Abstract
Context
Perfluoroalkyl-substances (PFAS) are chemical additives considered harmful for humans. We recently showed that accumulation of perfluoro-octanoic acid (PFOA) in human semen of exposed subjects was associated with altered motility parameters of sperm cells, suggesting direct toxicity.
Objectives
To determine whether direct exposure of human spermatozoa to PFOA was associated to impairment of cell function.
Patients and methods
Spermatozoa isolated from semen samples of ten normozoospermic healthy donors were exposed up to 2 h to PFOA, at concentrations from 0.1 to 10 ng/mL. Viability and motility parameters were evaluated by Sperm Class Analyser. Cell respiratory function was assessed by both mitochondrial probe JC-1 and respiratory control ratio (RCR) determination. Sperm accumulation of PFOA was quantified by liquid chromatography–mass spectrometry. Expression of organic ion-transporters OATP1 and SLCO1B2 was assessed by immunofluorescence and respective role in PFOA accumulation was evaluated by either blockade with probenecid or membrane scavenging through β-cyclodextrin (β-CD). Plasma membrane fluidity and electrochemical potential (ΔΨp) were evaluated, respectively, with Merocyanine-540 and Di-3-ANEPPDHQ fluorescent probes.
Results
Compared to untreated controls, a threefold increase of the percentage of non-motile sperms was observed after 2 h of exposure to PFOA regardless of the concentration of PFOA, whilst RCR was significantly reduced. Only scavenging with β-CD was effective in reducing PFOA accumulation, suggesting membrane involvement. Altered membrane fluidity, reduced ΔΨp and sperm motility loss associated with exposure to PFOA were reverted by β-CD treatment.
Conclusion
PFOA alters human sperm motility through plasma-membrane disruption, an effect recovered by incubation with β-CD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a family of chemical compounds featured by a high degree or complete substitution of hydrogen atoms with fluoride. This characteristic confers chemical stability and thermal inertia, allowing their use in a large number of industrial processes, including textiles, paper, leather processing, paints, general cleaning products, carpets and drapery. On the base of their amphiphilic properties, lack of degradation by environmental physical factors and wide distribution, PFAS have risen many concerns for their bio-accumulation in body tissues and potential harmful effects in humans [1,2,3]. In fact, inhalation of air particles and/or ingestion of contaminated food products and drinking water have been claimed as major routes of exposure to PFAS. Accordingly, PFAS have been found in several human tissues, such as the brain, placenta, semen and testis even in the presence of acknowledged blood/tissue barriers [4,5,6,7,8]. Importantly, exposure to PFAS have been largely recognized in several human populations all over the countries, with considerable differences in terms of geographical distribution, ethnicity, molecular weight (long-chain or short-chain PFAS) and degree of fluorination [9,10,11,12,13,14,15,16,17]. To this regard, perfluoro-octanoic acid (PFOA) and perfluoro-octanesulfonic acid (PFOS) are the most common and most studied PFAS in toxicological terms. In a recent study, we investigated the possible association between the exposure to PFOA and PFOS and endocrine disruption through the evaluation of developmental alterations and reproductive disorders in a group of 212 young males from the Veneto Region, a wide area in the northeast of Italy featured by high environmental exposure to these chemicals [18]. Compared to 171 age-matched controls residing outside of the exposed area, subjects from the contaminated area showed increased levels of circulating testosterone (T) and LH, together with altered anthropometrics, lower mean testicular volume, shorter penile length and anogenital distance. Importantly, quantification of PFOA and PFOS levels in biological fluids showed that PFOA was more represented than PFOS in semen of exposed subjects, despite the pattern of serum concentrations was essentially reversed. In particular, average PFOA levels retrieved in semen samples from exposed subjects were 0.67 ng/mL, ranging from 0 to nearly 6 ng/mL. In addition, semen levels of PFOA were significantly correlated with the presence of altered sperm parameters, in particular motility. This evidence was suggestive of a direct effect of PFOA on gamete function. However, data on this possible direct functional interference are lacking.
On these bases, in this study we investigated the possible disrupting role PFOA on human sperm function such as viability and motility. In particular, since PFAS are suggested to interfere with cell function through the alteration of plasma-membrane characteristics [19], we evaluated the possible site of accumulation of PFOA in sperm cells by the use of specific membrane transport-inhibition and cyclodextrin-based scavenging approach. Finally, we provided mechanisms insight underpinning the observed effect on cell motility by the evaluation of plasma membrane properties through specific fluorescent probes.
Materials and methods
Chemicals and reagents
Probenecid, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benimidazolylcarbocyanine iodide (JC-1), Merocyanine 540 (MC540), methyl-β-cyclodextrin (β-CD) and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich S.r.l. (Milan, Italy). Di-3-ANEPPDHQ was purchased from Thermo Fisher Scientific (Milano, Italy). Perfluoro-octanoic acid native compound was purchased from Wellington Laboratories (Southgate, Ontario, Canada) Chemicals were dissolved in (DMSO, Sigma-Aldrich S.r.l.) to a stock concentration of 0.01 M and stored at − 20 °C until use. Stock solutions were then diluted in Sperm Wash Medium (SWM, Irvine Scientific, Santa Ana, CA) immediately before use at the final concentrations detailed below. Rabbit polyclonal anti OATP1 and SLCO2B1 antibodies were purchased from Thermo Fisher Scientific.
Patients, sample collection and preparation
This study was approved by the Institutional Ethics Committee of the Hospital of Padova, Italy, (protocol number 2208P and successive amendments). The investigation was performed according to the principles of the Declaration of Helsinki. Ten normozoospermic healthy donors, attending the University Andrology Unit as participants in an infertility survey program (age ≥ 20 and ≤ 35 years) were included in the study after provided written consent. To reduce any inter-subject variability, patients underwent 4 separate sessions of semen donation by masturbation, taking care to maintain at least 3 days of sexual abstinence between each donation. Cervical mucus was obtained from 10 women resident in highly exposed areas Veneto region and from 10 age-matched control subjects from low exposure areas, during routine outpatient gynecological evaluation.
