Abstract
Purpose
Low testosterone (T) in Klinefelter’s syndrome (KS) can contribute to typical features of the syndrome such as reduced bone mineral density, obesity, metabolic disturbances and increased cardiovascular risk. The aim of the present study is to review and meta-analyze all available information regarding possible differences in metabolic and bone homeostasis profile between T treated (TRT) or untreated KS and age-matched controls.
Methods
We conducted a random effect meta-analysis considering all the available data from observational or randomized controlled studies comparing TRT-treated and untreated KS and age-matched controls. Data were derived from an extensive MEDLINE, Embase, and Cochrane search.
Results
Out of 799 retrieved articles, 21 observational and 22 interventional studies were included in the study. Retrieved trials included 1144 KS subjects and 1284 healthy controls. Not-treated KS patients showed worse metabolic profiles (including higher fasting glycemia and HOMA index as well as reduced HDL-cholesterol and higher LDL-cholesterol) and body composition (higher body mass index and waist circumference) and reduced bone mineral density (BMD) when compared to age-matched controls. TRT in hypogonadal KS subjects was able to improve body composition and BMD at spinal levels but it was ineffective in ameliorating lipid and glycemic profile. Accordingly, TRT-treated KS subjects still present worse metabolic parameters when compared to age-matched controls.
Conclusion
TRT outcomes observed in KS regarding BMD, body composition and glyco-metabolic control, are similar to those observed in male with hypogonadism not related to KS. Moreover, body composition and BMD are better in treated than untreated hypogonadal KS. Larger and longer randomized placebo-controlled trials are advisable to better confirm the present data, mainly derived from observational studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Klinefelter’s syndrome (KS) is the most frequent sex chromosomal aneuploidy observed in men, with an estimated prevalence of 1:500-1:1000 [1, 2]. Much evidence has clearly documented that the traditional phenotype attributed to the syndrome and characterized by tall stature, small testes, gynecomastia, gynoid aspects of the hip, sparse body hairs, primary hypogonadism and mental retardation is rarely observed in the clinical setting [1, 2]. In the vast majority of cases, KS presents with a mild phenotype, often difficult to distinguish from the general population. Accordingly, it has been reported that only 1 out of 4 patients with KS are correctly diagnosed during their life [1]. The final KS phenotype is probably the result of a combination among the severity of the genetic defects, androgen production and age at diagnosis [1]. Despite these considerations, the clinical hallmark of KS is still represented by small testes. Although some authors have suggested that subclinical signs of hypogonadism, can be observed even during prepubertal period, the majority of KS subjects show normal pubertal development with a physiological rise of testosterone (T) levels and enlargement of testes [3]. Very soon, however, a testis shrinkage occurs despite an increase in LH and FSH levels so that the testis size in KS remains much lower than normal adult males [2, 3]. In adulthood, serum T concentrations fall to the mid-low range of the young adult, although the age-onset of overt hypogonadism is quite variable [1, 2]. However, although direct prospective comparisons between KS and age-matched controls are lacking, it can be speculated that the age-depended decline of T observed in the general population occurs earlier in KS [1, 2]. In line with this hypothesis it has been suggested that low T in KS can contribute to the pathogenesis of typical KS features such as reduced bone mineral density, obesity, metabolic disturbances and increased cardiovascular risk [4]. Similar considerations have been also reported for the general population [5]. Data derived from long-term registry studies have suggested that T replacement therapy (TRT) might improve metabolic profile and body composition in overall hypogonadal men [6,7,8,9]. Although the latter results have not completely been confirmed when randomized controlled trials (RCTs) have been considered [10,11,12], a possible role of hypogonadism in the stratification of CV risk in the general population has been suggested [5, 13, 14]. Similarly, although a positive increment of bone mineral density (BMD), particularly at spine level, has been reported after TRT in overall hypogonadal men, its contribution in reducing fracture risk in the general population is more conflicting [15].
Few placebo controlled RCTs have investigated the role of TRT in patients with KS. A recent double-blind, placebo controlled RCT, involving 13 KS patients receiving oral T-undecanoate 160 mg per day or placebo for 6 months, showed that TRT produced favorable changes in body composition but limited effects on glucose homeostasis [16].
The aim of the present study is to review and meta-analyze all available information regarding possible differences in metabolic and bone homeostasis profile between TRT-treated or untreated KS and age-matched controls.
Methods
This meta-analysis was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (see Supplementary file 1).
Search strategy
An extensive Medline, Embase and Cochrane search was performed including the following words ("testosterone" [MeSH Terms] OR "testosterone" [All Fields]) AND Klinefelter [All Fields] for the selection of studies evaluating the relationship between T and Klinefelter’s Syndrome on several outcomes. The search, which accrued data from January 1, 1969 up to August 31, 2019, was restricted to English-language articles and studies of human participants. The identification of relevant studies was performed independently by six of the authors (A.P, W.V, R.C, R.P, A.R, G.R), and conflicts resolved by the other investigators. We did not employ search software. We hand-searched bibliographies of retrieved papers for additional references. The principal source of information was derived from published articles.
