Abstract
Purpose
Screening for vertebral fractures (VF) in primary hyperparathyroidism (PHPT) is recommended, but there are limited data regarding which patients are at greatest risk for VF. We evaluated risk factors for VF in PHPT.
Methods
This is a retrospective cross-sectional analysis of 117 participants with PHPT. We assessed Grades 2 and 3 VF by vertebral fracture assessment (VFA) and the association of VF with the trabecular bone score (TBS), other skeletal parameters and clinical risk factors. VFA was performed only in those who met National Osteoporosis Foundation criteria for VFA screening.
Results
T-scores were in the osteopenic range and TBS was degraded. Overall VF rate based on VFA or other imaging was 12.8%. Serum PTH, calcium and TBS were not associated with VF. Those with VF were older (p = 0.04), had worse renal function (p = 0.04), were more likely to have received osteoporosis treatment (p = 0.03), and tended to have had a prior fracture (p = 0.06). T-scores did not differ by fracture status at any skeletal site. Those with VF had nine times the odds of osteoporosis at the hip (95% CI 2.4–34.5), but this risk factor had low sensitivity (46.7%) for VF. Hip T-score < −2.6, Age > 78.6 years, and GFR < 58.8 ml/min/1.73 m2 (thresholds maximizing sensitivity and specificity) had areas under the curve of 0.60–0.67 for VF (all p < 0.05) and low sensitivity. Findings were similar when analyses were limited to women.
Conclusions
In PHPT, VF risk factors included older age, prior fracture, worse renal function and osteoporosis at the hip, but not osteoporosis at other sites, TBS or biochemical indices of PHPT. Since identified risk factors had low sensitivity and were generally inaccurate for categorizing those with VF, the data do not support limiting screening to PHPT patients with these specific VF risk factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary hyperparathyroidism (PHPT) is a common condition characterized by hypercalcemia and elevated or inappropriately normal parathyroid hormone (PTH). Today, PHPT is usually asymptomatic and detected incidentally in countries where calcium is routinely measured [1]. Despite being asymptomatic, PHPT often causes bone loss that can be detected by dual energy X-ray absorptiometry (DXA). In PHPT, while mean T-scores as measured by DXA are typically in the osteopenic range, there is a preferential loss of bone mineral density (BMD) at skeletal sites rich in cortical bone such as the distal 1/3-radius. In contrast, DXA indicates BMD at skeletal sites composed predominantly of trabecular bone, such as the lumbar spine (LS), are relatively spared [2, 3].
Despite this, most studies indicate increased risk of VF in PHPT compared to age- and sex-matched controls, with data suggesting a prevalence between 12.1% and 43.5% [4,5,6,7,8,9]. Further, new data indicate that many PHPT patients have silent VFs [10]. Visualization of bone microarchitecture using high-resolution peripheral quantitative computed tomography (HRpQCT) has elucidated the paradox of increased VF risk in PHPT despite preserved spine BMD. Studies indicate microstructural deficits in the trabecular and the cortical skeletal compartments leading to reduced whole bone and trabecular stiffness [11, 12].
Although HRpQCT is valuable, it is not widely available. TBS, an indirect index of trabecular microarchitecture, can be obtained using DXA. TBS evaluates pixel gray-level variations in the 2-dimensional spine DXA image. Lower TBS is associated with worse bone microarchitecture (fewer, poorly-connected, and more widely spaced trabeculae) [13]. In prospective studies, TBS predicts VF and nonvertebral fractures in non-PHPT patients, with some studies showing it performs as well as BMD [14,15,16]. TBS added to the predictive value of BMD for fracture in two studies [14, 16]. There are limited data regarding the utility of TBS for identifying those with VFs in PHPT. While two studies found that TBS is associated with prevalent VF in PHPT, both were conducted in Europe. Given that the presentation of PHPT varies geographically, with some regions of the world having more severe, symptomatic PHPT than is typically seen in the United States (US), it is not clear if these results are applicable to US PHPT patients [8, 17].