Human semen samples were obtained by masturbation after 2–5 days of sexual abstinence, in sterile containers. Samples were allowed to liquefy for 30 min and were examined for seminal parameters according to the World Health Organization criteria [20]. All samples had normal viscosity and leukocyte count was recorded. All semen cultures were negative and anti-sperm antibodies were absent in all subjects. Population characteristics and semen parameters of healthy donors are summarized in supplemental Table S1. Each semen sample was washed with Sperm Washing Medium, centrifuged at 1000 ×g for 10 min and resuspended in SWM. In agreement with PFOA levels found in semen from exposed subjects [18], sperm cells were then incubated with PFOA at a final concentration ranging from 0.1 to 10 ng/mL, for 1 or 2 h as detailed in the results section.
Sperm motility parameters were assessed using the Sperm Class Analyser (SCA, Microptic S.L., Barcelona, Spain) as previously described [21]. For the evaluation of sperm hyperactivation, the SCA cutoff points defined were: capture at 50 frames/s, curvilinear velocity (VCL) > 150 μm/s, linearity < 40% and amplitude of lateral head displacement > 3.5. In accordance with WHO guidelines, 200 spermatozoa were analyzed which is approximately equivalent to the capture of 5 or 6 randomly selected microscopic fields. After every scan, video sequences were assessed to validate whether all spermatozoa had been identified and that their trajectory had been correctly reconstructed by the SCA system. Sperm motility parameters considered were: progressive motility, non-progressive motility and absent motility; curvilinear velocity (VLC), straight-line velocity (VSL), average path velocity (VAP), amplitude of lateral head displacement (ALH), linearity of a curvilinear path (LIN, defined as VSL/VLC), straightness (STR, defined as VSL/VAP), wobble (WOB, defined as VAP/VCL), beat-cross frequency (BCF), hypermotile sperm fraction (Hyper) with the Sperm Class Analyser (SCA, Microptic S.L., Barcelona, Spain) as previously described [21].
Immunofluorescence
For immunofluorescence assay, aliquots of sperm cell samples were fixed in 4% paraformaldehyde/PBS solution for 15 min at room temperature, smeared on poly-lysine-coated microscope glasses and let dry. Specimens were then saturated with 5% BSA/5% normal donkey serum-PBS solution for 1 h at room temperature and then incubated with anti OATP1 or SLCO2B1 antibodies (final concentration 1 μg/mL for both) at 4 °C overnight. Primary immunoreaction was then detected with FITC-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Heidelberg, Germany). Samples were finally counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich), mounted with anti-fade buffer and the analyzed with Video Confocal-ViCo fluorescence microscope (Nikor-Firenze, Italy).
PFOA quantification in sperm cells by liquid chromatography–mass spectrometry
The quantification of sperm content of PFOA was measured through reversed-phase (RP) liquid chromatography coupled with high-resolution mass spectrometry (LC–MS) Agilent Varian 320 (Agilent Technologies, Santa Clara, CA, USA). Briefly, each sample was extracted with acetonitrile and fixed amounts of the stable isotope labeled internal standard were added (MPFOA, Wellington Laboratories, Ontario, Canada). To test the analytical response and to optimize calibration curve standard mixture was used at increasing concentrations (PFAC-MXB, Wellington Laboratories) together with isotope-labeled internal standards (MPFOA) at fixed concentrations. This solution was analyzed by LC–MS. The perfluoroalkyl specie was identified by comparing the retention time and mass spectra (i.e., m/z value and isotopic pattern). Quantification was calculated using the corresponding calibration curve.
Flow cytometry
After experimental treatment, sperm cells were evaluated for functional parameters by flow cytometry as follows. Mitochondrial membrane potential (ΔΨm) and plasma-membrane electrochemical potential (ΔΨp) were evaluated with JC-1 and Di-3-ANEPPDHQ fluorescent probes, respectively, as previously described [22]. Evaluation of plasma membrane fluidity was evaluated by MC540 probe [23]. Briefly, DMSO-stock solution of MC540 was diluted in sperm suspension at the final concentration of 4 μM and incubated for 15 min at 37 °C in the dark. Cells were finally analyzed by BD FACScan System (Becton–Dickinson, Milano, Italy) as previously described [21]. At least 105 events per session, corresponding to sperm cells, were recorded.
Mitochondria respiration studies
Spermatozoa were collected by centrifugation at 800g for 10 min at room temperature and washed by resuspension in isotonic salt medium (2 g/L bovine serum albumin, 113 mM KCl, 12.5 mM KH2PO4, 2.5 mM KH2PO4, 3 mM MgCl2, 0.4 mM ethylenediaminetetraacetic acid, and 20 mM Tris adjusted to pH 7.4 with HCl). Subsequently, cells were subjected to hypotonic treatment and their oxygen uptake was measured as previously described [24] The respiration capacity of hypotonically treated spermatozoa was tested polarographically, in the presence of a solution of 10 mM pyruvate and 10 mM malate (respiratory substrates) and 0.76 μM of adenosine diphosphate (ADP). The respiratory control ratio (RCR), an index of mitochondrial respiration efficiency, was calculated by dividing V3 (rate of oxygen uptake measured in the presence of respiratory substrates + ADP) by V4 (rate of oxygen uptake measured with respiratory substrates alone).
Statistical analysis
The results were expressed as means ± standard deviations (SD). Prior to data analysis, the Kolmogorov–Smirnov test was used to check for normality of distribution. Parameters not showing normal distribution were log-transformed. The Levene’s test was used to test the homogeneity of variance among groups. If homogeneity of variance assumption was violated, Welch test was performed and the respective p value was reported. Differences between 2 or more groups were evaluated respectively using Student’s t test or ANOVA for comparison of multiple parameters with Bonferroni correction. p values < 0.05 were considered as significant.