Study selection
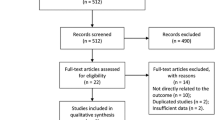
We included all cross-sectional either retrospective or prospective studies, comparing adulthood KS subjects and age-matched healthy controls (observational studies). In addition, we also included all interventional studies evaluating the effect of TRT on adulthood KS when compared to untreated age-matched hypogonadal KS or healthy controls. All studies without any arbitrary restriction were included (see also Supplementary Figure 1 and Tables 1, 2, 3; [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]). Studies comparing KS with other populations than healthy controls were excluded from the analysis (see Supplementary Figure 1). Similarly, those studies investigating the effects of an early T treatment in adolescents or newborn KS subjects were also excluded from the analysis (Supplementary Figure 1).
Outcome
The principal outcome of this analysis was the comparison between untreated KS patients and age-matched controls on several body composition and metabolic parameters. In addition, the analysis of BMD on lumbar and neck sites as well as the evaluation of some safety parameters including prostate volume, PSA and hematocrit levels was also performed whenever possible. Secondary outcomes included the evaluation of the effect of TRT on several parameters including body composition, metabolic parameters and BMD (see also Supplementary Table 3) in KS individuals when compared to untreated age-matched KS patients or healthy subjects.
Quality assessment
The quality of the studies was assessed using the Cochrane parameters [51]. In particular, in observational and pharmaco-epidemiological studies we evaluated the following criteria: the weaknesses of the designs that have been used (such as noting their potential to ascertain causality), the execution of the studies through a careful assessment of their risk of bias, especially the potential for selection bias and confounding to which all observational studies are susceptible, and the potential for reporting biases, including selective reporting of outcomes (Supplementary Table 1).
For each interventional study, we also assessed how the population was selected, the duration and route of TRT, the adequacy of study follow-up, and the funding source.
Statistical analysis
Heterogeneity was assessed using I2 statistics. Even when a low heterogeneity was detected, a random-effects model was applied, because the validity of tests of heterogeneity can be limited with a small number of component studies. When possible data were extracted as mean ± standard deviation. In all other cases adequate analyses were used. To estimate possible publication or disclosure bias we used funnel plots and the Begg adjusted rank correlation test [51, 52]. However, because these tests have low statistical power when the number of trials is small, undetected bias may still be present. In addition, to be more conservative, all endpoint values were evaluated in a non-paired fashion (non-paired analysis).
A meta-regression analysis was performed to test the effect of different parameters whenever indicated. All analyses were performed using Comprehensive Meta-analysis Version 2, Biostat (Englewood, NJ, USA). Multivariate analyses were performed on SPSS (Statistical Package for the Social Sciences; Chicago, USA) for Windows 22.0.
Results
Out of 799 retrieved articles, 21 observational and 22 interventional studies were included in the study (Supplementary Figure 1).
Observational studies
Among observational reports, 20, 6, and 7 reported information on metabolic, bone and safety parameters, respectively. The characteristics of the retrieved trials (including parameters on trial quality) are reported in Table 1 and Supplementary Tables 1, 2). Retrieved trials included 1149 KS subjects and 1,304 healthy controls. The mean age, baseline T levels and body mass index (BMI) of KS patients were 31.5 years, 10.4 nmol/L, and 25.8 kg/m2, respectively. The studies differed in the mean T levels and baseline characteristics of enrolled individuals (Table 1). In particular, 5 studies considered only hypogonadal KS patients, whereas 16 surveys included a mixed population of eugonadal and hypogonadal KS individuals (Table 1). In addition, 4 studies included a mixed population of T treated and untreated KS, whereas 16 studies evaluated only untreated KS patients and in one study this information was not available (Table 1).
Body composition and metabolic parameters
The 20 surveys reporting information on BMI included 1144 KS subjects and 1284 controls. I2 was 76.5 (p < 0.001). Funnel plot and Begg adjusted rank correlation test (Kendall’s τ: 0.02; p = 0.92) suggested no major publication bias.
When compared to age-matched healthy controls, patients with KS had significantly higher BMI and waist circumference (Fig. 1 and Supplementary Figure 2, panels A and B). The difference in BMI was confirmed when heterogeneity was reduced by removing outliers (I2 = 36.4; 1.74 [1.12; 2.36]; p < 0.0001).
Similarly, body fat was significantly higher in KS individuals when compared to age-matched healthy controls (Fig. 1 and Supplementary Figure 2, panel C). Lean mass was reduced in KS when compared to healthy controls, but the difference did not reach statistical significance (Fig. 1 and Supplementary 1, panel D). All these data were confirmed even when those trials with mean baseline T levels above 12 nmol/L were excluded from the analysis (Table 1) or when those surveys including patients on TRT were excluded (not shown). Meta-regression analysis showed that the differences between KS and controls on BMI were independent of KS subject age and hormonal profile, including T, E2, LH or FSH levels (not shown).