The 2014 International Guidelines for the Management of Asymptomatic PHPT recommend screening for VFs by obtaining X-ray, computed tomography (CT), magnetic resonance imaging (MRI), or vertebral fracture assessment (VFA) [18]. Parathyroidectomy is recommended if a VF is detected. The guidelines also suggest obtaining TBS if available, although it is not currently used as a criterion for recommending parathyroidectomy. The purpose of this study is to evaluate risk factors for VF in a US cohort with PHPT, including bone quality by TBS, as well as risk factor accuracy for categorizing those with VF. Ultimately, such information could allow spine imaging to be targeted to those most likely to have VFs.
Methods
Design
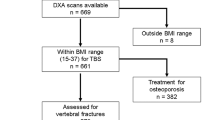
This is a cross-sectional retrospective analysis of 117 patients with PHPT who were evaluated at Columbia University Medical Center (CUMC), a tertiary referral center, between January 1, 2013 and December 31, 2017 and additionally had VFA. During this period, VFA was obtained on all patients having DXA who met 2013 National Osteoporosis Foundation (NOF) screening criteria: women age ≥70 or men ≥80; or individuals 65–69 with a T-score ≤ −1.5; or those ≥50 years with other risk factors (low trauma fracture, height loss, or long-term steroid use) [19]. Those enrolled represent a non-consecutive series of patients because VFA was obtained only on those who met NOF criteria. This study was approved by the CUMC Institutional Review Board.
Participants
We initially identified potential subjects with PHPT via CUMC PHPT registries and the electronic medical record (EMR): those with VFA at CUMC within the study period and International Classification of Diseases (ICD) codes ICD-9 252.01 (PHPT), ICD-9 252.00 (unspecified hyperparathyroidism), ICD-10 E21.0 (primary or familial hyperparathyroidism), or ICD-10 E21.3 (unspecified hyperparathyroidism) in order to broadly screen for potential patients with PHPT.
Records were rigorously reviewed to verify biochemical PHPT criteria. We included those with PHPT, defined as a recurrent pattern of hypercalcemia for ≥1 year with concurrent PTH within or above the normal range (15.1–85.7 pg/ml). We excluded those with normocalcemic PHPT, secondary PHPT, familial hypocalciuric hypercalcemia by history or genetic testing, lacking biochemical data within 6 months of VFA, with a VFA that was uninterpretable (due to scoliosis, bowel gas, etc.) or achieving surgical cure of PHPT before VFA. Patients on thiazides were included if they had confirmed PHPT off thiazides. We did not exclude those with history of osteoporosis medication use as doing so would have biased us against including those at highest risk of VF. 548 potential participants were screened. Four hundred and thirty-one were excluded based on biochemistries or other criteria (parathyroidectomy prior to the VFA).
Clinical data were abstracted from the EMR: demographics, classical fracture risk factors [age, sex, weight, height, prior fracture, glucocorticoid use >3 weeks at physiological doses or above (prednisone equivalent ≥5 mg daily), rheumatoid arthritis], diabetes, estimated PHPT duration (time from onset of hypercalcemia to VFA), osteoporosis medication use (including antiresorptives, teriparatide, raloxifene) for ≥1 year or estrogen use, symptoms of PHPT (urolithiasis, fragility fracture), biochemistries closest to the date of VFA, and BMD and T-scores by DXA at the LS, femoral neck (FN), total hip (TH) and distal 1/3-radius.
DXA and TBS
Areal BMD was measured at the LS (L1–L4), TH, FN, and the distal 1/3-radius using a QDR 4500 (Hologic Inc., Waltham, MA). T-scores were calculated using the manufacturer’s norms. In vivo precision is 1.28%, 1.36%, and 0.70% for the LS, TH and distal 1/3-radius, respectively. Spine TBS was calculated from subjects’ spine DXA image acquired on the day of VFA using TBS iNsight software (version 1.9; medimaps, Geneva, Switzerland) [20]. TBS was classified as degraded (≤1.200), partially degraded (1.200 < TBS < 1.350) or normal (≥1.350) [20]. TBS was assessed in 115 subjects. Spine DXA images could not be retrieved in 2 participants.