Results
Direct exposure to PFOA alters sperm motility parameters
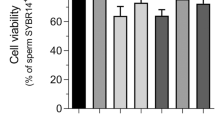
To disclose a possible direct effect of PFOA on sperm function, cells were exposed up to 2 h with the chemical compound at different concentrations. In particular, on the base of PFOA levels detected in semen samples [18], three levels of concentration were chosen: 0.1, 1 and 10 ng/mL. In control condition, PFOA was omitted (Fig. 1a). Compared to control condition, incubation with PFOA was associated with negligible effects on sperm viability, independently from the exposure time and concentration. Differently, progressive motility was significantly impaired by PFOA even at the lowest concentration of 0.1 ng/mL since the percentage of sperm cells with progressive forward motion was significantly reduced after 1 h of incubation compared to control. No significant differences were observed among the three levels of concentration at different time points. On the other hand, the percentage of cells with non-progressive motility showed to be significantly increased only after 2 h of exposure to PFOA at either 1 or 10 ng/mL, with no differential effect between the two conditions. These data reflected on the percentage of non-motile sperm cells that showed a significant increase after 1 h of exposure, since the lowest concentration of 0.1 ng/mL. After 2 h, a nearly threefold increase of the percentage of non-motile sperms was observed compared to control, with no significant dependence on the concentration of PFOA.
a Time course of sperm viability, progressive motility, non-progressive motility and absent motility in human sperm cells exposed to perfluoro-octanoic acid (PFOA), at different concentrations, up to 2 h at 37 °C. Comparisons are made with PFOA-free control condition (CTRL) at each time point. Significance: *P < 0.05 and **P < 0.01 vs CTRL. b Effect of exposure to PFOA 10 ng/mL for 2 h at 37 °C on detailed sperm motility parameters evaluated at sperm class analyser (SCA) such as: Hyper, hypermotile sperm fraction; VLC, curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity; ALH, amplitude of lateral head displacement; LIN, linearity of a curvilinear path (LIN, defined as VSL/VLC); STR, straightness (STR defined as VSL/VAP); wobble (WOB, defined as VAP/VCL); BCF, beat-cross frequency. SCA data of sperm cells prior to PFOA exposure (Basal) and cells cultured in absence of PFOA for 2 h at 37 °C (CTRL) are also reported. Significance: *P < 0.05 and ***P < 0.001 vs CTRL. Effect of exposure to PFOA 10 ng/mL for 2 h at 37 °C on mitochondrial membrane potential (ΔΨm, c), evaluated by JC-1 staining, and respiratory control ratio (RCR, D). Data of sperm cells prior to PFOA exposure (Basal) and cells cultured in absence of PFOA for 2 h at 37 °C (+ 2 h PFOA 0 ng/mL) are also reported. Significance: *P < 0.05 and n.s. P > 0.05 among indicated conditions. Data show the mean value of three independent experiments
The analysis of detailed sperm motility parameters, evaluated by SCA, was considered for the comparison among freshly ejaculated sperms at basal condition and sperm cells maintained for 2 h in SWM either absence or presence of PFOA at the concentration of 10 ng/mL (Fig. 1B). As expected, prolonged maintenance of sperms in culture media containing capacitating substances, like albumin, was associated with the increase of all parameters characterizing progressive motility at SCA analysis, such as hyper motility, curvilinear velocity, straight-line velocity and average path velocity, that showed higher values compared to basal conditions [25]. On the other hand, incubation with PFOA was associated with significant reduction of the aforementioned parameters compared to control conditions, reflecting an evident influence of PFOA on the motility gain of sperm. To investigate the molecular base subtending this evidence, we focused on the effect of PFOA on cell respiration evaluating both mitochondrial membrane potential (ΔΨm) and cell-oxygen consumption, respectively (Fig. 1c, d). Whilst sperm cells cultured in absence or presence PFOA showed no significant variation on the percentage of sperm cells with altered ΔΨm compared to basal, mitochondrial respiration efficiency was significantly affected by PFOA as depicted by the reduction of RCR, in comparison to both basal and control condition without PFOA.
Taken together, these results suggest a direct influence of PFOA on sperm motility, likely related to the impaired metabolic performance associated with a decreased mitochondrial respiratory activity.
Evidence of PFOA accumulation in the plasma membrane of sperm cells
To disclose a possible accumulation in spermatozoa, the cell amount of PFOA after continuous 2 h exposure at the concentration of 10 ng/mL was quantified by liquid chromatography–mass spectrometry (Fig. 2a). In comparison with very low levels detected in sperm cells at basal conditions, a significant and time-dependent increase of PFOA cell content was observed after 1 and 2 h of exposure, suggesting some degree of accumulation. Further experiments were thus performed to elucidate the accumulation site and the corresponding mechanism.
a Liquid chromatography–mass spectrometry (LC–MS) quantification of perfluoro-octanoic acid (PFOA) content in sperm cells exposed to PFOA 10 ng/mL up to 2 h at 37 °C. Quantitative data of sperm cells prior to PFOA exposure (Basal) are also reported. Significance: *P < 0.05, **P < 0.01 and ***P < 0.001 among indicated conditions. b Representative images of the expression of organic anion transporters (OAT) OAT1 and OATP4 in human sperm cells, assessed by immunofluorescence. Cell nuclei are counterstained with DAPI. Bright-field merges are provided as insets. In blank samples (Neg), primary antibodies are omitted. c LC–MS quantification of PFOA in sperm cells exposed to PFOA 10 ng/mL for 2 h at 37 °C, preceded or not by incubation with OAT inhibitor Probenecid 500 μM. In control conditions, PFOA was omitted. Significance: *** P < 0.001 and n.s. P > 0.05 among indicated conditions. d LC–MS quantification of PFOA in sperm cells exposed to PFOA 10 ng/mL for 2 h at 37 °C, followed by incubation with β-cyclodextrin (CD), at a concentration ranging from 0 to 10 mM, for 30 min at 37 °C. In basal conditions (Basal), PFOA was omitted. Significance: ***P < 0.001 vs Basal. Data show the mean value of three independent experiments
Previous studies focused on mechanistic elucidation of PFOA renal elimination, showed that tubular re-absorption of PFOA in kidney is sensitive to probenecid [26]. Probenecid is a known inhibitor of active renal transport and is effective on SLC22A6 (OAT1) and SLCO1B2 (OATP4) members of the organic anion transporter family [27,28,29,30,31]. Accordingly, we evaluated the possible expression of these OATs in sperm cells by immunofluorescence, finding a specific immune-staining for these membrane transporters at cell neck and flagellum (Fig. 2b). However, sperm exposure to PFOA in presence of 500 μM probenecid, was not associated with a significant reduction of the cell content of the perfluoroalkyl substance (Fig. 2d), ruling out a major involvement of OATs in the uptake of PFOA in spermatozoa.
