specific phantom. It analyzes the gray-level texture using pixels of the DXA images of the L1–L4 spine obtained by scanning time of 30 s per patient. TBS represents the average TBS of L1–L4 and is a unit-less measurement without gender effect [9]. If a lumbar vertebra was excluded during BMD analysis, the corresponding vertebra was also excluded from the TBS. The TBS > 1.310 reflects denser trabeculae with good microarchitecture connectivity. TBS < 1.230 indicates degraded microarchitecture with higher susceptibility to VFs. A score between 1.310 and 1.230 indicates partially degraded microarchitecture with a moderate risk of VFs [4, 9]. The CV for TBS was 1.39%, with the least significant change at 95% confidence being 0.038.
Assessment of vertebral fracture
VFs were assessed with patient in the supine position and rotation of the arm of the DXA machine. The VFA software calculated the deformity and severity of VFs based on six points marked on the anterior, posterior, and middle of the superior and inferior surface of each vertebra. These points were reviewed by an expert radiologist to exclude developmental variation, degenerative changes, and syndesmophytes [3, 17]. Wedge and biconcave deformities were calculated from the ratios of anterior and posterior heights of the vertebrae. Crush deformity was calculated using ratios of anterior, mid, and posterior heights of the adjacent vertebrae. The severity of VFs was graded as per Genant’s method, with grade 1 deformity being 20 to 24.9% reduction, grade 2 = 25 to 39.9%, and grade 3 = 40% or more [26]. VFA was limited to T7 to L4 vertebrae to maximize the stability of the results. T4 to T6 vertebrae were not analyzed because of their poor visualization in several patients due to overlapping with scapula and ribs [23, 27].
Hip structural analysis (HSA)
The hip structure analysis (HSA) assessed bone strength based on its geometric properties of the hip region using the APEX software of the DXA machine. The regions assessed were: (1) narrowest part of the femoral neck (NN), (2) femoral shaft (FS) at 2.0 cm distal to the midpoint of the lesser trochanter, and (3) inter-trochanter (IT). These three areas have differing cortical bone content of 60, 100, and 70%, respectively [24]. Cross-sectional area (CSA), cross-sectional moment of inertia (CSMI), cortical thickness (CTh), section modulus (SM), and bucking ratio (BR) were measured at each region [24]. Briefly, CSA indicates the total bone surface area after subtracting the area of voids, spaces, and marrow cavity. The CSMI indicates mass in the center of bone proportional to its structural rigidity. SM (Z) indicated the bending stress in a cross section at the maximum distance from the neutral axis. BR represented the ratio of the outer radius of the bone to the wall thickness in a tubular cross section. Bone with high BR indicates lower cortical stability. Thus, a higher value of CSA, CSMI, and SM but lower BR would be advantageous against fracture [24]. The CVs of these variables in the current study at NN were 2.1, 4.8, 3.6%, and 3.8%, respectively.
To understand the association of TBS with serum total calcium, phosphorus, and their product, the values were analyzed (1) during current study, (2) at initial presentation to the endocrine clinic, and (3) during follow-up.
The average of various biochemical parameters during follow-up was calculated using measurements available for each patient from their initial enrollment in the endocrine clinics till the present study.
Controls
Controls were selected from a large number of healthy subjects undergoing DXA to assess TBS and its relationship with vitamin D status at our center from 2019 to 2021. Subjects with a history of diabetes mellitus, blood glucose value > 7.8 mmol/L, and TSH > 10.0 µIU/ml were not included. Gender and age-matched controls (± two for ≤ 50 years and ± 5 years for those > 50 years) were included in a 1:1 ratio with cHypoPT patients. Their mean iPTH, serum total calcium, and phosphorus were within the normal range.
The study was approved by Institute Ethics Committee (IECPG-170/19.04.2018). Written consent was obtained from patients and controls after an explanation of the study purpose and investigations.
Biochemical parameters
The serum total calcium and inorganic phosphorus were measured on Hitachi 917, Roche, Germany (normal range 2.03 to 2.60 mmol/L and 0.81 to 1.45 mmol/L, respectively). The intra-assay and inter-assay coefficients of variation were 3.5 to 5.0%. Serum iPTH was measured by chemiluminescence (Elecsys-2010, Roche, Germany; normal range, 15–65 ng/L). Serum 25(OH) D was measured by chemiluminescence (LAISON, DiaSorin, Inc., MN) with the coefficient of variation being 2.9–5.5%.
