Abstract
Purpose
Prolactin regulatory element-binding protein (PREB), a member of the WD-repeat protein family, has been recognized as a transcriptional factor that regulates prolactin promoter activity in the anterior pituitary of rats. PREB is expressed not only in the pituitary but also in various other tissues, including the adipose tissue. Previous studies have shown that PREB acts as a transcriptional regulator and suppresses the expression of the adiponectin gene in cultured 3T3L1 preadipocytes. The aim of this study was to further examine the potential role of PREB in adipose tissue in vivo.
Methods
Transgenic mice that overexpressing PREB (PREB transgenic mice) were generated. Insulin resistance was evaluated in PREB transgenic mice using glucose and insulin tolerance tests. Adiponectin expression in the adipose tissue was examined by western blot analysis and quantitative polymerase chain reaction (qPCR). The expression levels of stearoyl-CoA desaturase (Scd) and adiponectin receptor 2(ADIPOR2) were quantified by qPCR.
Results
Glucose and insulin tolerance tests revealed insulin resistance in PREB transgenic mice. Serum adiponectin and leptin concentrations were decreased. Adiponectin gene expression was decreased in the adipose tissue, which was confirmed by the downregulation of the adiponectin-dependent hepatic Scd gene and upregulation of the ADIPOR2 gene in the liver of PREB transgenic mice. We also found that pioglitazone, an agonist for the peroxisome proliferator-activated receptor-r, improved the insulin resistance in the PREB transgenic mice after a 10-day feeding period.
Conclusions
These results demonstrated that PREB might contribute to the regulation of adiponectin gene expression in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prolactin regulatory element-binding protein (PREB) gene encodes a 1.9-kb mRNA, which is translated into a transcription factor that binds to and activates the basal prolactin promoter [1, 2]. The primary sequence of the PREB protein contains two potential transregulatory PQ-rich domains and three regions that are highly similar to the WD repeat, thus making it a member of the eukaryotic family of WD-repeat proteins. Members of this ever-expanding family of proteins are involved in multiple cellular functions, including signal transduction, RNA processing, cytoskeletal assembly, and vesicle trafficking [3]. The PREB protein has similarities to a subset of proteins belonging to the WD-repeat family of proteins, which play a role in gene regulation. Although PREB is ubiquitously expressed in humans, its expression levels vary greatly among tissues, with very high levels detected in the pituitary gland, pancreas, adrenal gland, and adipose tissue [4,5,6,7,8].
Adiponectin (APN), also called GBP28, apM1, AdipoQ, and Acrp30, is a 244-amino-acid-long polypeptide and is recognized as an adipocytokine that is highly specific to adipose tissue; it is also secreted into circulating blood and influences systemic metabolism [9]. Previous studies reported that APN acts as an anti-diabetic and anti-atherosclerotic molecule [10, 11]. Furthermore, a number of clinical trials have shown that subjects with high levels of circulating APN tend to be protected against type 2 diabetes mellitus (T2DM) and myocardial infarction. A previous study also reported that higher concentrations of APN protected against later development of T2DM (incidence rate ratio 0.63; 95% confidence interval: 0.43–0.92, p = 0.02) [12]. Several factors regulate APN gene expression, including other adipocytokines such as TNFa and IL6 [13], and transcription factors such as peroxisome proliferator-activated receptor-r (PPARr) [14]. Moreover, cAMP inhibited APN gene expression in human visceral adipose tissue [15].
APN has been shown to be a key regulator of insulin sensitivity through a number of studies that have revealed a strong connection between insulin resistance and APN levels [16]. We previously reported that PREB can function as a transcriptional regulator of the APN promoter and thus mediate the effect of cAMP in 3T3L1 cells. PREB binds to the APN promoter, and in cells expressing PREB, it decreases APN expression in vitro [8]. The findings that PREB inhibits APN in adipose cell lines led us to hypothesize that PREB plays an important role in adipose tissue function in mice, which may in turn affect other proper functions. In this study, we generated transgenic mice that overexpress PREB to further examine the potential role of PREB in adipose tissue in vivo.
Materials and methods
Generation of PREB transgenic mice
PREB cDNA bearing a FLAG-tag was ligated into a plasmid vector carrying the CAG promoter along with polyadenylation signals; the expression cassette with the CAG promoter was excised from the construct and injected into pronuclear-stage embryos of C57BL/6 J mice (UNITECH). Successful generation was confirmed by PCR analysis using DNA from blood obtained from mouse tail veins. Transgenic lines were maintained by back crossing with PREB mice. All animals were kept in a pathogen-free environment.
