Abstract
Physiological changes that endow mammalian sperm with fertilizing capacity are known as sperm capacitation. As part of capacitation, sperm develop an asymmetrical flagellar beating known as hyperactivation and acquire the ability to undergo the acrosome reaction. Together, these processes promote fertilizing competence in sperm. At the molecular level, capacitation involves a series of signal transduction events which include activation of cAMP-dependent phosphorylation pathways, removal of cholesterol, hyperpolarization of the sperm plasma membrane, and changes in ion permeability. In recent years, new technologies have aided in the study of sperm signaling molecules with better resolution, at both spatial and temporal levels, unraveling how different cascades integrate and cooperate to render a fertilizing sperm. Despite this new information, the molecular mechanisms connecting capacitation with acrosomal exocytosis and hyperactivation are not well understood. This review brings together results obtained in mammalian species in the field of sperm capacitation with special focus on those pathways involved in the preparation to undergo the acrosomal reaction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
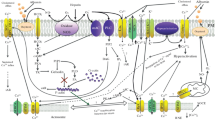
To acquire fertilizing capacity, mammalian sperm require two post-testicular maturation steps, one occurring in the male epididymis, known as epididymal maturation, and the other one occurring after ejaculation in the female tract known as capacitation. Capacitation was first observed independently by Chang (1951) and Austin (1952) in the 1950s. Their observations were crucial for future development of in vitro fertilization techniques, first in rabbit (Chang 1959) and later on in humans (Steptoe and Edwards 1978) with the birth of Louise Brown, the first “test-tube baby.” At the cell biology level, capacitation induces changes in the sperm motility pattern known as hyperactivated movement and prepares the sperm to undergo an exocytotic process known as acrosome reaction. At the molecular level, capacitation is associated with cholesterol loss from the sperm plasma membrane, increased membrane fluidity, changes in intracellular ion concentrations (Visconti et al. 2011), hyperpolarization of the sperm plasma membrane (Hernandez-Gonzalez et al. 2006), increased activity of the Protein Kinase A (PKA) (Krapf et al. 2010), and protein tyrosine phosphorylation (Arcelay et al. 2008). Although each of these events has been studied independently, information regarding how they interconnect to regulate sperm motility and to prepare the sperm to undergo the acrosome reaction is still unavailable. It is known that PKA plays a key role coordinating the majority of the events related to capacitation. Blockade of this kinase prevents the acrosome reaction, probably through inhibition of processes needed for the acquisition of acrosomal exocytotic competence (Visconti et al. 1995). Due to this essential role of PKA, it was surprising to find that the PKA catalytic subunit is absent in the head of the mouse sperm where all events related to acrosome reaction occur (Wertheimer et al. 2013). Interestingly, other molecules known to be essential for acrosomal reaction responsiveness such as SLO3 K+ channels are also found exclusively in the sperm flagellum. These results suggest that activation of signaling pathways in the sperm tail plays a role in the regulation of events happening in the head compartment. The finding that injection of Lucifer Yellow dye promptly diffuses throughout the whole interior of the sperm indicates that molecules can pass from one compartment to the other (Navarro et al. 2007). Nevertheless, it should be considered that in the case of second messengers such as cAMP, their diffusion throughout the sperm is limited and highly controlled by hydrolyzing enzymes (i.e., phosphodiesterases). An alternative possibility is that changes in ion permeability and the consequent modification of the sperm membrane potential in the whole cell can orchestrate the activation of signaling pathways throughout the sperm. Therefore, both inter-compartmental diffusion and changes in the membrane potential could play a role in coordinating events in the tail with those occurring in the sperm head. Although diffusion of molecules cannot be discarded and is likely to be involved in energy transfer from the tail to the head, little experimental evidence of this process is available. On the other hand, it is clear that mammalian sperm undergo hyperpolarization of the sperm plasma membrane and that these changes appear to be involved in the preparation of sperm for exocytosis of their acrosomes (De La Vega-Beltran et al. 2012; Hernandez-Gonzalez et al. 2006). This review will focus on the signaling events involved in the regulation of the changes in the sperm membrane potential that occur during capacitation and their connection with the acrosome reaction. For more information regarding capacitation, see other reviews on this topic (Aitken and Nixon 2013; Buffone et al. 2014; Harayama 2013; Bailey 2010). Discussion of the acrosome reaction and the molecular mechanisms involved in this event can be found in different chapters of this book.
5.2 Signaling Pathways Connecting Capacitation with the Acrosome Reaction
Spermatozoa undergo significant changes in their ionic environment during their journey to the egg. In the epididymis, sperm are surrounded by epididymal fluid containing concentrations that differ significantly from those found in other biological milieus such as blood. Remarkably, the epididymal fluid contains low levels of Na+ (~40 mM), Cl− (~40 mM), and HCO3 − (~4 mM) and high concentrations of K+ ions (Pastor-Soler et al. 2005). In particular, low pH and low HCO3 − concentrations are essential to maintain the sperm in a quiescent state before ejaculation (Pastor-Soler et al. 2005). Strikingly, the pH of the epididymis is kept low by the action of a battery of transporters in the epididymis epithelial cells including Na+/H+ antiporters, Na+/HCO3 − co-transporter, and a series of carbonic anhydrases and most importantly through the regulation of vacuolar H+-ATPase (Pastor-Soler et al. 2005). Once ejaculated, sperm encounter first seminal fluid and later on other fluids secreted by the female tract epithelium. In their new environment, the pH and concentrations of Na+, Cl−, and HCO3 − are increased while those of K+ are decreased (Kavanagh 1985). Sperm decode these changes on environmental ions promoting the activation of signaling cascades leading to capacitation.