When glyco-metabolic parameters were considered, patients with KS showed higher fasting glucose levels and HOMA index when compared to healthy controls (Fig. 2 and Supplementary Figure 2, panels E, F). In addition, higher LDL cholesterol and lower HDL cholesterol levels were also observed in subjects with KS when compared to controls (Fig. 2 and Supplementary Figure 2, panels G, H). Differences related to glyco-metabolic parameters were confirmed even when those trials with mean baseline T levels above 12 nmol/L were excluded from the analysis (Table 1) or when those surveys including patients on TRT were excluded (not shown). Finally, no differences between KS and controls were observed when total cholesterol and triglycerides levels were considered (not shown).
Bone parameters
The 6 studies reporting information on bone status included 406 KS subjects and 303 controls. All studies used data derived from dual-energy X-ray absorptiometry (DEXA) to assess BMD (see Table 1 and Supplementary Tables 1, 2). I2 on lumbar BMD was 93.3 (p < 0.001). Funnel plot and Begg adjusted rank correlation test (Kendall’s τ: − 0.13; p = 0.71) suggested no major publication bias.
KS showed significantly reduced BMD when either lumbar, neck or hip sites were considered (Fig. 3 and Supplementary Figure 3, panels A–C). The difference in lumbar BMD was confirmed when heterogeneity was reduced by removing outliers (I2 = 11.5; − 0.53 [− 0.77; − 0.29]; p < 0.0001). No sufficient data were available to perform further sensitivity analyses.
Safety parameters
Among observational studies considered, 5, 2 and 3 controls reported data on prostate volume, PSA and HTC levels, respectively (see Table 1 and Supplementary Tables 1, 2). I2 on prostate volume was 96.2 (p < 0.001). Funnel plot and Begg adjusted rank correlation test (Kendall’s τ: 0.0; p = 1.0) suggested no major publication bias. The difference in prostate volume was confirmed when heterogeneity was reduced by removing outliers (I2 = 0.0; − 9.15 [− 9.71; − 8.60]; p < 0.0001).
KS showed significantly reduced prostate volume and HCT when compared to controls (Fig. 4 and Supplementary Figure 3, panels E, F). In addition, lower PSA levels were also detected in KS when compared to age-matched controls but this difference did not reach statistical significance (Fig. 4, Supplementary Figure 3, panel D).
Interventional studies
Among the included studies, 20 and 10 surveys investigated possible differences between T-treated KS subjects and untreated KS individuals or age-matched healthy controls. The characteristics of the retrieved trials (including parameters on trial quality) are reported in Tables 2, 3 and Supplementary Tables 1, 3 and 4. Retrieved trials comparing treated and untreated KS patients included 530 subjects and 536 controls with a mean follow-up of 68.2 weeks and the mean age of the enrolled treated cohort was 29.8 years. In addition, studies evaluating the effects of TRT on KS in comparison to healthy controls included 373 KS individuals (mean age 31.9 years with a mean follow up of 77.6 weeks) and 359 controls.
KS treated vs. untreated
I2 performed on BMI was 77.6 (p < 0.0001). Funnel plot and Begg adjusted rank correlation test (Kendall’s τ: − 0.14; p = 0.50) suggested no major publication bias. By meta-analyzing these studies we found that TRT resulted in a significant improvement of body composition (reduction in fat mass and increase in lean mass; Fig. 1 and Supplementary Figure 4, panels A, B). Conversely, we did not observe any modification in BMI, waist circumference (Fig. 1 and Supplementary Figure 4, panels C, D) or in several of the metabolic parameters considered, including fasting glycaemia, HOMA index, LDL-cholesterol, HDL cholesterol (Fig. 2 and Supplementary Figure 4, panels E, H), total cholesterol and triglyceride levels (not shown). The lack of the difference in BMI was confirmed when heterogeneity was reduced by removing outliers (I2 = 28.4; − 0.18 [− 0.89; − 0.53]; p = 0.62).
In addition, a significant increase in lumbar but not in neck BMD was also observed (Fig. 3 and Supplementary Figure 4, panels I–L). Finally, KS treated subjects had significantly higher PSA, prostate volume, and HTC levels when compared to untreated KS patients (Fig. 4 and Supplementary Figure 4, panels M–O).
KS treated vs. controls
I2 performed on BMI was 63.03 (p < 0.001). Funnel plot and Begg adjusted rank correlation test (Kendall’s τ: 0.19; p = 0.47) suggested no major publication bias. When compared to age-healthy controls KS patients under TRT still had higher BMI but no difference in fat and lean body mass was observed (Fig. 1 and Supplementary Figure 5, panels A–C). In addition, higher fasting glucose levels, HOMA index as well as LDL-cholesterol and reduced HDL cholesterol were also observed in T treated KS patients when compared to controls (Fig. 2 and Supplementary Figure 5, panels D–G). Finally, no difference in total cholesterol and triglycerides levels between the two groups was observed (not shown).
The difference in BMI was confirmed when heterogeneity was reduced by removing outliers (I2 = 5.41; 1.01 [0.60; 1.42]; p < 0.0001). No enough data were available to perform possible analyses for bone and safety parameters.
Discussion
In this study we systematically reviewed and meta-analyzed, for the first time, all studies comparing metabolic profile and BMD status in TRT-treated and untreated KS patients in comparisons to age-matched controls. We found that not-treated KS patients have worse metabolic profile and body composition as well as a reduced BMD when compared to age-matched controls. TRT in hypogonadal KS subjects is able to improve body composition and BMD but its role is ineffective in ameliorating lipid and glycemic profile. Accordingly, TRT-treated KS subjects still present worse metabolic parameters when compared to age-matched controls.