Six-week-old male PREB mice were housed five per cage and maintained with free access to water and a regular chow diet or a HFD (60% by kcal; HFD-60; Oriental Yeast). Mice weighted 22 ± 2 g were sacrificed at 8 weeks of age after an overnight fasting, and their liver and adipose tissue were collected, snap-frozen in liquid nitrogen, and stored at −80 °C until processed for experimentation.
Metabolic experiments
All tests were performed after a 6-h fasting. For the glucose tolerance test (GTT), conscious mice were given 1 mg of glucose/g of body weight by intraperitoneal injection. Following successful infusion, blood was drawn from a tail snipping and blood glucose was measured using a Glucocard Diameter (ARKRAY) at 0, 0.5, 1, and 2 h. For insulin tolerance testing, 1 IU/kg of insulin was given by intraperitoneal injection, and blood was collected from tail snippings at 0, 0.5, 1, 2, and 2.5 h for blood glucose measurement.
Measurement of APN and Leptin
Blood samples were collected from the enucleated eyeball of mice under anesthesia, serum level of adiponectin (MRP 300; R&D Systems) and leptin (ab100718; Abcam) were measured using a commercially available enzyme-linked ELISA kits. All steps were performed under the manufacturer’s instructions.
Western blot analysis
Antibodies for APN and PREB were purchased from Abcam (ab3455, ab42501), and anti-GAPDH was purchased from TREVIGEN (2275-PC-100). Adipose tissue was homogenized with a bead mill homogenizer (Tomy Digital Biology) in TNE buffer (10 mmol l−1 Tris–HCl, 1% NP-40 v/v, 0.15 mol l−1 NaCl, 1 mmol l−1 EDTA) at 4 °C. Homogenates (10.3 µg) were separated on 4–15% SDS–polyacrylamide gels (Bio-Rad) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). After blocking with 7.5% w/v skim milk (Difco Laboratories) in PBS buffer containing 0.1% v/v Tween 20 (PBS-T) at 4 °C overnight, the membrane was washed and incubated overnight at 4 °C in 3% w/v BSA in PBS–Tween buffer containing rabbit anti-PREB (dilution 1:4000) or rabbit anti-GAPDH (1:5000) as the primary antibody, and polyclonal goat-anti-rabbit immunoglobulin–HRP (Dako Cytomation) as the secondary antibody for 1 h at 4 °C. To confirm equal loading, blots were stripped (western blot stripping buffer, Thermo scientific) after exposure and re-probed with the APN antibodies (1:250). Antibody binding was visualized using a chemiluminescence detection kit (ECL; Amersham Pharmacia Biotech).
Quantitative real-time PCR
Total RNA was extracted with RNA-Bee-RNA isolation reagent (TEL-TEST Inc.) from the mouse adipose and liver tissue, and reverse transcription was performed using Superscript II (Invitrogen) and 6 µg of total RNA; cDNA was used for qPCR using the Fast Start DNA Master SYBR Green I kit (Roche) in a CFX96 Real-time PCR Detection system (Bio-Rad) as previously described [8]. GAPDH was used as the housekeeping gene. The qPCR was performed using the following specific primer sequences:
-
GAPDH forward: 5′-TGAACGGGAAGCTCACTGG-3′, and reverse: 5′-TCCACCACCCTGTTGCTG TA-3′;
-
APN forward: 5′-AAGGACAAGGCCGTTCTC-3′, and reverse: 5′-AGAGTCGTTGACGTTATCTGCATA G-3′;
-
ADIPOR2 forward: 5′-GCCAAACACCGATTGGGGT-3′, and reverse: 5′-GGCTCCAAATCTCCTTGGTAGTT-3′;
-
Scd forward: 5′-GCTGTCAAAGAGAAGGGCGG-3′, and reverse: 5′-CTCTGGAACATCACCAGCTTCT-3′.
Treatment of thiazolidinedione
For PPARr agonist experiments, mice were weighed daily and gavaged with pioglitazone (10 mg/kg, Takeda) in 0.25% w/v hydroxypropyl methylcellulose (Alfa Aesar) for a period of 10 days. GTTs were done before and after pioglitazone treatment. Blood glucose was measured at 0, 0.5, 1, and 1.5 h.
Statistical analysis
Data shown are presented as the mean ± SE. Statistical analyses were performed using a one-way ANOVA and two-tailed Student’s t test. p values <0.05 were considered statistically significant.
Results
Expression of PREB in the adipose tissue of mice
We examined the expression of PREB in transgenic mice. Mice were sacrificed, and protein extracts from the adipose tissue were prepared and analyzed by western blotting. A strong protein band of approximately 41 kDa, matching the predicted size of the PREB protein, was detected. We also examined PREB expression in the adipose tissue of wild-type mice as a control; a significant increase in PREB expression was observed in the transgenic mice compared to that of the control group (Fig. 1).