5.2.1 Role of HCO3 − and cAMP
When sperm are exposed to higher HCO3 − concentrations in the seminal fluid or in in vitro capacitation media, an increased transport of this anion is observed. One of the main targets of HCO3 − inside the cell is the atypical adenylyl cyclase Adcy10 (aka sAC, aka SACY). This enzyme becomes stimulated upon binding of HCO3 − (Kleinboelting et al. 2014; Chen et al. 2000) with the consequent increase in intracellular cAMP and activation of PKA. This whole signaling process occurs very fast (~1 min) and multiple evidence indicate that this initial PKA activation is upstream of most of the other known events associated with capacitation including increase in intracellular pH (Wang et al. 2003, 2007; Zeng et al. 1996), hyperpolarization of the plasma membrane (Demarco et al. 2003), actin polymerization (Romarowski et al. 2015), and the promotion of tyrosine phosphorylation (Visconti et al. 1995). In addition, pharmacological and genetic loss of function experiments showed that this initial activation of PKA prepares the sperm for the acrosome reaction. However, as mentioned above, the PKA catalytic subunit is localized to the flagellum and it is not found in the sperm head. This finding strongly suggests that PKA modulates the acrosome reaction indirectly through other downstream signaling pathways. Among them, we will concentrate on the regulation of the increase in intracellular pH (pHi) and on the hyperpolarization of the sperm membrane potential (Em) associated with capacitation.
5.2.2 Increase of Intracellular pH
As sperm leave the acidic vagina (pH ~5) and travel through the utero tubal junction, they face a significant increase of pH to a value of approximately 8 (Suarez and Pacey 2006). This change in extracellular pH has been proposed to promote sperm intracellular alkalinization (Hamamah and Gatti 1998). An increase in pHi is also observed in sperm incubated in conditions that support capacitation, despite the fact that extracellular pH is maintained constant. In this case, the intracellular alkalinization suggests the presence of active mechanisms controlling pHi. In sperm, three main molecular systems have been reported to control their pHi. First, direct electrophysiological recordings identified the voltage-gated channel Hv1 (Lishko et al. 2010). This channel is expressed in the flagellum of human sperm, suggesting a direct impact on Ca2+ homeostasis through modulation of pH-sensitive channels such as the Ca2+ channel CatSper and the K+ channel SLO3, also located in the principal piece of the flagellum (see below). Hv1 is a H+ transporter that promotes movement of protons across the membrane through voltage-gated mechanisms (Takeshita et al. 2014) and is highly sensitive to Zn2+, an abundant ion in seminal plasma (Khan et al. 2011). It has been hypothesized that this high Zn2+ concentration plays a role in maintaining Hv1 closed until the seminal fluid is diluted in the female tract allowing its activation by still unknown mechanisms (Lishko et al. 2010). Added to the effect of Zn2+ removal, the endogenous cannabinoid anandamide found in the female tract (Gervasi et al. 2013) might facilitate Hv1 opening (Lishko et al. 2010). In contrast to human sperm, Hv1 is not found in mouse sperm. Consequently, the finding that Hv1 KO mice are fertile (Ramsey et al. 2009) is silent on the role of Hv1 in human sperm fertility.
A second possible mechanism for pHi alkalinization has been proposed in mouse. In particular, mice lacking the sperm-specific Na+/H+ exchanger (NHE-10 or sNHE) are sterile (Wang et al. 2007) and their sperm are unable to fertilize in vitro. In addition to the significant increase in HCO3 − anions upon leaving the epididymis, sperm also encounter higher Na+ concentrations in the seminal fluid and in the female tract. This increase in Na+ would favor interchange of this cation for H+ through sNHE. Interestingly, sNHE primary structure has a cyclic nucleotide binding domain, suggesting that it can respond to increased cAMP production during the initial steps of capacitation. Moreover, sperm from sNHE−/− have reduced levels of Adcy10 (Wang et al. 2003) and addition of permeable cAMP analogues restored protein tyrosine phosphorylation of sNHE-null sperm cells to a pattern similar to that of wild-type spermatozoa (Wang et al. 2007), suggesting a cross talk between cAMP synthesis and the regulation of sperm pHi.