The role of TRT in aging men represents a debatable issue [53, 54]. In particular, data derived from epidemiological population-based studies have suggested that associated morbidities influence the age-dependent reduction of T, which can be potentially reversible with the improvement of the underlying disorders [55,56,57]. In addition, cardiovascular (CV) safety of TRT has been questioned [58,59,60]. In line with this evidence the concept of functional vs. organic hypogonadism is emerging. In particular, the former represents a condition potentially reversible with borderline low T levels, mainly associated with sexual symptoms, whereas the latter includes the well-known organic conditions causing irreversible primary or secondary HG [61]. Lifestyle changes and/or removing the underlying conditions is the recommended strategy to increase endogenous T in functional hypogonadism, whereas the role of TRT is debatable [61]. This position has been endorsed by the US Endocrine Society [62] and by the Endocrine Society of Australia [63]. In addition, the US Food and Drug Administration (FDA) [64] along with Health Canada [65] recommend TRT only in those subjects with proven “organic” damage of the HPT axis.
KS represents probably one the most frequent cause of “organic” HG. Present study showed that TRT in men with KS resulted in similar outcomes than that observed in patients with functional hypogonadism. In particular, when overall population is considered, TRT has been found to reduce fat mass and improve lean mass either when both observational and placebo-controlled studies are considered [9,10,11]. Data derived from a meta-analysis of available RCTs documented that the TRT-induced modification of fat mass and lean muscle is essentially of the same amount preventing the observation of body weight loss [11]. The only meta-analysis which systematically analyzed all the results obtained from observational and registry studies documented that TRT can result even in weight loss, particularly after 2 years of treatment [9]. The presence of different baseline clinical characteristics of the patients enrolled, including lower T levels, larger BMI and longer follow up, have been advocated to justify the different outcomes observed between observational studies and RCTs [9,10,11]. However, it is important to highlight that data derived from observational studies are recognized to be characterized by important limitations and a high risk selection bias due to the non-random assignment of T exposure. Accordingly, physicians often prefer to treat healthier individuals, and healthier individuals more often request treatment for their hypogonadism-related problems. In addition, other limitations rely on the lack of information regarding the level of T before and during TRT, as well as on the limited data regarding the type of T preparation used and the follow-up performed during treatment.
Despite these considerations, available data seem to confirm the hypothesis that TRT can act mainly by modifying body composition (increasing lean mass and reducing fat mass) without any effect on body weight. Accordingly, data from experimental studies have documented that T is able to regulate the commitment of fat tissue stem cells supporting their development to muscle lineage [11].The initial improvement of muscle mass, can, in turn, help obese patients with hypogonadism to overcome their overfed, inactive state and to become physically and psychologically ready for changing their lifestyle which can eventually result in weight loss.
Interestingly, despite the body composition improvement observed in T-treated KS subjects, no modification of the metabolic parameters analyzed has been observed. In addition, T-substituted KS patients still present worse glyco-metabolic profile when compared to age-matched controls. Much evidence has documented that KS is associated with a higher risk for developing metabolic disorders and obesity, although the underlying etiological factors have not been completely clarified so far. The specific genetic profile, the association with hypogonadism and the unfavorable socio-economic conditions, which characterizes many KS subjects, are all factors used to explain the latter association [2, 4]. Present results seem to suggest that the genetic factors play a more important role than hypogonadism. Accordingly, the specific genetic pattern of KS has been correlated to the clinical phenotype [1, 2]. Unfortunately, no data were available to investigate the contribution of TRT in KS according to different genetic backgrounds.
Besides metabolic disturbances, bone impairment represents another important feature of KS. Fractures and osteoporosis are more frequent among KS individuals when compared to the general population [2]. Accordingly, present data showed that BMD was reduced in KS when compared to healthy controls. Sex steroids are, in fact, crucial for the maintenance of a good balance bone metabolism either in men or women [15]. Severe hypogonadism (total T < 3.5 nmol/L) is frequently associated with bone loss and osteoporosis, independently from the patient’s age, whereas the association between milder forms of hypogonadism and osteoporosis/osteopenia is less evident [15]. Available meta-analyses performed in the general population have documented that TRT resulted in increasing BMD, particularly at the lumbar level [66, 67]. Similar data have been derived from the TTrials, a coordinated set of 7 randomized placebo-controlled trials including 788 hypogonadal (TT < 9.4 nmol/L) men older than 65 years (mean age 72 years) treated with T gel 1% for 52 weeks [54]. Present data performed in KS are in line to what observed in men with hypogonadism not related to KS with better results observed at spine level. Some authors have suggested that KS is characterized by reduced levels of 25-hydroxy-vitamin D and low insulin-like factor 3 which can both contributed to the observed reduced BMD [2]. Nonetheless, no sufficient information on these parameters was available. Similarly, no enough data were available to evaluate possible difference in bone markers or in the risk of bone fractures.