Existence of PREB protein in PREB transgenic mice and control. Protein extract from mouse adipose tissue was subjected to western blot analysis; the expression level of GAPDH was assayed as a control and is shown at the bottom of each lane. Lanes 1, 3, 5 protein extract from wild-type mouse adipose tissue; lanes 2, 4, 6 protein extract from PREB mouse adipose tissue. The ratio of PREB to GAPDH is shown as a percentage of the control. Results are mean ± SE (n = 3) of separate experiments. *p values indicate significant differences
Glucose tolerance testing in PREB transgenic mice
To analyze the ability of the PREB transgenic mice to break down glucose, we performed a glucose tolerance test (GTT). Results revealed that the glucose tolerance in PREB transgenic mice was significantly attenuated after a glucose load test as compared with that of wild-type mice (Fig. 2a). We subsequently performed an insulin tolerance test, which revealed that PREB mice showed a significant resistance to the glucose-lowering effects of insulin, which was in accordance with the GTT data (Fig. 2b). These results indicated that PREB mice might exhibit insulin resistance. To better understand their glucose metabolism, we selected a HFD to induce the insulin resistance in PREB mouse. We compared the two groups of PREB mice that were or were not fed with HFD for 2 months. The HFD-fed mice were subjected to glucose tolerance (Fig. 3a) and insulin tolerance tests (Fig. 3b). Results showed that there were no significant differences in either glucose tolerance or insulin sensitivity between the two groups.
a Glucose tolerance test, b insulin tolerance test in wild-type (open circle) and PREB (solid circle) mice. Mice with standard chow diet were fasted for 6 h and glucose (1 mg/g) or insulin (1 IU/kg) was administered via intraperitoneal injection; plasma glucose was checked in a time-dependent manner as described in the methods. Results are expressed as mean plasma glucose ± SE. Number of mice: normal n = 5 and PREB n = 5. *p < 0.05
a Glucose tolerance test, b insulin tolerance test in PREB mice with (solid circle) or without HFD (open circle). Mice were fasted for 6 h, and glucose (1 mg/g) or insulin (1 IU/kg) was administered via intraperitoneal injection; plasma glucose was checked in a time-dependent manner as described in the methods. Results are expressed as mean plasma glucose ± SE. Number of mice: control n = 5 and HFD n = 5. *p < 0.05
Expression of APN in adipose tissue
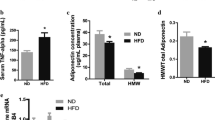
Previously, we reported that PREB binds to the APN promoter, and in cells expressing PREB, overexpression of the PREB gene decreases APN expression in vitro [8]. Therefore, we examined the expression of APN in the adipose tissue of PREB transgenic mice. To examine whether APN expression was affected in PREB transgenic mice, we examined the APN levels in the adipose tissue by western blot analysis (Fig. 4a) and qPCR (Fig. 4b). As seen in Fig. 4a, the western blots probed with an APN-specific antibody showed decreased concentrations of APN in the PREB transgenic mice as compared to the wild-type mice. In contrast, the basal levels of GAPDH remained unchanged in both the groups. APN mRNA levels were also decreased in the PREB transgenic mice. These results suggested that APN expression decreases in PREB transgenic mice. Based on the aforementioned results, indicating that overexpression of PREB induces a decrease in both the protein and mRNA levels of APN in PREB mice, we examined the serum levels of APN (Fig. 5a) and the other adipokine, leptin (Fig. 5b). The serum concentrations of APN and leptin in PREB transgenic mice were 6988.8 ± 672.88 ng/mL and 1.05 ± 0.12 ng/mL, respectively, which were significantly lower than that in control mice (10,266.4 ± 1341.89 and 2.55 ± 0.63 ng/mL, respectively). These results showed that PREB transgenic mice display reduced levels of serum APN and leptin.
a Expression of adiponectin (APN) in mouse adipose tissue. Protein was extracted and purified from the adipose tissue of mice, and western blot analysis was performed to measure APN expression; expression of GAPDH served as a control and is shown in the bottom lanes. Lanes 1, 3, 5 protein extract from wild-type mouse adipose tissue; lanes 2, 4, 6 protein extract from PREB mouse adipose tissue. The ratio of APN to GAPDH is shown as a percentage of the control. Results are mean ± SE of three separate experiments. *p values indicate significant differences. b mRNA expression of APN in mouse adipose tissue. APN mRNA purified from adipose tissue was measured by quantitative real-time PCR. The level of GAPDH served as a control, and the ratio of APN to GAPDH is shown as a percentage of the control. Results are expressed as the mean ± SE of three independent experiments. *p < 0.05
PREB mice displayed a significantly decreased expression of the stearoyl-CoA desaturase (Scd) gene in the liver tissue
APN is known to affect various organs including the liver, where it directly acts on the hepatic tissue and inhibits glucose production [11]. A previous report indicated that the Scd gene expression was suppressed in the liver of APN knockout mice [17]. We thereby examined the expression of this gene in PREB transgenic mice. Scd mRNA levels were analyzed by qPCR (Fig. 6). Using Scd-specific primers, qPCR results demonstrated a suppressed expression of Scd in PREB transgenic mice. However, the expression of GAPDH was not altered in either of the two groups (Fig. 6). These results suggested that PREB mice express a significantly decreased level of the APN-dependent Scd gene.