VFA
Lateral VFA was acquired from T4 to L5. Subjects were categorized as having VF(s) based on an International Society for Clinical Densitometry-certified densitometrist’s reading of the interpretable image (typically T10-L5) using the Genant semi-quantitative method: mild, moderate, and severe compression fractures were defined as a 20–25, 26–40, or >40% reduction in vertebral height, respectively [21]. The Genant visual semi-quantitative method is the current clinical technique of choice for diagnosing vertebral fracture with VFA. However, only grades 2 and 3 fractures were typically reported as VFs by readers due to VFA’s low specificity for grade 1 VF. At CUMC, equivocal fractures were typically confirmed by spine radiographs. We extracted available spine imaging by other modalities (X-Ray, CT, or MRI) from the EMR. Fifty-five participants (39.3%) had other imaging within 3 years before or 1 year after VFA. Ultimately those categorized as having VF for the analysis comparing those with vs. without VF had fractures identified by either (1) VFA (with equivocal fractures categorized as fractures only if confirmed by another modality) or (2) other imaging.
Biochemistries
Biochemistries were abstracted from the EMR and the value closest to the date of VFA/DXA was utilized. In some cases serum calcium was normal on the date closest to DXA but all patients had a recurrent pattern of hypercalcemia. The majority were performed at CUMC. However, in some cases, biochemistries were measured in outside commercial laboratories. At CUMC, serum calcium (normal: 8.8–10.3 mg/dL), phosphorus (2.5–4.5 mg/dL), and creatinine (0.50–0.95 mg/dL) were measured by auto-analyzer. PTH (15.1–85.7 pg/mL) was measured by two-site IRMA and 25-hydroxyvitamin D (20.0–50.0 ng/mL) was measured by liquid chromatography-tandem mass spectrometry (LC–MS/MS). The glomerular filtration rate was calculated using the modification of diet in renal disease (MDRD) equation [22].
Statistical analysis
Descriptive statistics were expressed as absolute (n) and relative (%) frequency and mean and standard deviation (SD) for categorical and continuous variables. We assessed the normality of all variables with the Shapiro–Wilk test. The Student’s t test, Chi-squared test or Fisher’s exact test were used, as appropriate, to assess between-group differences in normally distributed continuous or categorical variables, respectively. We assessed non-normally distributed continuous variables with the Wilcoxon Rank Sum test (BMI, estimated PHPT duration, calcium and vitamin D intake, serum calcium, serum PTH, BMD/T-scores at the FN and TH). Means and SD were used to describe normally distributed variables while medians and ranges were used for non-normally distributed variables. Comparisons, adjusted for age and/or history of osteoporosis treatment (for ≥1 year), were assessed with analysis of covariance (ANCOVA). The ability of TBS, DXA, etc. to categorize VF was evaluated with receiver operating characteristic curves and calculation of area under the curve (AUC) as well as sensitivity and specificity. Forward logistic regression was used to assess independent predictors of VF. We assessed the whole cohort and women only. SAS Version 9.4 and R 64-bit, version 3.5.1 was used for analysis. A two-tailed p-value of <0.05 was considered statistically significant for all analyses.
Results
Whole cohort
In the whole cohort (N = 117), mean age (±SD) was 72.9 ± 9.3 years and most were women (Table 1). The majority were non-Hispanic white (62%) but 28% were Hispanic, Black, or Asian. All women were postmenopausal. Serum calcium (median 10.5, range 9.0–11.7 mg/dl) and PTH (median 74, range 19–265 pg/ml,) tended to be mildly elevated. As shown in Table 1, mean 25-hydroxyvitamin D was normal (≥30 ng/ml). Osteoporosis (T-score ≤ −2.5) was present in 54.7%, but mean or median T-scores were in the osteopenic range at all sites. Mean TBS was in the degraded range and 94% had values that were degraded (48.7%) or partially degraded (45.3%). Median estimated duration of PHPT was 7.2 years (range 0.2–44 years). History of fragility fracture was reported by 35% and 15% had a history of nephrolithiasis.
VFs were identified in 10 participants (8.5%) by VFA between T6 and L5. Based on other imaging or VFA, 12.8% (n = 15) had a VF, 10 of whom had no clinical history of VF. Fractures not identified by VFA were mild, outside the interpretable VFA region or discovered on other imaging performed for pulmonary complaints, a fall, and back pain. All VFs occurred in women. Of those with VF, 66.6% had osteoporosis by DXA at any site (Table 1), but only 21% had a LS T-score ≤ −2.5.