On the other hand, on the base of the strict similarity to fatty acids, the possible interaction of PFOA with plasma membrane lipid bilayer has been suggested [32,33,34]. To verify this hypothesis, we took advantage of β-cyclodextrins (β-CD), cyclic oligo-saccharides made of 7 glucose units. The structural peculiarity of these compounds is having a large molecular size, that render β-CD impermeable to intact cell membranes, to be highly water-soluble but to display an inner hydrophobic cavity able to host several lipophilic substances. Due to these characteristics, β-CD have been widely used to experimentally modify the composition of plasma membranes through the removal of lipid components, such as sterols, from the lipid bilayer [35]. In addition, PFOA has been recently shown to bind with high affinity to β-CD [36]. On these bases, we assessed the possible accumulation of PFOA within cell membrane through the ability of β-CD to reduce the sperm PFOA content after previous incubation. Accordingly, sperm cells were exposed for 2 h with PFOA and were successively treated with β-CD at concentrations ranging from 0 to 10 mM for additional 30 min. Quantification by liquid chromatography–mass spectrometry showed a sharp reduction of sperm PFOA content, even at the lowest β-CD concentration of 1 mM (Fig. 2d).
Taken together, these results suggest that PFOA accumulates in sperm membranes, a process reverted by water-soluble lipid-sequestrants like β-CD.
PFOA impairs sperm motility through the likely alteration of plasma membrane fluidity and electrochemical potential
To address the downstream consequences associated with PFOA accumulation, specific properties of plasma membrane were evaluated by the use of fluorescent probes.
Membrane fluidity, acknowledged as a major determinant of sperm motility and fertilization potential, is associated with decreased packing order of phospholipids in the outer layer of the plasma membrane and can be evaluated by the use of the hydrophobic dye Merocyanine 540 (MC540, [37,38,39,40]. Membrane fluidity of sperm was assessed with MC540 after exposure to PFOA for 2 h, eventually followed by further 30 min incubation with 1 mM β-CD. The staining pattern for MC540 outlined a two cell populations with, respectively, “bright” staining and “mild” staining, corresponding to cells with high and low membrane fluidity (respectively, M1 and M2 domains in Fig. 3a, panel I). Compared to control condition (no exposure to PFOA and no β-CD treatment), exposure to PFOA was associated with a significant increase of both the cell fractions with bright staining for MC540 and the corresponding mean fluorescence value, with complementary decrease of the same parameters for the fraction with mild staining (Fig. 3a, panel II). This evidence was suggestive of a significant increase of membrane fluidity due to PFOA accumulation. Incubation with the sole β-CD also showed an increase of the population with bright staining and the corresponding mean fluorescence value, confirming previous data on increased membrane fluidity due to sterol withdrawal by β-CD [41]. However, the extent of this variation was lower compared with the exposure to PFOA. After PFOA incubation, addition of β-CD was apparently ineffective on the staining pattern for MC540 compared with the sole exposure to PFOA, compatible with a decreased packing order of phospholipids in the outer layer operated by both chemicals.
a Representative histogram plots of merocyanine 540 staining (MC540, panel I), evaluated by flow cytometry, of human sperm cells exposed or not to perfluoro-octanoic acid (PFOA) 10 ng/mL for 2 h at 37 °C, followed or not by incubation with β-cyclodextrin (CD) 1 mM CD for 30 min at 37 °C. In control conditions (CTRL), both PFOA and CD were omitted. Panel II summarizes the percentage of cells owning to M1 or M2 domains, corresponding to cells with bright or low staining for MC540, and the respective mean value of fluorescence intensity in arbitrary units (A.U.). Significance: *P < 0.05 and **P < 0.01 vs CTRL. b Representative histogram plots of Di-3-ANEPPDHQ staining (Di-3-ANEPPDHQ, panel I), evaluated by flow cytometry, of human sperm cells exposed or not to PFOA 10 ng/mL for 2 h at 37 °C, followed of not by incubation CD for 30 min at 37 °C. In control conditions (CTRL), both PFOA and CD were omitted. Panel II summarizes the percentage of cells owning to M1 or M2 domains, corresponding to cells with bright or low staining for Di-3-ANEPPDHQ. Significance: *P < 0.05 vs CTRL. Data show the mean value of three independent experiments
Increased permeability to ions and reduction of electrochemical potential have been claimed as major consequences of increased membrane fluidity in different cell models [42, 43]. Accordingly, by the use of Di-3ANEPPDHQ fluorescent dye [22], we evaluated whether variations of membrane fluidity due to the exposure to PFOA were also associated with altered electrochemical potential (ΔΨp). As for MC540, staining for Di-3ANEPPDHQ identified two cell populations: one with bright staining and one with mild staining, corresponding to cells high ΔΨp and low ΔΨp, respectively (M1 and M2 domains in Fig. 3b, panel I). Exposure to PFOA was associated with a significant increase of the cell population with low ΔΨp compared to control (Fig. 3b, panel II). However, post incubation of PFOA with β-CD restored the percentage of sperms high ΔΨp. Treatment with β-CD only was not associated with significant variation of the staining pattern for Di-3ANEPPDHQ compared control.