Statistical analysis
Data are presented as mean ± SD, median with interquartile range (IQR), and frequencies (%). The Student’s t test was performed for intergroup comparison of continuous variables with normal distribution. Mann–Whitney U test was performed for parameters that were not normally distributed. Fisher’s exact test was carried out to assess differences in the frequencies. The linear trend in the proportion of cases with VFs in the ordered categories of TBS was examined by a nonparametric trend test. Multivariable regression analysis was performed for VFs and TBS using relevant factors found to be significant in the univariate analysis, i.e., gender, duration of illness from the onset of symptoms, use of anticonvulsant at any time, age at current study, BMI, BMD lumbar spine, TBS, menopausal status, serum 25(OH)D, average serum total calcium, phosphorus and serum calcium–phosphorus product during follow-up. All analyses were performed using SPSS, version 20.0 (IBM, Armonk, NY). A two-tailed P value < 0.05 was considered significant.
Results
One hundred and seventy two patients with cHypoPT attended the endocrine clinics during the study period. 20 of them were not assessed for TBS due to: age < 20 years or > 75 yearrs (n = 16), and severe spine deformity, BMI > 37 kg/m2, > 2 lumbar vertebrae fractured, and pregnancy (n = 1 each for all). Finally, 152 patients (males 81, F = 71) were analyzed. Their clinical and biochemical characteristics at the initial presentation are shown in (Table 1). Twenty-one females were post-menopausal, and 24 males were ≥ 50 years of age in the current study (Table 1). Overall, 58 patients had used anticonvulsants for seizures, which were withdrawn in 21 of them in the past [28]. Fifteen patients had primary hypothyroidism but were euthyroid on thyroxine. Four patients were on a gluten-free diet for celiac disease. Other coexistent autoimmune illnesses were alopecia areata, alopecia totalis, vitiligo, and pulmonary alveolar proteinosis (n = 1, each) [29].
The mean age and BMI of the 152 controls (39.3 ± 12.2 years and 25.8 ± 3.76 kg/m2) were comparable to that of the cHypoPT group (P = 0.57 and 0.06). The mean serum total for calcium, phosphorus, and intact PTH of controls was in normal range (2.33 ± 0.13 mmol/L, 1.20 ± 0.16 mmol/L, and 55.6 ± 23.7 ng/L, respectively).
BMD and VFA in hypoparathyroidism and controls
The mean BMD at the lumbar spine, total hip, femoral neck, and trochanter was significantly higher in cHypoPT than in the controls (Table 2). The average BMD at lumbar spine and hip was higher by 25.4 and 13.4% in cHypoPT (P < 0.001 for both). The mean BMD values at the three forearm regions were comparable between cHypoPT and controls.
None of the cHypoPT patients had Z score ≤ − 2.0. In contrast, 17 of the 152 controls (11.2%) had Z score ≤ − 2.0 (P < 0.001). Paradoxically, the prevalence of VFs was higher in cHypoPT than controls (30.9 vs. 7.9%, P < 0.001). Frequency of multiple VFs was also higher in cHypoPT than controls (12.5 vs. 2.6%, P < 0.001).
TBS in hypoparathyroidism and controls
The mean TBS was higher in cHypoPT than controls (1.411 ± 0.091 vs. 1.334 ± 0.093, P < 0.001, Table 2). The mean age at presentation to the clinic was higher in cHypoPT patients with degraded TBS than those with normal TBS (37.4 ± 14.9 vs. 31.6 ± 11.4 years, P = 0.03). However, the median duration of illness was comparable in cHypoPT patients with and without degraded TBS [13.5 (10–23) vs. 13.2 (9–19) years, P = 0.61].