Finally, as mentioned in the previous section, there is inward transport of HCO3 − anions during capacitation. In addition to the well-established role in the regulation of Adcy10, HCO3 − is also a weak base and its transport inside the sperm is predicted to alkalinize pHi. Addition of HCO3 − to cells preincubated in the absence of this anion induced Em hyperpolarization accompanied by pHi increase (Demarco et al. 2003). Sodium replacement from the external media by the non-permeant cation choline does not allow the hyperpolarization induced by HCO3 − addition (Demarco et al. 2003), suggesting the presence of an electrogenic Na+/HCO3 − cotransporter in spermatozoa. Alternatively, it has also been proposed the presence of a neutral Na+/Cl−/HCO3 − cotransporter (Zeng et al. 1996) and the transport through CFTR (Chavez et al. 2012) or its coupled Cl−/HCO3 − exchangers (Chen et al. 2009). Equilibrium of HCO3 −/CO2 is modulated by the presence of sperm carbonic anhydrases, which catalyze the reversible hydration of CO2 to HCO3 −. In mouse sperm, carbonic anhydrases II and IV together contribute to almost all carbonic anhydrase activity detected (Wandernoth et al. 2015). When double knockout carbonic anhydrase II/IV male mice were mated with WT females, fertility was reduced by 90 % (Wandernoth et al. 2015).
Multiple evidence support the hypothesis that capacitation is accompanied by an increase in intracellular pH. However, how sperm alkalinization regulates other capacitation-associated events such as hyperactivation and the preparation for the acrosome reaction (AR) is not well established. Noteworthy, the activity of two of the best characterized sperm-specific ion channels is very sensitive to changes in pH. First, the sperm-specific Ca2+ channel CatSper, which is composed of multiple subunits including four pore-forming CatSper channel proteins, CatSper1–4, containing six transmembrane-spanning domains (Qi et al. 2007), and at least three auxiliary subunits β (Liu et al. 2007), γ (Wang et al. 2009), and δ (Chung et al. 2011). A His-rich domain found in the intracellular NH2 terminus of CatSper1 (Ren et al. 2001) has been proposed to be responsible for the pHi sensitivity of the channel (Ren and Xia 2010). Patch clamp measurements indicate a significant activation of flagellar calcium channel that is strongly potentiated by intracellular alkalinization (Kirichok et al. 2006). This current is not present in CatSper1−/− knockout mice.
A second sperm-specific channel highly dependent on pHi is the K+ channel SLO3 (Martinez-Lopez et al. 2009; Santi et al. 2010) which has an essential role in the regulation of the changes in plasma membrane potential associated with capacitation (see below). The possibility that these ion channels mediate the role of intracellular alkalinization in sperm is consistent with findings that CatSper and SLO3 functions are required for hyperactivation (Zeng et al. 2013) and that SLO3 is necessary for the acrosome reaction (Santi et al. 2010). Concurrent measurements of pHi, membrane potential, and intracellular Ca2+ concentrations using high-speed resolution and sperm from genetically modified knockout models will be required to conclusively test this hypothesis.
5.2.3 Changes in Membrane Potential
Capacitation is also associated with changes in the sperm plasma membrane potential (Em) (Zeng et al. 1995). Maintaining ionic gradients across their membranes requires considerable energy investment through modulation of pumps and ion transporters. As soon as cauda epididymal mouse sperm are suspended in a culture media, they have a relatively depolarized Em of ~ −40 mV. However, this membrane potential hyperpolarizes to ~ −60 mV when capacitation proceeds, whereas no change is observed when sperm are incubated in conditions that do not support capacitation (Demarco et al. 2003; Zeng et al. 1995; Espinosa and Darszon 1995). These measurements of sperm Em have been conducted in populations using fluorimetric measurements and constitute an average value. On the other hand, when the sperm Em was monitored using single cell microscopy (Arnoult et al. 1999) or flow cytometry (Escoffier et al. 2015; Lopez-Gonzalez et al. 2014), two sperm subpopulations were observed: one with an Em closer to that of the non-capacitated sperm population (~ −40 mV) and the other with a more hyperpolarized Em (~ −80 mV) (Arnoult et al. 1999). These findings are consistent with the observation that only a fraction of the sperm population are capable to undergo the acrosome reaction (Salicioni et al. 2007).
The mechanisms that regulate hyperpolarization of the sperm plasma membrane during capacitation are poorly understood. Membrane potential at any point is given by the relative permeability of the plasma membrane to ions present in the capacitation media. The study of how these permeabilities change during capacitation is crucial for the understanding of the molecular basis of Em changes. Under normal conditions, sperm maintain an internal ion concentration markedly different from that in the extracellular medium, and these differences establish the resting plasma membrane potential. Therefore, Em changes that occur during capacitation reflect parallel changes in ion permeability. The most relevant ions involved in Em regulation are Na+, K+, and Cl− which have equilibrium potentials of +40, −80, and −40 mV, respectively. Considering these ion’s equilibrium Em, changes in Em from −40 mV to −80 mV that occur in sperm during capacitation can be explained by either closure of electrogenic Na+ transport or by an opening of a K+ channel. Regarding the first possibility, non-capacitated sperm are hyperpolarized when Na+ ions are omitted from the incubation media. Moreover, hyperpolarization is also observed when sperm are treated with amiloride, a compound known to affect Na+ transport by epithelial Na+ channels (ENaC) (Hernandez-Gonzalez et al. 2006). ENaC channels contribute to the resting Em in many cell types by shifting the membrane potential toward the Na+ equilibrium potential (Butterworth 2010), whereas closing of these channels during capacitation mediated by activation of the CFTR (Hernandez-Gonzalez et al. 2007; Xu et al. 2007) would explain the observed hyperpolarization. Consistent with this possibility, both mouse spermatogenic cells and testicular sperm display amiloride-sensitive inward Na+ currents compatible with ENaC currents (Hernandez-Gonzalez et al. 2006), and fluorimetric measurements using Na+ concentration dyes indicate that intracellular Na+ concentrations decrease during capacitation (Escoffier et al. 2012).