Since plasma membrane potential is strictly related to sperm motility [22, 44], we evaluated whether the observed restoration of ΔΨp due to treatment with β-CD after exposure to PFOA was associated with a possible recovery of motility in sperm cells (Fig. 4a). Compared to control, 2 h of exposure to PFOA was associated with significant reduction of progressive motility and a corresponding increase of both the non-progressive motile and non-motile fraction as previously reported. However, further incubation with 1 mM β-CD for 30 min was associated with almost complete recovery of motility parameters, barely resulting in overlapping with the motility pattern of control samples at the same time point. Incubation with β-CD had non-significant effects on both progressive-, non-progressive motility and non-motile sperms, compared to matched time points of the control. Evaluation of all parameters characterizing progressive motility at SCA analysis, showed that incubation with β-CD after exposure to PFOA was associated with a significant recovery of the hyper-motile fraction, the curvilinear and the straight-line velocity, as well as of the straightness and wobble parameters (Fig. 4b). The same pattern was observed after the sole incubation with β-CD.
a Time course of sperm viability, progressive motility, non-progressive motility and absent motility in human sperm cells exposed to perfluoro-octanoic acid (PFOA) 10 ng/mL for 2 h at 37 °C, followed or not by incubation with β-Cyclodextrin (CD) 1 mM CD for 30 min at 37 °C. Comparisons are made with PFOA-free control condition (CTRL) at each time point. Significance: *P < 0.05 and **P < 0.01 vs CTRL. b Effect of exposure to PFOA 10 ng/mL for 2 h at 37 °C, followed or not by incubation with β-Cyclodextrin (CD) 1 mM CD for 30 min at 37 °C, on detailed sperm motility parameters evaluated at sperm class analyser (SCA) such as: Hyper, hypermotile sperm fraction; VLC, curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity; ALH, amplitude of lateral head displacement; LIN, linearity of a curvilinear path (LIN, defined as VSL/VLC); STR, straightness (STR, defined as VSL/VAP); WOB, wobble (WOB, defined as VAP/VCL); BCF, beat-cross frequency. SCA data of sperm cells prior to PFOA exposure (Basal) and cells cultured in absence of both PFOA and CD (CTRL) are also reported. Significance: aP < 0.05, bP < 0.01 and c P < 0.001 vs CTRL. Data show the mean value of three independent experiments
Taken together, these data suggest that the impairment of sperm motility associated with the exposure to PFAS, likely relies on the alteration of plasma-membrane potential possibly due to disruption of membrane fluidity. Treatment with β-CD, was observed to reduce the membrane content of PFOA and to essentially restore the electrochemical properties of plasma membrane and motility parameters.
Evidence of PFOA accumulation in cervical mucus of exposed women
On the base of the wide bio-accumulation of PFAS, it can be hypothesized that sperm cells may come into contact with PFAS in seminal plasma of exposed males and/or with PFAS in genital secretion of exposed females. Accordingly, we quantified PFOA by liquid chromatography–mass spectrometry in both serum and cervical mucus from 10 women resident in highly exposed areas in Veneto region (EXP). Results were compared results with those of 10 control subjects from low-exposure areas (CTRL). The two groups of subjects did not differ for age (EXP 20.56 ± 1.03 years vs CTRL 21.12 ± 0.98 years; P = 0.945), weight (EXP 61.11 ± 12.31 kg vs CTRL 59.74 ± 9.85; P = 0.431), height (EXP 1.71 ± 0.12 m vs CTRL 1.69 ± 0.21; P = 0.711) and BMI (EXP 21.11 ± 4.01 kg/m2 vs CTRL 20.95 ± 3.84 kg/m2, P = 0.33).
Both serum and cervical mucus from exposed women showed higher levels of PFOA compared to control subjects (serum: 36 ± 6.9 ng/mL exposed vs 5.1 ± 3.0 controls, P = 0.021; cervical mucus 5.9 ± 1.2 ng/mL exposed vs 0.14 ± 0.10 ng/mL controls, P = 0.009). These data are suggestive that female genital secretion may represent an accumulation site of PFAS and a possible source of exposure for ejaculated sperm cells.
Discussion
In this study we provide evidence of a direct detrimental effect of the exposure to perfluoro-octanoic acid on human sperm motility, associated with a significant increase of the non-motile cell fraction at concentrations comparable to average levels observed in human semen retrieved from subjects residing in highly exposed areas. In addition, we provide mechanistic insights of this evidence, showing that PFOA accumulates within the lipid bilayer of sperm plasma membrane, altering the fluidity and the electrochemical potential of this organelle and, in turn, the sperm oxygen consumption. This model is further supported by the fact that treatment with β-cyclodextrin, an acknowledged sequestrant of membrane lipids, effectively reduces sperm PFOA content, restoring cell biochemical and motility properties.
Widely and indiscriminately used over the past decades for several industrial applications, PFAS are currently concerned for their chemical stability to hydrolysis, photolysis, or microbial degradation that associates with persistent environmental accumulation [45,46,47]. In turn, PFAS have been recognized to accumulate in several human fluids and tissues [48], showing also some sex-dependent toxicodynamic since higher PFAS levels have been detected in adult men compared to women, an evidence possibly linked to lower clearance [49,50,51,52]. To this regard, association studies frequently linked PFAS exposure with alterations of male reproductive system. In particular, Joensen et al. [53] reported that young men with high serum levels of PFOS and PFOA had unexplained low semen quality, whilst a recently study from Governini et al. [54] reported altered sperm quality, higher index of chromatin fragmentation, as well as an increase of the chromosomal aneuploidy rate in sperm cells from subjects with elevate PFAS concentration in serum and seminal plasma. However, in spite of this evidence, a mechanistic hypothesis of PFAS toxicity has not been provided yet. Very recent data from our group showed a strong reduction of sperm motility in male subjects exposed to PFAS [18]. This evidence was highly suggestive of a direct effect of these substances on the gamete function. Here we provide a mechanistic model supporting this hypothesis. In particular, membrane scavengers β-cyclodextrins succeeded in abrogating PFAS accumulation in sperm cells. This capacity was not detected for probenecid, a known inhibitor of PFOA cell re-absorption through the blockade of organic anions transporting protein. This evidence suggests that PFOA accumulates within sperm plasma membrane altering, in turn, membrane fluidity, membrane potential, oxygen consumption and cell mobility. Plasma membrane is a key organelle involved in sperm physiology and the fine tuning of its composition, from ejaculation to fecundation, has been critically linked to the overall efficacy of the fertilization process (Reviewed in [55]). In particular, there is a general consensus that a progressive gain of membrane fluidity is gathered during the transit through the female reproductive tract thanks to cholesterol deprivation by sterols acceptors like albumin of HDL [56,57,58]. Importantly, cholesterol is not uniformly widespread on sperm membrane but generally accumulates in lipid rafts together with ion channels and membrane proteins. Hence, ordered removal of cholesterol from these highly functional interfaces triggers a cascade of cell events associated with the gain of progressive motility and fusogenic properties [59]. It can be speculated that, because of the high hydrophobicity of perfluoroalkyl substances, PFOA randomly accumulates in sperm membranes, altering local pH and permeability to ionic species and, in turn, membrane potential as recently observed also in somatic cell models [32]. In agreement with this model, the local perturbation of membrane composition may also result in the production of free radicals, as recently demonstrated for other chemical species such as graphene-oxide [60], possibly explaining the aforementioned association between PFAS exposure and sperm-DNA damage. On the other hand, incubation with β-CD likely restores membrane properties by widespread removal of both PFOA and cholesterol, with consequent activation of canonic pathways involved in sperm capacitation and gain of hypermotility [61]. Intriguingly, exposure to PFOA was associated with decreased mitochondrial respiratory activity, but unvaried mitochondrial membrane potential. Similar effects have been previously described for other chemical species having more physiological significance, such as Zinc (Zn). Secreted by the prostate, Zn is detected at very high concentration in seminal plasma where it exerts physiologic modulation of sperm motility. In fact, Zn reduces reduce sperm oxygen consumption through the direct inhibitory effects on specific receptors expressed on sperm flagellum, as well as reducing cell respiration through blockade of mitochondrial aconitase [62, 63]. Nevertheless, none of these events were associated with mitochondrial toxicity and reduction of ΔΨm [64, 65]. Hence, these results are not contradictory since ΔΨm represents a general marker of the energetic state of the mitochondria and the cell, whilst RCR values provides a much more detailed information of the efficiency of oxidative phosphorylation process.