Comparison of those with vs. without vertebral fracture
Median serum PTH [80 (range 19–265) vs. 73 (range 26–258) pg/ml, p = 0.95] and calcium levels [10.5 (range 9.9–11) vs.10.5 (range 9.0–11.7) mg/dl, p = 0.71] were not different in those with vs. without VF (Table 1). Those with fracture were older (mean 77.4 ± 11.0 vs. 72.2 ± 8.9 years, p = 0.04), had worse kidney function (GFR 61.8 ± 18.8 vs. 72.2 ± 17.6 mL/min/1.73 m2, p = 0.04), tended to be more likely to have had other fractures (p = 0.06) and were more likely to have been treated for osteoporosis (80% vs. 46%, p = 0.03). In contrast, those with vs. without VF did not differ by race (p = 0.97), sex (p = 0.64), BMI (p = 0.35), phosphorus (p = 0.45), 25-hydroxyvitamin D levels (p = 0.43), estimated duration of PHPT (p = 0.74), calcium (p = 0.57) or vitamin D supplementation dosage (p = 0.44) or co-morbidities including diabetes (p = 1.00), rheumatoid arthritis (p = 0.84) and steroid use (p = 1.00).
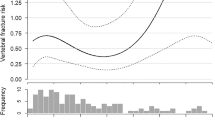
Those with vs. without VF did not differ by BMD or T-score at any site (Table 1), before or after adjusting for age and medication use. However, those with VF were much more likely to have osteoporosis at the hip (Table 1 and Fig. 1). TBS did not differ before (p = 0.73) or after adjusting for age (p = 0.95), or age and history of osteoporosis treatment (p = 0.96). Distributions of TBS categories were also similar between those with or without VF (p = 0.39; Fig. 2).
A logistic model that evaluated risk factors differing between groups (age, GFR, osteoporosis at hip, and history of medication use) indicated that having osteoporosis at the hip and prior osteoporosis medication use (Table 2) were independently associated with an increased odds of having a VF. Those with osteoporosis at the hip had nine times the odds of VF, whereas those with a history of medication use had 5.8 times the odds of VF.
ROC analysis indicated that age >78.6 years, eGFR < 58.8 mL/min/1.73 m2, hip T-score < −2.6 and (thresholds maximizing sensitivity and specificity) had AUCs of 0.67, 0.67, and 0.60 respectively for detecting VF (all p < 0.05), indicating they were fairly poor discriminators of fracture (Table 3). In contrast, TBS had an AUC of only 48.0% (p = 0.66), no better than a random classifier. Sensitivity and specificity for each significant risk factor is shown in Table 2. All risk factors had low sensitivity; specificity was >90% for hip T-score < −2.6 and the presence of osteoporosis at the hip.
Forty-three participants (36.8%) underwent parathyroidectomy. Pathologic examination (available for 41/43 patients) demonstrated adenomas or hyperplasia.
Women only (n = 110)
Women made up 94% of the cohort. As expected, female sex had high sensitivity for VF (100%), but the specificity of this association was low (6.9%). Analysis of the subset of women only did not show any differences from that of the cohort as a whole. Women with VF tended to be older (77.4 ± 11.0 vs. 72.3 ± 9.0 years, p = 0.047) and almost twice as likely to have prior fracture (60 vs. 32.6%, p = 0.04). They were also more likely to be treated for osteoporosis compared to those without VF (80.0 vs. 49.5%, p = 0.048) and had lower GFR (62 ± 19 vs. 72 ± 18 mL/min/1.73 m2, p = 0.046). They also had osteoporosis more frequently at the TH (46.7 vs. 9.5%, p = 0.001). There were no other between-group differences.
Discussion
This study investigated clinical, biochemical and skeletal risk factors associated with VF in PHPT. It is the first to be conducted in the US or include a significant number of racial minorities. We found that about 1 in 8–9 participants had grade 2 or 3 VF in the lower thoracolumbar spine based on VFA, most of which were clinically silent. Based on VFA and/or other imaging, the VF prevalence rate was 12.8%. In this PHPT cohort, VF was associated with age, osteoporosis at the hip, prior fractures, osteoporosis treatment, and poorer renal function. Of note, these risk factors for VF are similar to those in non-PHPT patients. In contrast to our expectations, there was no association between VF and TBS, spine BMD or the biochemical severity of PHPT.