The mean TBS was significantly less in females than the males with cHypoPT (1.386 ± 0.102 vs. 1.434 ± 0.074, P = 0.002). Postmenopausal cHypoPT women tended to have lower mean TBS than the pre-menopausal cHypoPT (1.343 ± 0.122 vs. 1.404 ± 0.088, P = 0.047, Fig. 1). 15.1% of the cHypoPT patients had degraded or partially degraded microarchitecture. The frequency of such degraded microarchitecture was higher in females than in males with cHypoPT (25.4 vs. 6.1%, P = 0.001). The proportion of degraded and partially degraded microarchitecture was also high in post than pre-menopausal cHypoPT (52.3 vs. 14.0%, P = 0.002). In contrast, the mean TBS and frequency of degraded TBS were comparable between males cHypoPT < 50 years and ≥ 50 years (1.443 ± 0.068 vs. 1.412 ± 0.086, P = 0.09 and; 3.5 vs. 12.5%, P = 0.15).
Hip structural analysis in hypoparathyroidism
The mean CSA and CTh were higher, whereas BR was lower in the cHypoPT than the controls at NN, IT, and FS (P < 0.01 for all sites, data for NN shown in Table 2). The mean CSMI and SM were higher in the cHypoPT group at the NN and IT region (P < 0.01). The mean neck–shaft angle was comparable between cHypoPT and the controls (127.0° ± 6.1° vs. 125.9° ± 5.8°, P = 0.10). However, there was no significant difference in various HSA parameters at all sites in the cHypoPT group with and without VFs. Similarly, HSA was comparable between pre- and post-menopausal cHypoPT.
Factors determining TBS in hypoparathyroidism
On univariate regression analysis, TBS showed a significant inverse association with female gender (P = 0.001), post-menopausal status (P < 0.001) and calcium–phosphorus product during follow-up (P = 0.03). There was significant correlation between TBS and lumbar spine BMD in the cHypoPT (r = 0.568, P < 0.001) and controls (r = 0.639, P < 0.001). There was no significant association of TBS with age at the current study, BMI, duration of illness, use of anticonvulsant, and follow-up serum total calcium and phosphorus and serum 25(OH)D measured during the current study.
On multivariable regression analysis, menopausal status and higher calcium–phosphorus product during follow-up were the significant determinants of subnormal TBS in cHypoPT (P < 0.01 and 0.02, Table 4). For every 5 year increase in duration of menopause, the risk of impaired TBS (< 1.310) increased by fivefold. For every 1 mmol2/L2 increase in mean follow-up serum calcium–phosphate product, the odds of impaired TBS increased by 9.1-fold. Lumbar BMD was the significant positive predictor of TBS (P < 0.001). Linear regression showed that for every gm/cm2 rise in lumbar spine BMD, the TBS increased by 0.513 in controls but only 0.227 in cHypoPT.
Factors determining VFs in hypoparathyroidism
The mean TBS, frequency of degraded TBS, HSA in cHypoPT with (n = 47) and without (n = 105) VFs are given in (Table 3). The proportion with degraded and partially degraded TBS tended to be higher in patients with VFs than without VFs (23.4 vs. 11.4%, P = 0.08). The difference in proportion with degraded TBS between patients with and without VFs was more apparent when only grade 2 and 3 VFs were considered, excluding milder grade 1 (35.0 vs. 11.6%, P = 0.01). There was a linear trend of increased frequency of VFs with a decrease in TBS (P = 0.04, Fig. 2). This linear trend was more significant when only moderate and severe VFs were considered (P = 0.004). Though univariate analysis showed significantly lower total and mid-forearm BMD in cHypoPT patients with VFs, these were not the significant determinants of VFs on multivariable regression analysis. Menopausal status was the only significant predictor of VFs in cHypoPT. For every 5 year increase in the duration of menopause, the risk of vertebral fracture increased by 1.6-fold (Table 4).
Discussion
Seeman et al. in 1982, first reported increased BMD of the lumbar spine in patients with surgical hypoparathyroidism [30]. Subsequently, a similar increase in BMD was also observed in cHypoPT [2]. Interestingly, Rubin et al. and Gafini et al. observed poor bone microarchitecture on histomorphometry in chronic hypoparathyroidism [31, 32]. The overall impact of increased BMD but poor microarchitecture on VFs is an area of current interest [1, 3, 33,34,35].