Alternatively, hyperpolarization can also be explained by an increased outward K+ current. In agreement with this hypothesis, sperm lacking SLO3, the aforementioned sperm-specific K+ channel, do not undergo hyperpolarization during capacitation. Moreover, treatment of wild-type sperm with the SLO3 inhibitor clofilium blocks the capacitation-induced hyperpolarization (Sanchez-Carranza et al. 2015; Chavez et al. 2013). Although sperm from SLO3−/− mice cannot hyperpolarize during capacitation, the hyperpolarization can be obtained when sperm are incubated in low Na+ or in the presence of amiloride (Chavez et al. 2013; Hernandez-Gonzalez et al. 2006), indicating that an electrogenic Na+ permeability is functionally present in mature mouse sperm and its elimination promotes hyperpolarization. These experiments suggest that a decrease in electrogenic Na+ transport is sufficient to hyperpolarize the sperm Em. However, the lack of capacitation-induced hyperpolarization of sperm from SLO3−/− mice, while maintaining the ability to hyperpolarize when Na+ transport is decreased, strongly argues in favor that an outward K+ transport is the one responsible for changes in Em observed during capacitation. Consistently, recent electrophysiological recordings showed that although increasing extracellular [Na+] (in a low sodium media) before capacitation produced larger depolarizations than after capacitation, this effect is not to be interpreted as changes of Na+ permeability during capacitation. Rather, explanation to this is found in the observed permeability ratios of K+ and Na+: a P K/P Na of 1.6/.13 = 12.3 before capacitation and P K/P Na of 4.38/.13 = 33 after capacitation. These values indicate that adding external Na+ after capacitation produces a much lower change in voltage than does the addition of external Na+ before capacitation and that this difference is due to the large increase in K+ permeability after capacitation, rather than a decrease in Na+ permeability (Chavez et al. 2013).
Since capacitation prepares the sperm for the acrosome reaction (Zeng et al. 1995; Florman et al. 1998), it was proposed that the Em hyperpolarization that accompanies capacitation may regulate the sperm’s ability to generate transient Ca2+ elevations in response to physiological agonists of the acrosome reaction (e.g., zona pellucida of the egg or progesterone). In support of this hypothesis, the presence of T-type Ca2+ channels has been documented in spermatogenic cells (Arnoult et al. 1996; Lievano et al. 1996) and in sperm (Escoffier et al. 2007). These channels were originally called low-voltage-activated (LVA) channels because they can be activated by a small depolarization of the plasma membrane. However, they are usually found in an inactive state at membrane potentials of ~ −40 mV that are observed in non-capacitated sperm. To release inactivation, Em should be hyperpolarized to potentials between −80 mV and −60 mV, from where they can be readily activated (Arnoult et al. 1996; Lievano et al. 1996). Accordingly, a depolarized membrane potential of sperm before capacitation would prevent a premature AR, provided that T-type Ca2+ channels are involved in the regulation of this event (Arnoult et al. 1996; Florman et al. 1998).
In recent years, patch clamp measurements became possible in sperm cells. These measurements were able to conclusively demonstrate using solid electrophysiological tools the presence of CatSper, SLO3, and Hv1 in mammalian sperm from different species (Kirichok et al. 2006; Lishko et al. 2010; Lishko and Kirichok 2010; Navarro et al. 2007; Strunker et al. 2011; Brenker et al. 2012, 2014; Sumigama et al. 2015). However, T channel currents from Cav 3.2 which are readily observed in testicular sperm cells (Arnoult et al. 1996; Lievano et al. 1996; Publicover and Barratt 1999; Martinez-Lopez et al. 2009) cannot be detected in epididymal sperm. Inward Ca2+ currents elicited by depolarization were fully abolished in spermatogenic cells from Cav 3.2-null mice revealing the molecular identity of the T channel inward current in spermatogenic cells. However, mice lacking Cav 3.2 channels are fertile (Escoffier et al. 2007) which argue against an essential role of this channel in sperm. Despite these results, antibodies against Cav 3.2 stained the head of mouse sperm by immunofluorescence. This staining appears to be specific because it completely disappears in sperm from Cav 3.2 KO mice (Escoffier et al. 2007). Why this channel is active in testicular sperm and then becomes inactivated during epididymal transit without being degraded is still not understood.