When safety parameters were investigated, KS was associated with reduced HTC levels as well as reduced prostate volume and PSA. TRT induced a rise in all the aforementioned parameters without inducing the development of serious adverse events. All these data are in line to what observed in men with hypogonadism not related to KS and revised elsewhere [58,59,60]. Hence when correctly used TRT is not associated with serious adverse events.
The interpretation of the results of the present meta-analysis should be cautious because of the relevant potential biases. Although specific analyses seem to exclude a publication bias in this case, high heterogeneity among studies was documented. Few placebo controlled RCTs are available and the majority of the data were derived from observational studies. In observational studies the completeness of follow-up and the management of missing data represent another potential source of bias. Meta-analyses are based on the synthetic reports of the average results obtained in each study, without access to patient-level data. For this reason, some of the original information of each study is lost in meta-analyses. On the other hand, the possibility of combining a large number of investigations allows for a much greater statistical power, limiting the problem of casual results because of small sample size. It is also possible that some of the results noticed here are caused by the effects of unadjusted confounders. Hence, great caution is required in the interpretation of results, which should be confirmed in large-scale placebo controlled RCTs. The cardiovascular safety of TRT, especially in men with functional hypogonadism still represents a conflicting topic [58,59,60, 68]. No sufficient information regarding long-term CV safety in KS is available in this study [69]. Some authors have suggested that earlier TRT can result in better outcomes especially considering neurobiological outcomes. No information regarding this issue can be derived from the present meta-analysis. No sub-analysis of non-mosaic or mosaic KS patients, was possible due to missing information. The contribution of the duration of TRT on the different outcome analyzed was not possible due to limited available data. Some observational studies included a mixed population of treated and untreated KS patients. No specific sub-analysis comparing eugonadal or hypogonadal KS to controls was possible. However, the main results were confirmed when those trials with mean baseline T levels above 12 nmol/L or when those surveys including patients on TRT were excluded from the analysis. Finally, the present meta-analysis was not previously registered on PROSPERO.
In conclusion present data, performed on one of the most frequent and well recognized organic form of hypogonadism, indicate that TRT outcomes observed in KS regarding BMD, body composition and glyco-metabolic control are similar to that observed in other organic forms of hypogonadism. Moreover, our analysis shows that TRT improves both body composition and BMD when comparing treated and untreated hypogonadal KS. Larger and longer randomized placebo-controlled trials are advisable to better confirm present data, mainly derived from observational studies.
References
Bonomi M, Rochira V, Pasquali D, Balercia G, Jannini EA, Ferlin A, Klinefelter ItaliaN Group (KING) (2017) Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Investig 40:123–134. https://doi.org/10.1530/EJE-17-0902
Gravholt CH, Chang S, Wallentin M, Fedder J, Moore P, Skakkebæk A (2018) Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocr Rev 39:389–423. https://doi.org/10.1210/er.2017-00212
Aksglaede L, Skakkebaek NE, Almstrup K, Juul A (2011) Clinical and biological parameters in 166 boys, adolescents and adults with nonmosaic Klinefelter syndrome: a Copenhagen experience. Acta Paediatr 100:793–806. https://doi.org/10.1111/j.1651-2227.2011.02246.x
Calogero AE, Giagulli VA, Mongioì LM, Triggiani V, Radicioni AF, Jannini EA, Pasquali D, Klinefelter ItaliaN Group (KING) (2017) Klinefelter syndrome: cardiovascular abnormalities and metabolic disorders. J Endocrinol Investig 40:705–712. https://doi.org/10.1007/s40618-017-0619-9
Corona G, Vignozzi L, Sforza A, Maggi M (2013) Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health 31:103–125. https://doi.org/10.5534/wjmh.2013.31.2.103
Haider A, Saad F, Doros G, Gooren L (2014) Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract 8:e339–349. https://doi.org/10.1016/j.orcp.2013.10.005
Yassin A, Haider A, Haider KS, Caliber M, Doros G, Saad F, Garvey WT (2019) Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: eight-year data from a registry study. Diabetes Care 42:1104–1111. https://doi.org/10.2337/dc18-2388
Corona G, Maseroli E, Maggi M (2014) Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother 15:1903–1926. https://doi.org/10.1517/14656566.2014.944896
Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M (2016) Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Investig 39:967–981. https://doi.org/10.1007/s40618-016-0480-2
Grossmann M, Ng Tang Fui M, Cheung AS (2019) Late-onset hypogonadism: metabolic impact. Andrology. https://doi.org/10.1111/andr.12705
Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M (2016) Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol 174:99–116. https://doi.org/10.1530/EJE-15-0262
Grossmann M (2018) Hypogonadism and male obesity: focus on unresolved questions. Clin Endocrinol (Oxf) 89:11–21. https://doi.org/10.1111/cen.13723
Goodale T, Sadhu A, Petak S, Robbins R (2017) Testosterone and the heart. Methodist Debakey Cardiovasc J 13:68–72. https://doi.org/10.14797/mdcj-13-2-68
Rastrelli G, Maggi M, Corona G (2018) Pharmacological management of late-onset hypogonadism. Expert Rev Clin Pharmacol 11:439–458. https://doi.org/10.1080/17512433.2018.1445969
Rochira V, Antonio L, Vanderschueren D (2018) EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology 6:272–285. https://doi.org/10.1111/andr.12470
Høst C, Bojesen A, Erlandsen M, Groth K, Kritstensen K, Jurik AG, Birkebæk N, Gravholt C (2019) A placebo-controlled randomized study with testosterone in Klinefelter syndrome—beneficial effects on body composition. Endocr Connect 8:1250–1261. https://doi.org/10.1530/EC-19-0323
Watanabe H, Igari D, Tanahashi Y, Harada K, Saito M (1974) Measurements of size and weight of prostate by means of transrectal ultrasonotomography. Tohoku J Exp Med 114:277–285. https://doi.org/10.1620/tjem.114.277
Horowitz M, Wishart JM, O'Loughlin PD, Morris HA, Need AG, Nordin BE (1992) Osteoporosis and Klinefelter's syndrome. Clin Endocrinol (Oxf) 36:113–118. https://doi.org/10.1111/j.1365-2265.1992.tb02910.x
Kamischke A, Baumgardt A, Horst J, Nieschlag E (2003) Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J Androl 24:41–48. https://doi.org/10.1002/j.1939-4640.2003.tb02638.x
Yesilova Z, Ozata M, Oktenli C, Sanisoglu SY, Erbil MK, Dagalp K (2004) Effect of supraphysiologic doses of testosterone on fasting plasma total homocysteine concentrations in men with Klinefelter's syndrome. Fertil Steril 81:1278–1282. https://doi.org/10.1016/j.fertnstert.2003.11.029
Yesilova Z, Oktenli C, Sanisoglu SY, Musabak U, Cakir E, Ozata M, Dagalp K (2005) Evaluation of insulin sensitivity in patients with Klinefelter's syndrome: a hyperinsulinemic euglycemic clamp study. Endocrine 27:11–15. https://doi.org/10.1385/ENDO:27:1:011
Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, Laurberg P, Frystyk J, Flyvbjerg A, Christiansen JS, Gravholt CH (2006) The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 29:1591–1598. https://doi.org/10.2337/dc06-0145
Seo JT, Lee JS, Oh TH, Joo KJ (2007) The clinical significance of bone mineral density and testosterone levels in Korean men with non-mosaic Klinefelter's syndrome. BJU Int 99:141–146. https://doi.org/10.1111/j.1464-410X.2006.06584.x
Høst C, Bojesen A, Frystyk J, Flyvbjerg A, Christiansen JS, Gravholt CH (2010) Effect of sex hormone treatment on circulating adiponectin and subforms in Turner and Klinefelter syndrome. Eur J Clin Investig 40:211–219. https://doi.org/10.1111/j.1365-2362.2009.02250.x
Bojesen A, Høst C, Gravholt CH (2010) Klinefelter's syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod 16:396–401. https://doi.org/10.1093/molehr/gaq016
Ferlin A, Schipilliti M, Vinanzi C, Garolla A, Di Mambro A, Selice R, Lenzi A, Foresta C (2011) Bone mass in subjects with Klinefelter syndrome: role of testosterone levels and androgen receptor gene CAG polymorphism. J Clin Endocrinol Metab 96:E739–E745. https://doi.org/10.1210/jc.2010-1878
Hiéronimus S, Lussiez V, Le Duff F, Ferrari P, Bständig B, Fénichel P (2011) Klinefelter's syndrome and bone mineral density: is osteoporosis a constant feature? Ann Endocrinol (Paris) 72:14–18. https://doi.org/10.1016/j.ando.2010.10.002
Bak CW, Byun JS, Lee JH, Park JH, Lee KA, Shim SH (2012) Clinical and social characteristics of Korean men with Klinefelter syndrome. Int J Urol 19:443–449. https://doi.org/10.1111/j.1442-2042.2012.02964.x
Foresta C, Caretta N, Palego P, Ferlin A, Zuccarello D, Lenzi A, Selice R (2012) Reduced artery diameters in Klinefelter syndrome. Int J Androl 35:720–725. https://doi.org/10.1111/j.1365-2605.2012.01269.x
Pasquali D, Arcopinto M, Renzullo A, Rotondi M, Accardo G, Salzano A, Esposito D, Saldamarco L, Isidori AM, Marra AM, Ruvolo A, Napoli R, Bossone E, Lenzi A, Baliga RR, Saccà L, Cittadini A (2013) Cardiovascular abnormalities in Klinefelter syndrome. Int J Cardiol 168:754–759. https://doi.org/10.1016/j.ijcard.2012.09.215
Selice R, Caretta N, Di Mambro A, Torino M, Palego P, Ferlin A, Foresta C (2013) Prostate volume and growth during testosterone replacement therapy is related to visceral obesity in Klinefelter syndrome. Eur J Endocrinol 169:743–749. https://doi.org/10.1530/EJE-13-0488