Guidelines for the management of asymptomatic PHPT recommend spine imaging for patients with PHPT. One of the goals of this analysis was to determine if clinical or skeletal risk factors could accurately categorize those with VF so that spine imaging could be targeted to those most likely to have VFs. Despite some risk factors, such as osteoporosis at the hip, being strongly associated with VF, all identified risk factors had generally poor ability (low AUC) to categorize those with fracture. Since none of these risk factors discriminated those with VF well, our results suggest routine spine imaging is needed.
Further, targeting only those with osteoporosis at the hip or GFR < 58 ml/min/1.73 m2 for VF screening would miss half of those with VF. Importantly, from a clinical stand-point, finding a positive result would not change the recommendation for PTX, as PHPT patients with osteoporosis or low GFR already meet guidelines for recommending parathyroidectomy. Identification of VF in these subgroups may, however, be a useful clinical adjunct in patients who are unsure about parathyroidectomy, in whom such knowledge might influence their acceptance of surgery.
Our ability to accurately assess the prevalence of VF was limited by the fact that VFA was obtained only on a subset of “high risk” PHPT patients, did not include mild VF, and assessed the lower thoracolumbar spine only. Despite this, the observed prevalence is similar to that of a Japanese study in which 12.1% had VF as determined by lateral radiographs (VF defined as height loss >20%) [9]. The VF rate is also in the range (11.8–34.5%) reported for US adults age ≥50 years of various races without PHPT [23,24,25,26]. In contrast, our VF rate is lower than that in some recent PHPT studies (21–43.5%) conducted in Europe and Canada in white women [8, 10, 17, 27], all of which utilized lateral radiographs but defined VF variably as vertebral height loss >20 or ≥25%.
There are several possible reasons for the disparity in rates. This study has a significant proportion (24.3%) of racial minorities who may have lower rates of VF than white women [28, 29], though there was no difference in participants’ racial distribution between those with and without VF in our study. Secondly, our cohort had a milder biochemical profile (lower serum calcium and PTH) than many other studies, although neither our study nor several others found biochemistries to be associated with VF in PHPT [8,9,10, 17, 27].
As noted above, differences in imaging modality may have also contributed. Many studies used standard radiographs to detect VFs. Our study utilized VFA, corroborated by other modalities when available. VFA cannot visualize vertebra above T7 well [30]. However, most VFs related to osteoporosis occur between T10 and L2 [31, 32]. Although VFA is sensitive (87–93%) and specific (93–95%) for moderate to severe VFs, it has low sensitivity for mild VFs compared to radiographs [33]. The prevalence rate in our study, therefore, reflects that of moderate to severe fractures within the lower thoracolumbar spine. Whether mild VFs have the same clinical significance as more severe VFs is unclear. Grade 1 fractures may not predict fracture to the same degree as higher grade fractures and may not be an indication for treatment if isolated [32, 34]. Despite these limitations, VFA is attractive for screening because of its low cost and radiation as well as the ease of obtaining it simultaneously with BMD measurement.
In contrast to our study, two European studies indicated TBS is associated with prevalent VFs in PHPT [8, 17]. In the general population, TBS predicts incident VFs in postmenopausal women [14, 16, 35]. In the general population as well as those with PHPT, studies indicate TBS performs the same or better at detecting or predicting VF than BMD by DXA and some studies have found that TBS adds to the predictive power of BMD [8, 14, 16, 17, 35]. For these reasons, recent guidelines recommend VF assessment in PHPT and obtaining TBS when possible [18].
There are several potential explanations for why TBS was not useful in our study. Differences in PHPT cohorts may have played a role. We assessed only a subset who had VFA at our center and on average these patients were older and “higher risk” compared to other PHPT studies [8, 17]. Further, most had degraded or partially degraded TBS. Few participants with normal TBS may have contributed to the inability of TBS to discriminate those with and without fracture. It is unlikely that study sample size was a factor. Our study was similar in size to other published studies on PHPT and TBS and was able to detect a TBS difference of 0.0856, similar to other studies, with 80% power and a two-tailed alpha of 5% [8, 9, 17].