This study showed that with an average of 23% higher lumbar BMD, one-third of cHypoPT had VFs. These fractures were clinically asymptomatic and more prevalent in post-menopausal women. Though lumbar spine BMD and TBS showed a good correlation, the latter provided more information on bone health in cHypoPT. This was indicated by normal BMD Z scores in all the cHypoPT but degraded bone microarchitecture in 15.1% of the cHypoPT group which increased to 50% of post-menopausal cHypoPT. The present study also revealed a high prevalence of VFs (42.9%) in post-menopausal females. The overall trend of increasing VFs with a decreasing TBS further supported the role of poor TBS in the VFs in cHypoPT. Previously, Sakane et al. reported a similar low prevalence of osteoporosis on BMD (2.9%) but subnormal TBS in up to 30% of hypoparathyroid cases [11]. Iglesias et al. also observed lower TBS in postsurgical hypoparathyroidism [36]. In contrast, Cipriani et al. reported high TBS in hypoparathyroidism with comparable TBS between pre- and post-menopausal women [37]. The lack of differences in the TBS between pre- and post-menopausal hypoparathyroid cases observed by Cipriani et al. could be due to an age gap of only eight years between pre- and post-menopausal women compared to the 23 years in the present study [37].
This study also showed that males had higher TBS with a four-fold lower prevalence of degraded TBS than females. However, unlike females, they showed no age-related decline in TBS after 50 years. There is no previous study assessing the relationship between TBS and VFs including a wide age spectrum of cHypoPT, i.e., young and old males and females with cHypoPT.
Thus, the trend of bone fragility in cHypoPT better indicated by abnormal TBS than BMD is akin to that reported in type 2 diabetes, endogenous or exogenous Cushing syndrome, and acromegaly [5,6,7, 38,39,40]. Vinolas et al. observed an association of bone fragility fractures in Cushing’s disease with low TBS rather than BMD [6]. Calatayud et al. observed normal lumbar BMD in 73% of patients with acromegaly but normal TBS in only 38% of them [7]. Similarly, Hong et al. observed lower TBS in acromegaly patients than in healthy controls despite comparable BMD values [38].
In this study, menopausal years and higher calcium–phosphorus product during follow-up were significant determinants of subnormal TBS in cHypoPT. Lower TBS in post-menopausal cHypoPT could reflect deteriorating bone microarchitecture due to declining estrogen levels. The inverse relation of calcium–phosphorus product with TBS indicates the possible effect of excess serum phosphate on TBS and thus the importance of better serum phosphate control in the management of hypoparathyroidism. However, this needs to be further assessed as serum phosphate alone was not the significant predictor of TBS.
The present study revealed another interesting aspect of bone health in cHypoPT, i.e., a nearly 50% lower rise in TBS with rising BMD in cHypoPT than in healthy controls. Though the current study was not designed to investigate this lower rise of TBS in cHypoPT, it could reflect their low bone turnover and inappropriate trabecular mineralization due to PTH deficiency in hypoparathyroidism [41]. Regression analysis of VFs revealed a significant association with menopausal status but not with TBS. However, both menopausal status and calcium–phosphorus product during follow-up were the significant determinants of TBS. This indicated a complex interlink of multiple factors determining the VFs in cHypoPT.
This study also analyzed HSA in cHypoPT and its relationship with VFs. There is only one previous case–control study on HSA in 31 hypoparathyroidism females [42]. Park et al. observed high CSA and CTh and low BR in hypoparathyroidism at the femoral neck and trochanter region. Paradoxically, at the femoral shaft, CSA and CTh were low whereas BR was high [42]. The above study did not assess VFs in hypoparathyroidism. In the present study, the mean CSA and CTh were higher, and BR was lower in cHypoPT than in controls at all sites. However, parameters of HSA were comparable between patients with and without VFs or with menopausal status. The lack of correlation of HSA with VFs in cHypoPT could either be due to the different content of cancellous and cortical bone at vertebral and hip regions. While vertebral bone is 75% trabecular and 25% cortical, this ratio in the femoral head is 50:50 [43]. It is possible that higher CSA and CTh at the hip might be advantageous to patients with hypoparathyroidism against hip fractures. There is no systematic information to date on the prevalence of hip fractures in hypoparathyroidism.