Shanbhogue VV, Hansen S, Jørgensen NR, Brixen K, Gravholt CH (2014) Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in Klinefelter syndrome. J Bone Miner Res 29:2474–2482. https://doi.org/10.1002/jbmr.2272
Chang S, Skakkebæk A, Gravholt CH (2015) Klinefelter Syndrome and medical treatment: hypogonadism and beyond. Hormones (Athens) 14:531–548. https://doi.org/10.14310/horm.2002.1622
Ferlin A, Selice R, Di Mambro A, Ghezzi M, Di Nisio A, Caretta N, Foresta C (2015) Role of vitamin D levels and vitamin D supplementation on bone mineral density in Klinefelter syndrome. Osteoporos Int 26:2193–2202. https://doi.org/10.1007/s00198-015-3136-8
Jørgensen IN, Skakkebaek A, Andersen NH, Pedersen LN, Hougaard DM, Bojesen A, Trolle C, Gravholt CH (2015) Short QTc interval in males with klinefelter syndrome-influence of CAG repeat length, body composition, and testosterone replacement therapy. Pacing Clin Electrophysiol 38:472–482. https://doi.org/10.1111/pace.12580
Lee HS, Park CW, Lee JS, Seo JT (2017) Hypogonadism makes dyslipidemia in Klinefelter's syndrome. J Korean Med Sci 32:1848–1851. https://doi.org/10.3346/jkms.2017.32.11.1848
Sasagawa I, Nakada T, Kazama T, Terada T, Katayama T (1989) Testosterone replacement therapy and prostate/seminal vesicle volume in Klinefelter's syndrome. Arch Androl 22:245–249. https://doi.org/10.3109/01485018908986780
Choi HR, Lim SK, Lee MS (1995) Site-specific effect of testosterone on bone mineral density in male hypogonadism. J Korean Med Sci 10:431–435. https://doi.org/10.3346/jkms.1995.10.6.431
Ozata M, Yildirimkaya M, Bulur M, Yilmaz K, Bolu E, Corakci A, Gundogan MA (1996) Effects of gonadotropin and testosterone treatments on Lipoprotein(a), high density lipoprotein particles, and other lipoprotein levels in male hypogonadism. J Clin Endocrinol Metab 81:3372–3378. https://doi.org/10.1210/jcem.81.9.8784099
Ozata M, Bulur M, Beyhan Z, Sengül A, Saglam M, Turan M, Corakci A, Ali Gundogan M (1997) Effects of gonadotropin and testosterone treatments on prostate volume and serum prostate specific antigen levels in male hypogonadism. Endocr J 44:719–724. https://doi.org/10.1507/endocrj.44.719
Ozata M, Ozisik G, Caglayan S, Yesilova Z, Bingöl N, Saglam M, Turan M, Beyhan Z (1998) Effects of gonadotropin and testosterone treatments on plasma leptin levels in male patients with idiopathic hypogonadotropic hypogonadism and Klinefelter's syndrome. Horm Metab Res 30:266–271. https://doi.org/10.1055/s-2007-978881
Shibasaki T, Sasagawa I, Suzuki Y, Yazawa H, Ichiyanagi O, Matsuki S, Miura M, Nakada T (2001) Effect of testosterone replacement therapy on serum PSA in patients with Klinefelter syndrome. Arch Androl 47:173–176. https://doi.org/10.1080/014850101753145861
Jiang-Feng M, Hong-Li X, Xue-Yan W, Min N, Shuang-Yu L, Hong-Ding X, Liang-Ming L (2012) Prevalence and risk factors of diabetes in patients with Klinefelter syndrome: a longitudinal observational study. Fertil Steril 98:1331–1335. https://doi.org/10.1016/j.fertnstert.2012.07.1122
Condorelli RA, Calogero AE, La Vignera S (2013) Different profile of endothelial cell apoptosis in patients with Klinefelter's syndrome. J Endocrinol Investig 36:84–91. https://doi.org/10.3275/8287
Jo DG, Lee HS, Joo YM, Seo JT (2013) Effect of testosterone replacement therapy on bone mineral density in patients with Klinefelter syndrome. Yonsei Med J 54:1331–1335. https://doi.org/10.3349/ymj.2013.54.6.1331
Chang S, Skakkebæk A, Trolle C, Bojesen A, Hertz JM, Cohen A, Hougaard DM, Wallentin M, Pedersen AD, Østergaard JR, Gravholt CH (2015) Anthropometry in Klinefelter syndrome—multifactorial influences due to CAG length, testosterone treatment and possibly intrauterine hypogonadism. J Clin Endocrinol Metab 100:E508–E517. https://doi.org/10.1210/jc.2014-2834
Garolla A, Selice R, Menegazzo M, Valente U, Zattoni F, Iafrate M, Prayer-Galetti T, Gardiman MP, Ferlin A, Di Nisio A, Foresta C (2018) Novel insights on testicular volume and testosterone replacement therapy in Klinefelter patients undergoing testicular sperm extraction. A retrospective clinical study. Clin Endocrinol (Oxf) 88:711–718. https://doi.org/10.1111/cen.13572
Granato S, Barbaro G, Di Giorgio MR, Rossi FM, Marzano C, Impronta F, Spaziani M, Anzuini A, Lenzi A, Radicioni AF (2019) Epicardial fat: the role of testosterone and lipid metabolism in a cohort of patients with Klinefelter syndrome. Metabolism 95:21–26. https://doi.org/10.1016/j.metabol.2019.03.002
Van den Bergh JP, Hermus AR, Spruyt AI, Sweep CG, Corstens FH, Smals AG (2001) Bone mineral density and quantitative ultrasound parameters in patients with Klinefelter's syndrome after long-term testosterone substitution. Osteoporos Int 12:55–62. https://doi.org/10.1007/s001980170158