Despite the lack of solid electrophysiology evidence on the presence of Ca2+ T channels in mature sperm, hyperpolarization appears to be required for exocytosis of the sperm acrosome. As mentioned in the introduction, the ability of agonists to induce exocytosis is obtained only after sperm undergo capacitation (Buffone et al. 2012). Similarly, only capacitated sperm undergo acrosome reaction when exposed to high K+ (60 mM for mouse sperm) (Florman et al. 1992). If sperm are incubated in the absence of both albumin and HCO3 −, they do not acquire the ability to undergo acrosome reaction. However, if non-capacitated sperm are hyperpolarized with pharmacological agents such as valinomycin or with the aforementioned Na+ transport blocker amiloride, they acquire the ability to react when exposed to high K+, to solubilized ZP (De La Vega-Beltran et al. 2012), or to progesterone (Stival et al. 2015). Interestingly, the hyperpolarizing reagents did not induce other capacitation-associated processes such as PKA activation or the increase in tyrosine phosphorylation (De La Vega-Beltran et al. 2012). Moreover, inhibition of the tyrosine kinase cSrc, which prevents acrosome reaction (see below), could also be bypassed with K+ ionophore, restoring Em hyperpolarization and the ability to undergo acrosome reaction (Stival et al. 2015). To analyze the necessity of hyperpolarization for the acrosome reaction, sperm were incubated in conditions that support capacitation (media containing both albumin and HCO3 −) but in the presence of high K+ concentrations (70 mM) to clamp the Em in a depolarized state. These sperm underwent normal PKA activation and the increase in tyrosine phosphorylation but were unable to undergo acrosome reaction when exposed to solubilized zona pellucida (De La Vega-Beltran et al. 2012). Finally, sperm from SLO3 KO mice do not hyperpolarize during capacitation and cannot undergo acrosome reaction (Santi et al. 2010). Altogether, these pharmacological and genetic approaches strongly suggest that hyperpolarization is essential to prepare the sperm for the acrosome reaction during capacitation.
5.3 Link Between Em Hyperpolarization and Other Capacitation Associated Events
At the starting of this review we asked how signaling events occurring during capacitation prepare mammalian sperm for a physiological acrosome reaction. As described in previous sections, a link between these processes is likely to be at the level of the Em hyperpolarization observed during capacitation. One of the first signaling pathways observed during capacitation is the HCO3 −-dependent stimulation of cAMP synthesis which activates PKA. In turn, PKA activation appears to be necessary for Em hyperpolarization (Escoffier et al. 2015; Hernandez-Gonzalez et al. 2006). However, how PKA signals Em changes is not well understood. Considering the evidence mentioned above, hyperpolarization changes are due to SLO3 opening which increased K+ permeability almost three times (Chavez et al. 2013). Therefore, to better understand the link between these processes, it will be necessary to investigate the connection between the cAMP pathway and SLO3 modulation. One possibility is that PKA directly phosphorylates SLO3 and induces its activation. Although this possibility cannot be discarded, there is no direct evidence of its occurrence. An alternative possibility is that the capacitation-associated increase in pHi mediates the action of cAMP on SLO3 activation. Consistently, the aforementioned sperm-specific NHE contains a cAMP-binding consensus sequence; it can be speculated that upon the increase in cAMP, this exchanger is activated, and therefore, the consequent intracellular alkalinization directly activates SLO3 (Lishko et al. 2012). This hypothesis is sound; however, conclusive demonstration will require further experimentation. In addition, this possibility does not take into account the role of PKA. A third alternative to explain the observed results is that SLO3 activation is mediated by other enzymes downstream of the cAMP/PKA pathway.
One of the possible candidates to mediate the connection between PKA and SLO3 activation is cSrc, a tyrosine kinase known to be present in mammalian sperm (Krapf et al. 2010; Baker et al. 2006; Lawson et al. 2008; Battistone et al. 2013; Krapf et al. 2012; Bragado et al. 2012). Although this enzyme was initially proposed to be the tyrosine kinase mediating the capacitation-associated increase in tyrosine phosphorylation (Baker et al. 2006), it was later shown that sperm from knockout mice models lacking cSrc display normal levels of tyrosine phosphorylation upon capacitation (Krapf et al. 2010). Despite not being involved in the regulation of the capacitation-associated increase in tyrosine phosphorylation, cSrc KO sperm are barely motile and they are unable to fertilize (Krapf et al. 2010), indicating an essential role of this enzyme for male reproduction. More recently, we used a combination of pharmacology and antibodies that detect the activated form Tyr(P)416-Src to evaluate the role of this tyrosine kinase in capacitation (Stival et al. 2015). Our results indicated that cSrc was activated downstream PKA in mouse sperm (Stival et al. 2015). In addition, we showed that cSrc inhibitors blocked Em hyperpolarization and the ability of progesterone to induce the acrosome reaction in capacitated sperm. Moreover, acrosome reaction responsiveness was rescued by inducing hyperpolarization with the K+ ionophore valinomycin (Stival et al. 2015). Consistently, in human (Varano et al. 2008), bovine (Etkovitz et al. 2009), and porcine sperm (Bragado et al. 2012), cSrc was shown to be involved in the acquisition of acrosomal responsiveness, species in which cSrc inhibition completely blocked the progesterone-induced acrosome reaction. Altogether, these results support the hypothesis that cSrc is involved in the regulation of Em downstream of PKA activation. However, the extent by which the effect of cSrc is mediated by SLO3 is still not known. Interestingly, electrophysiological recordings derived from Xenopus oocytes expressing mammalian SLO3 suggest that inhibition of cSrc leads to decrease of SLO3 currents (Stival et al. 2015). Whether this decrease is due to direct or indirect phosphorylation pathways will require direct electrophysiological assays of mature sperm.