The limitation of this study was the inability to include controls with matched serum 25(OH)D values who were using calcium and vitamin D therapy for a long duration akin to that of the cHypoPT group. However, serum 25(OH)D was not a significant determinant of TBS or VFs in cHypoPT in this study. Besides, all the patients in this study were on conventional therapy and their calcemic control was intermittently suboptimal [20, 44]. A subset of patients was also using anticonvulsants for control of hypocalcemic seizures. The relationship between intermittent calcium control, seizures, related injury, antiepileptic therapy, and TBS could not be analyzed in the present study due to limited number of such cases and variability in the long-term calcemic control. Theoretically, all these factors could enhance the risk of VFs in hypoparathyroidism.
Thus, the present study showed that despite high BMD, patients with cHypoPT had a four-fold higher prevalence of VFs. TBS was subnormal in 50% of the post-menopausal cHypoPT women and one-fourth of the cHypoPT cases with VFs. Thus, impaired TBS provides a biological basis for increased VFs in cHypoPT, at least in one-fourth of cases. However, TBS has a limitation to detect abnormal bone microarchitecture in cHypoPT as the majority of patients with VFs had normal TBS.
Data availability
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cipriani C, Abraham A, Silva BC, Cusano NE, Rubin MR, McMahon DJ, Zhang C, Hans D, Silverberg SJ, Bilezikian JP (2017) Skeletal changes after restoration of the euparathyroid state in patients with hypoparathyroidism and primary hyperparathyroidism. Endocrine 55:591–598
Laway BA, Goswami R, Singh N, Gupta N, Seith A (2006) Pattern of bone mineral density in patients with sporadic idiopathic hypoparathyroidism. Clin Endocrinol (Oxford) 64:405–409
Chawla H, Saha S, Kandasamy D, Sharma R, Sreenivas V, Goswami R (2017) Vertebral fractures and bone mineral density in patients with idiopathic hypoparathyroidism on long-term follow-up. J Clin Endocrinol Metab 102:251–258
Cipriani C, Minisola S, Bilezikian JP, Diacinti D, Colangelo L, Piazzolla V, Angelozzi M, Nieddu L, Pepe J, Diacinti D (2021) Vertebral fracture assessment in postmenopausal women with postsurgical hypoparathyroidism. J Clin Endocrinol Metab 106:1303–1311
Yamamoto M, Yamauchi M, Sugimoto T (2019) Prevalent vertebral fracture is dominantly associated with spinal microstructural deterioration rather than bone mineral density in patients with type 2 diabetes mellitus. PLoS ONE 14(9):e0222571
Vinolas H, Grouthier V, Mehsen-Cetre N, Boisson A, Winzenrieth R, Schaeverbeke T, Mesguich C, Bordenave L, Tabarin A (2018) Assessment of vertebral microarchitecture in overt and mild Cushing’s syndrome using trabecular bone score. Clin Endocrinol (Oxf) 8:148–154
Calatayud M, Pérez-Olivares Martín L, Librizzi MS, Lora Pablos D, González Méndez V, Aramendi Ramos M, Martínez Diaz-Guerra G, Hawkins F (2021) Trabecular bone score and bone mineral density in patients with long-term controlled acromegaly. Clin Endocrinol (Oxford) 95:58–64
Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26:2762–2769
McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJM, Fujita Y, Glüer CC, Goltzman D, Iki M, Karlsson M, Kindmark A, Kotowicz M, Kurumatani N, Kwok T, Lamy O, Leung J, Lippuner K, Ljunggren Ö, Lorentzon M, Mellström D, Merlijn T, Oei L, Ohlsson C, Pasco JA, Rivadeneira F, Rosengren B, Sornay-Rendu E, Szulc P, Tamaki J, Kanis JA (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Min Res 31:940–948
Krohn K, Schwartz EN, Chung YS, Lewiecki EM (2019) Dual-energy X-ray absorptiometry monitoring with trabecular bone score: 2019 ISCD official position. J Clin Densitom 22:501–505