Di Minno MN, Esposito D, Di Minno A, Accardo G, Lupoli G, Cittadini A, Giugliano D, Pasquali D (2015) Increased platelet reactivity in Klinefelter men: something new to consider. Andrology 3:876–881. https://doi.org/10.1111/andr.12080
Higgins JPT, Green S (2008) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008]. The Cochrane Collaboration. https://www.cochrane-handbook.org. Accessed 6 Feb 2020
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101. https://doi.org/10.2307/2533446
Corona G, Rastrelli G, Reisman Y, Sforza A, Maggi M (2018) The safety of available treatments of male hypogonadism in organic and functional hypogonadism. Expert Opin Drug Saf 17:277–292. https://doi.org/10.1080/14740338.2018.1424831
Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Resnick SM, Budoff M, Mohler ER 3rd, Wenger NK, Cohen HJ, Schrier S, Keaveny TM, Kopperdahl D, Lee D, Cifelli D, Ellenberg SS (2018) Lessons from the testosterone trials. Endocr Rev 39:369–386. https://doi.org/10.1210/er.2017-00234
Mohr BA, Guay AT, O'Donnell AB, McKinlay JB (2005) Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 62:64–73. https://doi.org/10.1111/j.1365-2265.2004.02174.x
Corona G, Maseroli E, Rastrelli G, Francomano D, Aversa A, Hackett GI, Ferri S, Sforza A, Maggi M (2016) Is late-onset hypogonadotropic hypogonadism a specificage-dependent disease, or merely an epiphenomenon caused by accumulating disease-burden? Minerva Endocrinol 41(2):196–210
Tajar A, Forti G, O'Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Huhtaniemi IT, Wu FC, EMAS Group (2010) Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 95:1810–1818. https://doi.org/10.1210/jc.2009-1796
Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M (2018) Testosterone and cardiovascular risk: meta-analysis of interventional studies. J Sex Med 15:820–838. https://doi.org/10.1016/j.jsxm.2018.04.641
Corona G, Rastrelli G, Guaraldi F, Tortorici G, Reismann Y, Sforza A, Maggi M (2019) An update on heart disease risk associated with testosterone boosting medications. Expert Opin Drug Saf 18:321–332. https://doi.org/10.1016/j.jsxm.2018.04.641
Gagliano-Jucá T, Basaria S (2019) Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol 16:555–574. https://doi.org/10.1038/s41569-019-0211-4
Grossmann M, Matsumoto AM (2017) A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab 102:1067–1075. https://doi.org/10.1210/jc.2016-3580
Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA (2018) Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 103:1715–1744. https://doi.org/10.1210/jc.2018-00229
Yeap BB, Grossmann M, McLachlan RI, Handelsman DJ, Wittert GA, Conway AJ, Stuckey BG, Lording DW, Allan CA, Zajac JD, Burger HG (2016) Endocrine Society of Australia position statement on male hypogonadism (part 1): assessment and indications for testosterone therapy. Med J Aust 205:173–178. https://doi.org/10.5694/mja16.00393
FDA Drug Safety Communication: FDA cautions about using T products for low T due to aging requires labeling change to inform of possible increased risk of heart attack and stroke with use. Published 3 March 2015. https://www.fda.gov/Drugs/DrugSafety/ucm436259.htmforHumanMedicalProducts/ucm402054.htm. Accessed 6 Feb 2020
Summary safety review-testosterone replacement product- cardiovascular risk. Published 15 July 2014. https://www.hc-sc.gc.ca/dhp-mps/medeff/reviews-examens/testosterone-eng.php. Accessed 6 Feb 2020
Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A (2005) Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 63:280–293. https://doi.org/10.1111/j.1365-2265.2005.02339.x
Tracz MJ, Sideras K, Boloña ER, Haddad RM, Kennedy CC, Uraga MV, Caples SM, Erwin PJ, Montori VM (2006) Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab 91:2011–2016. https://doi.org/10.1210/jc.2006-0036
Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M (2018) Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med 15:1260–1271. https://doi.org/10.1016/j.jsxm.2018.06.012
Esposito D, Accardo G, Giallauria F, Bossone E, Vigorito C, Lenzi A, Pasquali D, Isidori AM, Cittadini A (2016) Klinefelter syndrome, cardiovascular system, and thromboembolic disease: Review of literature and clinical perspectives. Eur J Endocrinol. 175(1):R27–40. https://doi.org/10.1530/EJE-15-1025
Funding
No separate funding was necessary for the undertaking of this systematic review and meta-analysis.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is a review paper, therefore no ethical approval was necessary.
Informed consent
This is a review paper, therefore no informed consent was necessary.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pizzocaro, A., Vena, W., Condorelli, R. et al. Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest 43, 1675–1687 (2020). https://doi.org/10.1007/s40618-020-01299-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01299-1