We found prevalent VFs were strongly associated with osteoporosis at the hip but not osteoporosis or BMD at other sites. Other studies have shown an association between VF and lower FN and TH BMD [8, 10]. In addition, like our study, most have found PHPT patients with VFs to be older [9, 17, 27, 36]. Patients with VF in our study also had a higher rate of treatment for osteoporosis, which likely reflects that those with VF had lower BMD, were older and more likely to have other fractures. We also observed lower GFR in PHPT patients with VFs, similar to a 2018 Danish study [27].
All VF occurred in women in our study, though there was no statistically significant difference in the sex distribution between those with and without VF. Few studies have assessed the association between sex and VF in PHPT. One previous study found no overall difference in VF prevalence by sex, but when stratified by age, found younger men (<59 years) to have higher rates than women [27]. Our cohort may have contained too few men to allow any meaningful conclusions in this regard.
The prevalence of osteoporosis in our study (54.7%) is at the high end of the range (39–62.9%) reported in PHPT [3, 10, 37]. This likely reflects the VFA screening parameters at our center. Only older individuals or those >50 years old with risk factors were eligible for VFA, which would predispose to a greater likelihood of osteoporosis. The majority (66%) with VF had osteoporosis by DXA, however few (21%) had a LS T-score ≤ −2.5. Similarly, in the study by Cipriani et al, 62.9% of PHPT patients had osteoporosis but only 39.1% of those with VF had spine T-scores ≤ −2.5. This highlights the limitation of spine BMD to accurately classify those with VF in PHPT.
Our study has several limitations. We used ICD codes to identify subjects. While we only included those with biochemistries consistent with PHPT, this “screening” method may have missed patients with PHPT if they lacked appropriate coding. As noted this study used clinical data and we were only able to include those who had VFA as part of their clinical assessment. Because VFA was only obtained on a subset of patients with PHPT, results may only be applicable to older, higher risk patients or women, though this may have been expected to increase the yield of predictive risk factors, since this group was more at risk than all-comers. In addition, prevalence rates were likely affected by use of VFA which does not detect grade 1 fractures or assess the upper thoracic spine. Further, biochemistries were analyzed in multiple laboratories which may have introduced assay variability that limited our ability to detect differences in markers of PHPT severity between those with and without fracture. We did not have a non-PHPT control group and cannot formally compare if prevalence or risk factors for VF differ between those with and without PHPT. Compared to the prevalence rate for VF detected by VFA in the general population in other studies (4.3–11.8%), our rate appears at the high end reported, though direct comparison is difficult [38,39,40]. We also did not have information on smoking and parental hip fracture as risk factors. Lastly, a major limitation of this study is that it is retrospective. A prospective study assessing baseline risk factors for future fracture would have provided stronger evidence of associations.
Our study also has several strengths. We examined a large cohort of patients with PHPT in the USA as well as a racially diverse group of participants. In addition, we avoided surveillance bias in screening for VF by limiting the study period to that in which VFAs were routinely performed at the time of DXA.
In summary, we found VF to be associated with older age, lower GFR, history of fracture, higher rates of osteoporosis treatment, and osteoporosis at the hip but not osteoporosis at other skeletal sites, TBS or biochemical indices associated with severity of PHPT. Because identified risk factors were generally poor discriminators of those with VF, our data support the use of routine spine imaging to accurately identify subclinical VF in PHPT patients.