Sakane EN, Vieira MCC, Lazaretti-Castro M, Maeda SS (2019) Predictors of poor bone microarchitecture assessed by trabecular bone score in postsurgical hypoparathyroidism. J Clin Endocrinol Metab 104:5795–5803
Goswami R, Bhatia M, Goyal R, Kochupillai N (2002) Reversible peripheral neuropathy in idiopathic hypoparathyroidism. Acta Neurol Scand 105:128–131
Goswami R, Brown EM, Kochupillai N, Gupta N, Rani R, Kifor O, Chattopadhyay N et al (2004) Prevalence of calcium sensing receptor autoantibodies in patients with sporadic idiopathic hypoparathyroidism. Eur J Endocrinol 150:9–18
Goswami R, Sharma R, Sreenivas V, Gupta N, Ganapathy A, Das S (2012) Prevalence and progression of basal ganglia calcification and its pathogenic mechanism in patients with idiopathic hypoparathyroidism. Clin Endocrinol (Oxford) 77:200–206
Saha S, Gantyala SP, Aggarwal S, Sreenivas V, Tandon R, Goswami R (2017) Long-term outcome of cataract surgery in patients with idiopathic hypoparathyroidism and its relationship with their calcemic status. J Bone Minel Metab 35:405–411
Saha S, Kandasamy D, Sharma R, Bal C, Sreenivas V, Goswami R (2020) Nephrocalcinosis, renal dysfunction, and calculi in patients with primary hypoparathyroidism on long-term conventional therapy. J Clin Endocrinol Metab 105(4):dgz319
Goswami R, Ray D, Sharma R, Tomar N, Gupta R, Gupta N, Sreenivas V (2008) Presence of spondyloarthropathy and its clinical profile in patients with hypoparathyroidism. Clin Endocrinol (Oxford) 68:258–263
Tomar N, Bora H, Singh R, Gupta N, Kaur P, Chauhan SS, Sharma YD, Goswami R (2010) Presence and significance of a R110W mutation in the DNA-binding domain of GCM2 gene in patients with isolated hypoparathyroidism and their family members. Eur J Endocrinol 162:407–421
Tomar N, Gupta N, Goswami R (2013) Calcium-sensing receptor autoantibodies and idiopathic hypoparathyroidism. J Clin Endocrinol Metab 98:3884–3891
Shoback D (2008) Clinical practice hypoparathyroidism. New England J Med 359:391–403
Gafni RI, Collins MT (2019) Hypoparathyroidism. N Engl J Med 380:1738–1747
Saha S, Goswami R (2019) Auditing the efficacy and safety of alfacalcidol and calcium therapy in idiopathic hypoparathyroidism. J Clin Endocrinol Metab 104:1325–1335
Zeytinoglu M, Jain RK, Vokes TJ (2017) Vertebral fracture assessment: enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone 104:54–65
Broy SB, Cauley JA, Lewiecki ME, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 1: hip geometry. J Clin Densitom 18:287–308
Hong AR, Lee JH, Kim JH, Kim SW, Shin CS (2019) Effect of endogenous parathyroid hormone on bone geometry and skeletal microarchitecture. Calcif Tissue Int 104:382–389
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Malgo F, Hamdy NAT, Ticheler CHJM, Smit F, Kroon HM, Rabelink TJ, Dekkers OM, Appelman-Dijkstra NM (2017) Value and potential limitations of vertebral fracture assessment (VFA) compared to conventional spine radiography: experience from a fracture liaison service (FLS) and a meta-analysis. Osteoporos Int 28:2955–2965
Modi S, Tripathi M, Saha S, Goswami R (2014) Seizures in patients with idiopathic hypoparathyroidism: effect of antiepileptic drug withdrawal on recurrence of seizures and serum calcium control. Eur J Endocrinol 170:777–783
Saha S, Madan K, Jain D, Goswami R (2019) Pulmonary alveolar proteinosis (PAP) in idiopathic hypoparathyroidism. BMJ Case Rep. 12(11):e231053. https://doi.org/10.1136/bcr-2019-231053
Seeman E, Wahner HW, Offord KP, Kumar R, Johnson WJ, Riggs BL (1982) Differential effects of endocrine dysfunction on the axial and the appendicular skeleton. J Clin Investig 69:1302–1309
Rubin MR, Zhou H, Cusano NE, Majeed R, Omeragic B, Gomez M, Nickolas TL, Dempster DW, Bilezikian JP (2018) The effects of long-term administration of rhPTH(1–84) in hypoparathyroidism by bone histomorphometry. J Bone Miner Res 33:1931–1939