We acknowledge the exclusive in vitro nature of these results that have been obtained on the base of the detection of PFAS within seminal plasma of exposed subject, lacking of the exact source and time of exposure of sperm cells to PFAS within the male genital-urinary tract. However, in this study we applied levels of exposure to PFOA which are highly comparable with those retrieved in semen from subjects residing in areas of high environmental pollution, reporting conclusive findings about the possible direct toxicity of PFOA on sperm function. Furthermore, we provide evidence that exposure to the perfluoroalkyl substances may also derive from female genital secretions, opening novel scenarios about the reproductive toxicity of these pollutants and the corresponding screening for exposure. Of note, average levels of PFOA in cervical mucus were nearly tenfold of those observed in semen (~ 6 vs ~ 0.7 ng/mL, respectively). Accordingly, all experiments were performed at 10 ng/mL of PFOA, being an order of magnitude closer to the average exposure levels experienced by the sperm in the post-ejaculatory phase.
In conclusion, here we demonstrate that PFOA alters human sperm motility as a downstream effect of plasma-membrane disruption and that incubation with CD is associated almost complete recovery from PFOA toxicity. Further studies are required to disclose the specific influence on fertilization and embryo development.
References
ATSDR (2008) Agency for toxic substances and disease registry. In: Draft Toxicological Profile for Perfluoroalkyls, Atlanta
Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Env Assess Manag 7:513–541. https://doi.org/10.1002/ieam.258
Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, Andersen CY, Damdimopoulou P (2019) Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Env Int 124:482–492. https://doi.org/10.1016/j.envint.2019.01.010
Austin ME, Kasturi BS, Barber M, Kannan K, Mohan Kumar PS, Mohan Kumar SM (2003) Neuroendocrine effects of perfluorooctane sulfonate in rats. Env Health Perspect 111:1485–1489. https://doi.org/10.1289/ehp.6128
Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y,. Kishi R, Nakazawa H. (2004) Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect112(11):1204–1207. https://doi.org/10.1289/ehp.6864 (PMID: 15289168)
Fei C, McLaughlin JK, Tarone RE, Olsen J (2007) Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Env Health Perspect 115:1677–1682. https://doi.org/10.1289/ehp.10506
Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, Kim S, Park S, Hwang I, Jeon J, Yang H, Giesy JP (2011) Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Env Sci Technol 45:7465–7472. https://doi.org/10.1021/es202408a
Li N, Mruk DD, Chen H, Wong CKC, Lee WM, Cheng CY (2016) Rescue of perfluorooctanesulfonate (PFOS)–mediated Sertoli cell injury by overexpression of gap junction protein connexin 43. Sci Rep 6:29667. https://doi.org/10.1038/srep29667
Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL (2007) Polyfluoroalkyl chemicals in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Env Health Perspect 115:1596–1602. https://doi.org/10.1289/ehp.10598
EFSA (European Food Safety Authority) (2008) Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts: scientific opinion of the panel on contaminants in the food chain. EFSA J 653:1–131. https://doi.org/10.2903/j.efsa.2008.653
Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D (2009) Perfluorinated compounds—exposure assessment for the general population in Western countries. Int J Hyg Env Health 212:239–270. https://doi.org/10.1016/j.ijheh.2008.04.007
Roosens L, D’Hollander W, Bervoets L, Reynders H, Van Campenhout K, Cornelis C, Van Den Heuvel R, Koppen G, Covaci A (2010) Brominated flame retardants and perfluorinated chemicals, two groups of persistent contaminants in Belgian human blood and milk. Env Pollut 158:2546–2552. https://doi.org/10.1016/j.envpol.2010.05.022
Stahl T, Mattern D, Brunn H (2011) Toxicology of perfluorinated compounds. Env Sci Eur 23:38. https://doi.org/10.1186/2190-4715-23-38
Domingo JL (2012) Health risks of dietary exposure to perfluorinated compounds. Env Int 40:187–195. https://doi.org/10.1016/j.envint.2011.08.001
Cornelis C, D’Hollander W, Roosens L, Covaci A, Smolders R, Van Den Heuvel R, Govarts E, Van Campenhout K, Reynders H, Bervoets L (2012) First assessment of population exposure to perfluorinated compounds in Flanders, Belgium. Chemosphere 86:308–314. https://doi.org/10.1016/j.chemosphere.2011.10.034
Lindh CH, Rylander L, Toft G, Axmon A, Rignell-Hydbom A, Giwercman A, Pedersen HS, Góalczyk K, Ludwicki JK, Zvyezday V, Vermeulen R, Lenters V, Heederik D, Bonde JP, Jönsson BA (2012) Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations. Chemosphere 88:1269–1275. https://doi.org/10.1016/j.chemosphere.2012.03.049
Ding G, Xue H, Yao Z, Wang Y, Ge L, Zhang J, Cui F (2018) Occurrence and distribution of perfluoroalkyl substances (PFASs) in the water dissolved phase and suspended particulate matter of the Dalian Bay, China. Chemosphere 200:116–123. https://doi.org/10.1016/j.chemosphere.2018.02.093