References
M.D. Walker, S.J. Silverberg, Primary hyperparathyroidism. Nat. Rev. Endocrinol. 14(2), 115–125 (2018)
S.J. Silverberg, E. Shane, L. de la Cruz, D.W. Dempster, F. Feldman, D. Seldin et al. Skeletal disease in primary hyperparathyroidism. J. Bone Min. Res. 4(3), 283–291 (1989)
M.D. Walker, E. Cong, J.A. Lee, A. Kepley, C. Zhang, D.J. McMahon et al. Vitamin D in primary hyperparathyroidism: effects on clinical, biochemical, and densitometric presentation. J. Clin. Endocrinol. Metab. 100(9), 3443–3451 (2015)
S. De Geronimo, E. Romagnoli, D. Diacinti, E. D’Erasmo, S. Minisola, The risk of fractures in postmenopausal women with primary hyperparathyroidism. Eur. J. Endocrinol. 155(3), 415–420 (2006)
A.M. Kenny, D.C. MacGillivray, C.C. Pilbeam, H.D. Crombie, L.G. Raisz, Fracture incidence in postmenopausal women with primary hyperparathyroidism. Surgery 118(1), 109–114 (1995)
L.J. Melton III, E.J. Atkinson, W.M. O’Fallon, H. Heath III, Risk of age-related fractures in patients with primary hyperparathyroidism. Arch. Intern Med. 152(11), 2269–2273 (1992)
E. Vignali, G. Viccica, D. Diacinti, F. Cetani, L. Cianferotti, E. Ambrogini et al. Morphometric vertebral fractures in postmenopausal women with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 94(7), 2306–2312 (2009)
C. Eller-Vainicher, M. Filopanti, S. Palmieri, F.M. Ulivieri, V. Morelli, V.V. Zhukouskaya et al. Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur. J. Endocrinol. 169(2), 155–162 (2013)
H. Kaji, M. Yamauchi, K. Chihara, T. Sugimoto, The threshold of bone mineral density for vertebral fractures in female patients with primary hyperparathyroidism. Eur. J. Endocrinol. 153(3), 373–378 (2005)
C. Cipriani, F. Biamonte, A.G. Costa, C. Zhang, P. Biondi, D. Diacinti et al. Prevalence of kidney stones and vertebral fractures in primary hyperparathyroidism using imaging technology. J. Clin. Endocrinol. Metab. 100(4), 1309–1315 (2015)
S. Hansen, J.E. Beck Jensen, L. Rasmussen, E.M. Hauge, K. Brixen, Effects on bone geometry, density, and microarchitecture in the distal radius but not the tibia in women with primary hyperparathyroidism: a case-control study using HR-pQCT. J. Bone Min. Res. 25(9), 1941–1947 (2010)
E.M. Stein, B.C. Silva, S. Boutroy, B. Zhou, J. Wang, J. Udesky et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J. Bone Min. Res. 28(5), 1029–1040 (2013)
B.C. Silva, W.D. Leslie, H. Resch, O. Lamy, O. Lesnyak, N. Binkley et al. Trabecular Bone Score: A Noninvasive Analytical Method Based Upon the DXA Image. J. Bone Miner. Res. 29(3), 518–530 (2014)
D. Hans, A.L. Goertzen, M.A. Krieg, W.D. Leslie, Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J. Bone Min. Res. 26(11), 2762–2769 (2011)
S. Boutroy, D. Hans, E. Sornay-Rendu, N. Vilayphiou, R. Winzenrieth, R. Chapurlat, Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos. Int. 24(1), 77–85 (2013)
K. Briot, S. Paternotte, S. Kolta, R. Eastell, D.M. Reid, D. Felsenberg et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone 57(1), 232–236 (2013)
E. Romagnoli, C. Cipriani, I. Nofroni, C. Castro, M. Angelozzi, A. Scarpiello et al. “Trabecular Bone Score” (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone 53(1), 154–159 (2013)
J.P. Bilezikian, M.L. Brandi, R. Eastell, S.J. Silverberg, R. Udelsman, C. Marcocci et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J. Clin. Endocrinol. Metab. 99(10), 3561–3569 (2014)
F. Cosman, S.J. de Beur, M.S. LeBoff, E.M. Lewiecki, B. Tanner, S. Randall et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 25(10), 2359–2381 (2014)
D. Hans, N. Barthe, S. Boutroy, L. Pothuaud, R. Winzenrieth, M.A. Krieg, Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J. Clin. Densitom. 14(3), 302–312 (2011)
H.K. Genant, C.Y. Wu, C. van Kuijk, M.C. Nevitt, Vertebral fracture assessment using a semiquantitative technique. J. Bone Min. Res. 8(9), 1137–1148 (1993)
A.S. Levey, J.P. Bosch, J.B. Lewis, T. Greene, N. Rogers, D. Roth, A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern Med. 130(6), 461–470 (1999)