Gafni RI, Brahim JS, Andreopoulou P, Bhattacharyya N, Kelly MH, Brillante BA, Reynolds JC, Zhou H, Dempster DW, Collins MT (2012) Daily parathyroid hormone 1–34 replacement therapy for hypoparathyroidism induces marked changes in bone turnover and structure. J Bone Miner Res 27:1811–1820
Mendonça ML, Pereira FA, Nogueira-Barbosa MH, Monsignore LM, Teixeira SR, Watanabe PC, Maciel LM, de Paula FJ (2013) Increased vertebral morphometric fracture in patients with postsurgical hypoparathyroidism despite normal bone mineral density. BMC Endocr Disord 13:1
Formenti AM, Tecilazich F, Giubbini R, Giustina A (2019) Risk of vertebral fractures in hypoparathyroidism. Rev Endocr Metab Disord 20:295–302
Muschitz C, Kocijan R, Haschka J, Pahr D, Kaider A, Pietschmann P, Hans D, Muschitz GK, Fahrleitner-Pammer A, Resch H (2015) TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone 79:259–266
Iglesias SG, Dominguez MLM, Herrero EF, Martinez-Pueyo JI, Arroba CM, Diaz-Guerra GM, Hawkins Carranza F (2019) Trabecular bone score and bone mineral density in patients with postsurgical hypoparathyroidism after total thyroidectomy for differentiated thyroid carcinoma. Surgery 165:814–819
Cipriani C, Pepe J, Silva BC, Rubin MR, Cusano NE, McMahon DJ, Nieddu L, Angelozzi M, Biamonte F, Diacinti D, Hans D, Minisola S, Bilezikian JP (2018) Comparative effect of rhPTH(1–84) on bone mineral density and trabecular bone score in hypoparathyroidism and postmenopausal osteoporosis. J Bone Miner Res 33:2132–2139
Hong AR, Kim JH, Kim SW, Kim SY, Shin CS (2016) Trabecular bone score as a skeletal fragility index in acromegaly patients. Osteoporos Int 27:1123–1129
Florez H, Hernández-Rodríguez J, Carrasco JL, Prieto-González S, Muxi A, Filella X, Ruiz-Gaspà S, Gómez-Puerta JA, Cid M, Espinosa G, Monegal A, Guañabens N, Peris P (2020) Vertebral fracture risk in glucocorticoid-induced osteoporosis: the role of hypogonadism and corticosteroid boluses. RMD Open 6(2):e001355
Gao Y, Wang O, Guan W, Wu X, Mao J, Wang X, Yu W, Nie M (2021) Bone mineral density and trabecular bone score in patients with 21-hydroxylase deficiency after glucocorticoid treatment. Clin Endocrinol (Oxford) 94:765–773
Szulc P (2020) Biochemical bone turnover markers in hormonal disorders in adults: a narrative review. J Endocrinol Invest 43(10):1409–1427
Park HS, Seo DH, Rhee Y, Lim SK (2017) Site-specific difference of bone geometry indices in hypoparathyroid patients. Endocrinol Metab (Seoul) 32:68–76
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 3:S131–S139
Hamdy N, Decallonne B, Evenepoel P, Gruson D, van Vlokhoven-Verhaegh L (2021) Burden of illness in patients with chronic hypoparathyroidism not adequately controlled with conventional therapy: a Belgium and the Netherlands survey. J Endocrinol Invest 44(7):1437–1446
Acknowledgements
The help of Mr. Arun Chandran in measuring biochemical parameters and Mr. Sushil in DXA is acknowledged. The help of Mr. Ram Sumer and Mr. Rambabu in assisting patients is acknowledged.
Funding
This research did not receive any specific grant from a funding agency in the public commercial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All studies were performed according to the declaration of Helsinki, and approved by the Institute Ethics Committee of All India Institute of Medical Sciences, New Delhi, India.
Informed consent
All the participants gave written informed consent prior to participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saha, S., Mannar, V., Kandasamy, D. et al. Vertebral fractures, trabecular bone score and their determinants in chronic hypoparathyroidism. J Endocrinol Invest 45, 1777–1786 (2022). https://doi.org/10.1007/s40618-022-01818-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01818-2