Di Nisio A, Sabovic I, Valente U, Tescari S, Rocca MS, Guidolin D, Dall’Acqua S, Acquasaliente L, Pozzi N, Plebani M, Garolla A, Foresta C (2019) Endocrine disruption of androgenic activity by perfluoroalkyl substances: clinical and experimental evidence. J Clin Endocrinol Metab 104:1259–1271. https://doi.org/10.1210/jc.2018-01855
Qiao W, Zhang Y, Xie Z, Luo Y, Zhang X, Sang C, Xie S, Huang J (2019) Toxicity of perfluorooctane sulfonate on Phanerochaete chrysosporium: growth, pollutant degradation and transcriptomics. Ecotoxicol Env Saf 174:66–74. https://doi.org/10.1016/j.ecoenv.2019.02.066
World Health Organization (2010) Department of Reproductive Health and Research. In: WHO laboratory manual for the examination and processing of human semen; Fifth edition. Switzerland
Zuccarello D, Ferlin A, Garolla A, Menegazzo M, Perilli L, Ambrosini G, Foresta C (2011) How the human spermatozoa sense the oocyte: a new role of SDF1-CXCR4 signalling. Int J Androl 34(6 Pt 2):e554–e565. https://doi.org/10.1111/j.1365-2605.2011.01158.x
Grami D, Rtibi K, Selmi S, Jridi M, Sebai H, Marzouki L, Šabovic I, Foresta C, De Toni L (2018) Aqueous extract of Eruca Sativa protects human spermatozoa from mitochondrial failure due to bisphenol A exposure. Reprod Toxicol 82:103–110. https://doi.org/10.1016/j.reprotox.2018.10.008
Muratori M, Porazzi I, Luconi M, Marchiani S, Forti G, Baldi E (2004) AnnexinV binding and merocyanine staining fail to detect human sperm capacitation. J Androl 25:797–810. https://doi.org/10.1002/j.1939-4640.2004.tb02858.x
Ferramosca A, Focarelli R, Piomboni P, Coppola L, Zara V (2008) Oxygen uptake by mitochondria in demembranated human spermatozoa: a reliable tool for the evaluation of sperm respiratory efficiency. Int J Androl 31:337–345. https://doi.org/10.1111/j.1365-2605.2007.00775.x
Bedu-Addo K, Lefièvre L, Moseley FL, Barratt CL, Publicover SJ (2005) Bicarbonate and bovine serum albumin reversibly “switch” capacitation-induced events in human spermatozoa. Mol Hum Reprod 11:683–691. https://doi.org/10.1093/molehr/gah226
Alonso CAI, Osycka-Salut CE, Castellano L, Cesari A, Di Siervi N, Mutto A, Johannisson A, Morrell JM, Davio C, Perez-Martinez S (2017) Extracellular cAMP activates molecular signalling pathways associated with sperm capacitation in bovines. Mol Hum Reprod 23:521–534. https://doi.org/10.1093/molehr/gax030
Mor AL, Kaminski TW, Karbowska M, Pawlak D (2018) New insight into organic anion transporters from the perspective of potentially important interactions and drugs toxicity. J Physiol Pharmacol. https://doi.org/10.26402/jpp.2018.3.01
Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW (2012) Renal elimination of perfluorocarboxylates (PFCAs). Chem Res Toxicol 25(1):35–46. https://doi.org/10.1021/tx200363w
Vanden Heuvel JP, Davis JW 2nd, Sommers R, Peterson RE (1992) Renal excretion of perfluorooctanoic acid in male rats: inhibitory effect of testosterone. J Biochem Toxicol Spring 7:31–36
Ylinen M, Hanhijärvi H, Jaakonaho J, Peura P (1989) Stimulation by oestradiol of the urinary excretion of perfluorooctanoic acid in the male rat. Pharmacol Toxicol 65:274–277. https://doi.org/10.1111/j.1600-0773.1989.tb01172.x
Takeda M, Khamdang S, Narikawa S, Kimura H, Kobayashi Y, Yamamoto T, Cha SH, Sekine T, Endou H (2002) Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther 300:918–924. https://doi.org/10.1124/jpet.300.3.918
Kleszczyński K, Składanowski AC (2009) Mechanism of cytotoxic action of perfluorinated acids. I. alteration in plasma membrane potential and intracellular pH level. Toxicol Appl Pharmacol 234:300–305. https://doi.org/10.1016/j.taap.2008.10.008
Nouhi S, Ahrens L, Campos Pereira H, Hughes AV, Campana M, Gutfreund P (2018) Interactions of perfluoroalkyl substances with a phospholipid bilayer studied by neutron reflectometry. J Colloid Interface Sci 511:474–481. https://doi.org/10.1016/j.jcis.2017.09.102
Fitzgerald NJM, Wargenau A, Sorenson C, Pedersen J, Tufenkji N, Novak PJ, Simcik MF (2018) Partitioning and accumulation of perfluoroalkyl substances in model lipid bilayers and bacteria. Environ Sci Technol 52:10433–10440. https://doi.org/10.1021/acs.est.8b02912
Coisne C, Tilloy S, Monflier E, Wils D, Fenart L, Gosselet F (2016) Cyclodextrins as emerging therapeutic tools in the treatment of cholesterol-associated vascular and neurodegenerative diseases. Molecules 21:E1748. https://doi.org/10.3390/molecules21121748
Weiss-Errico MJ, Miksovska J, O’Shea KE (2018) β-Cyclodextrin reverses binding of perfluorooctanoic acid to human serum albumin. Chem Res Toxicol 31:277–284. https://doi.org/10.1021/acs.chemrestox.8b00002
Buffone MG, Brugo-Olmedo S, Calamera JC, Verstraeten SV, Urrutia F, Grippo L, Corbetta JP, Doncel GF (2006) Decreased protein tyrosine phosphorylation and membrane fluidity in spermatozoa from infertile men with varicocele. Mol Reprod Dev 73:1591–1599. https://doi.org/10.1002/mrd.20611
Williamson P, Mattocks K, Schlegal RA (1983) Merocyanine 540, a fluorescent probe sensitive to lipid packaging. Biochim Biophys Acta 732:387–393. https://doi.org/10.1016/0005-2736(83)90055-X
Langner M, Hui SW (1993) Merocyanine interaction with phosphatidylcholine bilayers. Biochim Biophys Acta 1149:175–179. https://doi.org/10.1016/0005-2736(93)90038-2