G. Ballane, J.A. Cauley, M.M. Luckey, G. El-Hajj Fuleihan, Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos. Int. 28(5), 1531–1542 (2017)
J.T. Schousboe, Epidemiology of vertebral fractures. J. Clin. Densitom. 19(1), 8–22 (2016)
J.T. Schousboe, T. Vo, B.C. Taylor, P.M. Cawthon, A.V. Schwartz, D.C. Bauer et al. Prediction of incident major osteoporotic and hip fractures by trabecular bone score (TBS) and prevalent radiographic vertebral fracture in older men. J. Bone Min. Res. 31(3), 690–697 (2016)
L.J. Melton III, S.H. Kan, M.A. Frye, H.W. Wahner, W.M. O’Fallon, B.L. Riggs, Epidemiology of vertebral fractures in women. Am. J. Epidemiol. 129(5), 1000–1011 (1989)
H. Ejlsmark-Svensson, L.S. Bislev, S. Lajlev, T. Harslof, L. Rolighed, T. Sikjaer et al. Prevalence and risk of vertebral fractures in primary hyperparathyroidism: a nested case-control study. J. Bone Min. Res. 33(9), 1657–1664 (2018)
J.A. Cauley, L. Palermo, M. Vogt, K.E. Ensrud, S. Ewing, M. Hochberg et al. Prevalent vertebral fractures in black women and white women. J. Bone Min. Res. 23(9), 1458–1467 (2008)
H.K. Genant, Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos. Int. 10(4), 259–264 (1999)
E.M. Lewiecki, A.J. Laster, Clinical review: clinical applications of vertebral fracture assessment by dual-energy x-ray absorptiometry. J. Clin. Endocrinol. Metab. 91(11), 4215–4222 (2006)
B.A. Christiansen, M.L. Bouxsein, Biomechanics of vertebral fractures and the vertebral fracture cascade. Curr. Osteoporos. Rep. 8(4), 198–204 (2010)
D.L. Kendler, D.C. Bauer, K.S. Davison, L. Dian, D.A. Hanley, S.T. Harris et al. Vertebral fractures: clinical importance and management. Am. J. Med. 129(2), 221, e1–10 (2016)
J.T. Schousboe, C.R. Debold, Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos. Int. 17(2), 281–289 (2006)
P.D. Delmas, H.K. Genant, G.G. Crans, J.L. Stock, M. Wong, E. Siris et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 33(4), 522–532 (2003)
M. Iki, J. Tamaki, E. Kadowaki, Y. Sato, N. Dongmei, R. Winzenrieth et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J. Bone Min. Res. 29(2), 399–407 (2014)
C. Cipriani, F. Biamonte, A.G. Costa, C. Zhang, P. Biondi, D. Diacinti et al. Prevalence of kidney stones and vertebral fractures in primary hyperparathyroidism using imaging technology. J. Clin. Endocrinol. Metab. 100(4), 1309–1315 (2015)
G. Viccica, F. Cetani, E. Vignali, M. Miccoli, C. Marcocci, Impact of vitamin D deficiency on the clinical and biochemical phenotype in women with sporadic primary hyperparathyroidism. Endocrine 55(1), 256–265 (2017)
S. Waterloo, L.A. Ahmed, J.R. Center, J.A. Eisman, B. Morseth, N.D. Nguyen et al. Prevalence of vertebral fractures in women and men in the population-based Tromso Study. BMC Musculoskelet. Disord. 13, 3 (2012)
M. Frost, K. Wraae, B. Abrahamsen, M. Hoiberg, C. Hagen, M. Andersen et al. Osteoporosis and vertebral fractures in men aged 60–74 years. Age Ageing 41(2), 171–177 (2012)
E. Kanterewicz, E. Puigoriol, J. Garcia-Barrionuevo, L. del Rio, M. Casellas, P. Peris et al. Prevalence of vertebral fractures and minor vertebral deformities evaluated by DXA-assisted vertebral fracture assessment (VFA) in a population-based study of postmenopausal women: the FRODOS study. Osteoporos. Int. 25(5), 1455–1464 (2014)
Funding
This study was funded by the Endocrine Fellows Foundation, T32 DK007271.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The Columbia University Medical Center Institutional Review Board granted a waiver of consent (45 CFR 46.116) for this study as this was a retrospective chart review of historical patients, the study involved no more than minimal risk to participants, the waiver did not adversely affect the rights and welfare of the subjects, and the research could not practicably be carried out without the waiver.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, M., Williams, J., Kuo, J. et al. Risk factors for vertebral fracture in primary hyperparathyroidism. Endocrine 66, 682–690 (2019). https://doi.org/10.1007/s12020-019-02099-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02099-1