5.4 Concluding Remarks
As stated in the introduction, how mammalian sperm acquire acrosome reaction responsiveness during capacitation is still not well understood. Although it is clear that two of the main physiological endpoints of capacitation are the ability to undergo acrosome reaction and the change in motility pattern known as hyperactivation, whether these processes are coordinately regulated or are independent is not known. Remarkably, PKA activation appears to be upstream most events associated with capacitation. However, this protein kinase has not been found in the sperm head, raising the question of how PKA could mediate events related to the acrosome reaction. In this review, we explored evidence suggesting a possible mechanism by which changes in the flagellum can signal the preparation for the acrosome reaction. Although it is not the only possible explanation, one hypothesis consistent with experimental observations is that PKA in the sperm tail regulates changes in the sperm membrane potential that will be felt in the complete cell including the plasma membrane surrounding the acrosome. As in all scientific inquiry, this hypothesis raises new questions. For example, it is not clear how a hyperpolarizing change in the sperm Em signals the preparation for the acrosome reaction. One possibility is that hyperpolarization mediates transformation of a Ca2+ channel from an inactive to a closed state as was proposed for the T channels (Arnoult et al. 1999). Another possibility is that these changes in Em favor contacts between the outer acrosomal membrane and the plasma membrane. This type of contacts has been observed using electronic microscopy (Zanetti and Mayorga 2009). At the molecular level, the identity of the molecules mediating these events has remained elusive. One of the reasons is the difficulties inherent to mammalian sperm which are not amenable to many relatively common techniques used in other cell types such as RNAi silencing or overexpression of molecules by cDNA transfection. In addition, many of the sperm compartments are at distances below the separation limits of normal immunofluorescence techniques. Promising new technical advances such as gene editing, super resolution, and helium ion microscopy are expected to significantly improve our understanding of those capacitation changes that regulate sperm exocytosis.
References
Aitken RJ, Nixon B (2013) Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod 19:785–793
Arcelay E, Salicioni AM, Wertheimer E, Visconti PE (2008) Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int J Dev Biol 52:463–472
Arnoult C, Cardullo RA, Lemos JR, Florman HM (1996) Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci U S A 93:13004–13009
Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, Florman HM (1999) Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc Natl Acad Sci U S A 96:6757–6762
Austin CR (1952) The capacitation of the mammalian sperm. Nature 170:326
Bailey JL (2010) Factors regulating sperm capacitation. Syst Biol Reprod Med 56:334–348
Baker MA, Hetherington L, Aitken RJ (2006) Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci 119:3182–3192
Battistone MA, Da Ros VG, Salicioni AM, Navarrete FA, Krapf D, Visconti PE, Cuasnicu PS (2013) Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol Hum Reprod 19:570–580
Bragado MJ, Gil MC, Martin-Hidalgo D, Hurtado DL, Bravo N, Moreno AD, Garcia-Marin LJ (2012) Src family tyrosine kinase regulates acrosome reaction but not motility in porcine spermatozoa. Reproduction 144:67–75
Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krahling M, Muller A, Kaupp UB, Strunker T (2012) The CatSper channel: a polymodal chemosensor in human sperm. EMBO J 31:1654–1665
Brenker C, Zhou Y, Muller A, Echeverry FA, Trotschel C, Poetsch A, Xia XM, Bonigk W, Lingle CJ, Kaupp UB, Strunker T (2014) The Ca2 + -activated K+ current of human sperm is mediated by Slo3. Elife 3, e01438
Buffone MG, Ijiri TW, Cao W, Merdiushev T, Aghajanian HK, Gerton GL (2012) Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev 79:4–18
Buffone MG, Wertheimer EV, Visconti PE, Krapf D (2014) Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim Biophys Acta 1842:2610–2620
Butterworth MB (2010) Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Biochim Biophys Acta 1802:1166–1177
Chang MC (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168:697–698
Chang MC (1959) Fertilization of rabbit ova in vitro. Nature 184(Suppl 7):466–467
Chavez JC, Hernandez-Gonzalez EO, Wertheimer E, Visconti PE, Darszon A, Trevino CL (2012) Participation of the Cl-/HCO(3)- exchangers SLC26A3 and SLC26A6, the Cl- channel CFTR, and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biol Reprod 86:1–14
Chavez JC, De La Vega-Beltran JL, Escoffier J, Visconti PE, Trevino CL, Darszon A, Salkoff L, Santi CM (2013) Ion permeabilities in mouse sperm reveal an external trigger for SLO3-dependent hyperpolarization. PLoS One 8, e60578
Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J (2000) Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289:625–628
Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, Zhou SC, Zhou WW, Tsang LL, Chung YW, Hoglund P, Chan HC, Shi QX (2009) Cl- is required for HCO3- entry necessary for sperm capacitation in guinea pig: involvement of a Cl-/HCO3- exchanger (SLC26A3) and CFTR. Biol Reprod 80:115–123
Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE (2011) A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun 2:153
De La Vega-Beltran JL, Sanchez-Cardenas C, Krapf D, Hernandez-Gonzalez EO, Wertheimer E, Trevino CL, Visconti PE, Darszon A (2012) Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J Biol Chem 287:44384–44393
Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE (2003) Involvement of a Na+/HCO-3 cotransporter in mouse sperm capacitation. J Biol Chem 278:7001–7009
Escoffier J, Boisseau S, Serres C, Chen CC, Kim D, Stamboulian S, Shin HS, Campbell KP, De Waard M, Arnoult C (2007) Expression, localization and functions in acrosome reaction and sperm motility of Ca(V)3.1 and Ca(V)3.2 channels in sperm cells: an evaluation from Ca(V)3.1 and Ca(V)3.2 deficient mice. J Cell Physiol 212:753–763
Escoffier J, Krapf D, Navarrete F, Darszon A, Visconti PE (2012) Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J Cell Sci 125:473–485
Escoffier J, Navarrete F, Haddad D, Santi CM, Darszon A, Visconti PE (2015) Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol Reprod 92:121
Espinosa F, Darszon A (1995) Mouse sperm membrane potential: changes induced by Ca2+. FEBS Lett 372:119–125
Etkovitz N, Tirosh Y, Chazan R, Jaldety Y, Daniel L, Rubinstein S, Breitbart H (2009) Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev Biol 334:447–457
Florman HM, Corron ME, Kim TD, Babcock DF (1992) Activation of voltage-dependent calcium channels of mammalian sperm is required for zona pellucida-induced acrosomal exocytosis. Dev Biol 152:304–314
Florman HM, Arnoult C, Kazam IG, Li C, O’Toole CM (1998) A perspective on the control of mammalian fertilization by egg-activated ion channels in sperm: a tale of two channels. Biol Reprod 59:12–16
Gervasi MG, Marczylo TH, Lam PM, Rana S, Franchi AM, Konje JC, Perez-Martinez S (2013) Anandamide levels fluctuate in the bovine oviduct during the oestrous cycle. PLoS One 8, e72521
Hamamah S, Gatti JL (1998) Role of the ionic environment and internal pH on sperm activity. Hum Reprod 13(Suppl 4):20–30
Harayama H (2013) Roles of intracellular cyclic AMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa. J Reprod Dev 59:421–430
Hernandez-Gonzalez EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, Lopez-Gonzalez I, Demarco I, Wertheimer E, Darszon A, Visconti PE (2006) Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem 281:5623–5633
Hernandez-Gonzalez EO, Trevino CL, Castellano LE, De La Vega-Beltran JL, Ocampo AY, Wertheimer E, Visconti PE, Darszon A (2007) Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J Biol Chem 282:24397–24406
Kavanagh JP (1985) Sodium, potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. J Reprod Fertil 75:35–41
Khan MS, Zaman S, Sajjad M, Shoaib M, Gilani G (2011) Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int J Appl Basic Med Res 1:93–96
Kirichok Y, Navarro B, Clapham DE (2006) Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439:737–740
Kleinboelting S, Diaz A, Moniot S, van den Heuvel J, Weyand M, Levin LR, Buck J, Steegborn C (2014) Crystal structures of human soluble adenylyl cyclase reveal mechanisms of catalysis and of its activation through bicarbonate. Proc Natl Acad Sci U S A 111:3727–3732
Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE (2010) Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem 285:7977–7985
Krapf D, Ruan YC, Wertheimer EV, Battistone MA, Pawlak JB, Sanjay A, Pilder SH, Cuasnicu P, Breton S, Visconti PE (2012) cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev Biol 369:43–53
Lawson C, Goupil S, Leclerc P (2008) Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod 79:657–666
Lievano A, Santi CM, Serrano CJ, Trevino CL, Bellve AR, Hernandez-Cruz A, Darszon A (1996) T-type Ca2+ channels and alpha1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett 388:150–154
Lishko PV, Kirichok Y (2010) The role of Hv1 and CatSper channels in sperm activation. J Physiol 588:4667–4672
Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y (2010) Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140:327–337
Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE (2012) The control of male fertility by spermatozoan ion channels. Annu Rev Physiol 74:453–475
Liu J, Xia J, Cho KH, Clapham DE, Ren D (2007) CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem 282:18945–18952
Lopez-Gonzalez I, Torres-Rodriguez P, Sanchez-Carranza O, Solis-Lopez A, Santi CM, Darszon A, Trevino CL (2014) Membrane hyperpolarization during human sperm capacitation. Mol Hum Reprod 20(7):619–629
Martinez-Lopez P, Santi CM, Trevino CL, Ocampo-Gutierrez AY, Acevedo JJ, Alisio A, Salkoff LB, Darszon A (2009) Mouse sperm K+ currents stimulated by pH and cAMP possibly coded by Slo3 channels. Biochem Biophys Res Commun 381:204–209
Navarro B, Kirichok Y, Clapham DE (2007) KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci U S A 104:7688–7692
Pastor-Soler N, Pietrement C, Breton S (2005) Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology (Bethesda) 20:417–428
Publicover SJ, Barratt CL (1999) Voltage-operated Ca2+ channels and the acrosome reaction: which channels are present and what do they do? Hum Reprod 14:873–879
Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE (2007) All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A 104:1219–1223
Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE (2009) Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci U S A 106:7642–7647
Ren D, Xia J (2010) Calcium signaling through CatSper channels in mammalian fertilization. Physiology (Bethesda) 25:165–175
Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE (2001) A sperm ion channel required for sperm motility and male fertility. Nature 413:603–609
Romarowski A, Battistone MA, La Spina FA, Puga Molina LC, Luque GM, Vitale AM, Cuasnicu PS, Visconti PE, Krapf D, Buffone MG (2015) PKA-dependent phosphorylation of LIMK1 and Cofilin is essential for mouse sperm acrosomal exocytosis. Dev Biol 405:237–249
Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, Visconti PE (2007) Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl 65:245–259
Sanchez-Carranza O, Torres-Rodriguez P, Darszon A, Trevino CL, Lopez-Gonzalez I (2015) Pharmacology of hSlo3 channels and their contribution in the capacitation-associated hyperpolarization of human sperm. Biochem Biophys Res Commun 466:554–559
Santi CM, Martinez-Lopez P, De La Vega-Beltran JL, Butler A, Alisio A, Darszon A, Salkoff L (2010) The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 584:1041–1046
Steptoe PC, Edwards RG (1978) Birth after the reimplantation of a human embryo. Lancet 2:366
Stival C, La Spina FA, Baro GC, Arcelay E, Arranz SE, Ferreira JJ Sr, Le Grand S, Santi CM, Visconti PE, Buffone MG, Krapf D (2015) Src is the connecting player between PKA activation and hyperpolarization through SLO3 regulation in mouse sperm. J Biol Chem 290(30):18855–18864
Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB (2011) The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471:382–386
Suarez SS, Pacey AA (2006) Sperm transport in the female reproductive tract. Hum Reprod Update 12:23–37
Sumigama S, Mansell S, Miller M, Lishko PV, Cherr GN, Meyers SA, Tollner T (2015) Progesterone accelerates the completion of sperm capacitation and activates CatSper channel in spermatozoa from the rhesus macaque. Biol Reprod 93:130
Takeshita K, Sakata S, Yamashita E, Fujiwara Y, Kawanabe A, Kurokawa T, Okochi Y, Matsuda M, Narita H, Okamura Y, Nakagawa A (2014) X-ray crystal structure of voltage-gated proton channel. Nat Struct Mol Biol 21:352–357
Varano G, Lombardi A, Cantini G, Forti G, Baldi E, Luconi M (2008) Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod 23:2652–2662
Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS (1995) Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 121:1139–1150
Visconti PE, Krapf D, De La Vega-Beltran JL, Acevedo JJ, Darszon A (2011) Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl 13:395–405
Wandernoth PM, Mannowetz N, Szczyrba J, Grannemann L, Wolf A, Becker HM, Sly WS, Wennemuth G (2015) Normal fertility requires the expression of carbonic anhydrases II and IV in sperm. J Biol Chem 290:29202–29216
Wang D, King SM, Quill TA, Doolittle LK, Garbers DL (2003) A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol 5:1117–1122
Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, Moe OW, Garbers DL (2007) A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc Natl Acad Sci U S A 104:9325–9330
Wang H, Liu J, Cho KH, Ren D (2009) A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol Reprod 81:539–544
Wertheimer E, Krapf D, De La Vega-Beltran JL, Sanchez-Cardenas C, Navarrete F, Haddad D, Escoffier J, Salicioni AM, Levin LR, Buck J, Mager J, Darszon A, Visconti PE (2013) Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. J Biol Chem 288:35307–35320
Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Yi LG, Zhu H, Ma ZG, Wang XF, Chen ZH, Zhou SC, Dong HS, Zhang XH, Chung YW, Yuan YY, Yang WX, Chan HC (2007) Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci U S A 104:9816–9821
Zanetti N, Mayorga LS (2009) Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol Reprod 81:396–405
Zeng Y, Clark EN, Florman HM (1995) Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev Biol 171:554–563
Zeng Y, Oberdorf JA, Florman HM (1996) pH regulation in mouse sperm: identification of Na(+)-, Cl(-)-, and HCO3(-)-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol 173:510–520
Zeng XH, Navarro B, Xia XM, Clapham DE, Lingle CJ (2013) Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. J Gen Physiol 142:305–313
Acknowledgments
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica de Argentina PICT 2014-2702 (to DK) and PICT 2012-1175 (to MGB); NIH R01 HD44044 and HD038082 (to PEV), R01-TW008662 (to MGB); and the National Research Council of Argentina PIP 112-201101-00740 (to MGB and DK).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Stival, C., Puga Molina, L.d.C., Paudel, B., Buffone, M.G., Visconti, P.E., Krapf, D. (2016). Sperm Capacitation and Acrosome Reaction in Mammalian Sperm. In: Buffone, M. (eds) Sperm Acrosome Biogenesis and Function During Fertilization. Advances in Anatomy, Embryology and Cell Biology, vol 220. Springer, Cham. https://doi.org/10.1007/978-3-319-30567-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-30567-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30565-3
Online ISBN: 978-3-319-30567-7
eBook Packages: MedicineMedicine (R0)