Rathi R, Colenbrander B, Bevers MM, Gadella BM (2001) Evaluation of in vitro capacitation of stallion spermatozoa. Biol Reprod 65:462–470. https://doi.org/10.1095/biolreprod65.2.462
van Gestel RA, Helms JB, Brouwers JF, Gadella BM (2005) Effects of methyl-beta-cyclodextrin-mediated cholesterol depletion in porcine sperm compared to somatic cells. Mol Reprod Dev 72:386–395. https://doi.org/10.1002/mrd.20351
Zavodnik IB, Lapshina EA, Palecz D, Bryszewska M (1996) The effects of palmitate on human erythrocyte membrane potential and osmotic stability. Scand J Clin Lab Invest 56:401–407. https://doi.org/10.3109/00365519609088794
Tillman TS, Cascio M (2003) Effects of membrane lipids on ion channel structure and function. Cell Biochem Biophys 38:161–190. https://doi.org/10.1385/CBB:38:2:161
Ritagliati C, Baro Graf C, Stival C, Krapf D (2018) Regulation mechanisms and implications of sperm membrane hyperpolarization. Mech Dev 154:33–43. https://doi.org/10.1016/j.mod.2018.04.004
Jensen AA, Leffers H (2008) Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 31:161–169. https://doi.org/10.1111/j.1365-2605.2008.00870.x
Chen MH, Ha EH, Wen TW, Su YN, Lien GW, Chen CY, Chen PC, Hsieh WS (2012) Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One 7:e42474. https://doi.org/10.1371/journal.pone.0042474
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35:1339–1342
Di Nisio A, Foresta C (2019) Water and soil pollution as determinant of water and food quality/contamination and its impact on male fertility. Reprod Biol Endocrinol. 17:4. https://doi.org/10.1186/s12958-018-0449-4
Kim SJ, Heo SH, Lee DS, Hwang IG, Lee YB, Cho HY (2016) Gender differences in pharmacokinetics and tissue distribution of 3 perfluoroalkyl and polyfluoroalkyl substances in rats. Food Chem Toxicol 97:243–255. https://doi.org/10.1016/j.fct.2016.09.017
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99:366–394. https://doi.org/10.1093/toxsci/kfm128
Kudo N, Kawashima Y (2003) Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci 28:49–57. https://doi.org/10.2131/jts.28.49
Kennedy GL Jr, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG (2004) The toxicology of perfluorooctanoate. Crit Rev Toxicol 34:351–384
Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jørgensen N (2009) Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect 117:923–927. https://doi.org/10.1289/ehp.0800517
Governini L, Guerranti C, De Leo V, Boschi L, Luddi A, Gori M, Orvieto R, Piomboni P (2015) Chromosomal aneuploidies and DNA fragmentation of human spermatozoa from patients exposed to perfluorinated compounds. Andrologia. 47:1012–1019. https://doi.org/10.1111/and.12371
Flesch FM, Gadella BM (2000) Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta 1469:197–235. https://doi.org/10.1016/S0304-4157(00)00018-6
Nikolopoulou M, Soucek DA, Vary JC (1985) Changes in the lipid content of boar sperm plasma membranes during epididymal maturation. Biochim Biophys Acta 815:486–498. https://doi.org/10.1016/0005-2736(85)90377-3
Toshimori K (1998) Maturation of mammalian spermatozoa: modifications of the acrosome and plasma membrane leading to fertilization. Cell Tissue Res 293:177–187. https://doi.org/10.1007/s004410051110
Haidl G, Opper C (1997) Changes in lipids and membrane anisotropy in human spermatozoa during epididymal maturation. Hum Reprod 12:2720–2723. https://doi.org/10.1093/humrep/12.12.2720
Vos JP, Lopes-Cardozo M, Gadella BM (1994) Metabolic and functional aspects of sulfogalactolipids. Biochim Biophys Acta 1211:125–149. https://doi.org/10.1016/0005-2760(94)90262-3
Arbo MD, Altknecht LF, Cattani S, Braga WV, Peruzzi CP, Cestonaro LV, Göethel G, Durán N, Garcia SC (2019) In vitro cardiotoxicity evaluation of graphene oxide. Mutat Res 841:8–13. https://doi.org/10.1016/j.mrgentox.2019.03.004
Stival C, Puga Molina Ldel C, Paudel B, Buffone MG, Visconti PE, Krapf D (2016) Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol 220:93–106. https://doi.org/10.1007/978-3-319-30567-7_5
Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y (2010) Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140:327–337. https://doi.org/10.1016/j.cell.2009.12.053
Lishko PV, Kirichok Y (2010) The role of Hv1 and CatSper channels in sperm activation. J Physiol 588(Pt 23):4667–4672. https://doi.org/10.1113/jphysiol.2010.194142
Foresta C, De Carlo E, Zorzi M, Rossato M, Finelli L (1990) Possible significance of seminal zinc on human spermatozoa functions. Acta Eur Fertil. 21:305–308
Costello LC, Liu Y, Franklin RB, Kennedy MC (1997) Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem 272:28875–28881. https://doi.org/10.1074/jbc.272.46.28875
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Ethics Committee of the Hospital of Padova, Italy, (protocol number 2208P and successive amendments). The investigation was performed according to the principles of the Declaration of Helsinki.
Informed consent
All patients provided signed informed consent at enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Šabović, I., Cosci, I., De Toni, L. et al. Perfluoro-octanoic acid impairs sperm motility through the alteration of plasma membrane. J Endocrinol Invest 43, 641–652 (2020). https://doi.org/10.1007/s40618-019-01152-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01152-0