Expression of Scd mRNA in mouse liver. Scd mRNA was purified from liver tissue and measured by quantitative real-time PCR. The level of GAPDH served as a control, and the ratio of Scd to GAPDH is shown as a percentage of the control. Results are represented as the mean ± SE of three separate experiments. *p < 0.05
Expression of ADIPOR2 in PREB transgenic mice
ADIPOR1 and ADIPOR2 have been shown to serve as the major APN receptors in vivo and mediate the metabolic actions of APN. ADIPOR1 is ubiquitously expressed, including abundant expression in the skeletal muscle, whereas ADIPOR2 is most abundantly expressed in the mouse liver [3]. Therefore, we examined the expression of ADIPOR2 in mouse liver by qPCR (Fig. 7). ADIPOR2 mRNA levels in the liver of PREB transgenic mice were significantly increased compared to that of the wild type (Fig. 7). The elevated ADIPOR2 expression may be in response to the decreased APN levels in PREB transgenic mice.
mRNA expression of the adiponectin receptor 2 (ADIPOR2) in mouse liver. Total RNA was extracted from the liver of mice, and quantitative real-time PCR was performed to analyze ADIPOR2 mRNA expression; the level of GAPDH served as a control, and the ratio of ADIPOR2 to GAPDH is shown as a percentage of the control in the plot. Results are represented as the mean ± SE of three separate experiments. *p < 0.05
Thiazolidinedione ameliorates insulin resistance in PREB mice
Thiazolidinediones (TZDs) have been shown to act as insulin sensitizers in animal models of obesity-linked insulin resistance and diabetes, and they have been widely used as therapeutic agents for the treatment of T2DM. Treatment with the TZD pioglitazone significantly increases the plasma APN concentration in patients with T2DM. This increase is associated with an improvement in hepatic insulin sensitivity [18]. We hypothesized that TZD treatment would reverse the insulin resistance observed in the PREB transgenic mice in our study. We treated the mice with a low dose of pioglitazone (10 mg/kg of body weight/day) by daily gavage for a period of 10 days. The GTT was performed before and after pioglitazone treatment (Fig. 8a, b). Prior to treatment with pioglitazone, PREB transgenic mice showed an increased glucose intolerance compared to that of the control group (Fig. 8a). However, glucose tolerance was substantially improved after treatment with pioglitazone (Fig. 8b). An improvement in insulin resistance with TZD treatment in PREB mice indicated that PREB expression might be correlated with impaired insulin sensitivity.
Glucose tolerance test in wild-type (open circle) and PREB (solid circle) mice before (a), and after (b) feeding of pioglitazone (10 mg/kg in 0.25% hydroxypropyl methylcellulose) for 10 days. Mice fed a standard chow diet were fasted for 6 h before glucose (1 mg/g) was administered via intraperitoneal injection, and the plasma glucose was checked in a time-dependent manner as described in the methods. Results represent mean glucose concentrations ± SE. Number of mice: normal n = 5 and PREB n = 5. *p < 0.05
Discussion
PREB cDNA was recently isolated from a rat pituitary cDNA library, and the protein product was shown to transactivate the prolactin promoter [1]. PREB mRNA transcripts were present not only in the pituitary but a strong signal was also present in both the pancreas and adipose tissue [5, 8]. In this study, we generated PREB transgenic mice. These mice showed significant resistance to the glucose-lowering effects of insulin, reduced concentrations of serum APN and leptin, and a decreased APN expression in the adipose tissue. TZD treatment improved insulin resistance in these mice. Previously, we examined the role of PREB in the regulation of APN expression in mouse adipocytes and their cell line, 3T3L1; we observed that the overexpression of PREB decreased the APN protein expression. These findings suggested that overexpression of PREB may be a negative regulator of APN in vivo.
The PREB protein has three motifs, WD I, WD II, and WD III, with significant degrees of homology to the consensus WD-repeat sequence; this suggests that PREB is a member of the WD-repeat protein superfamily [3]. The highly conserved WD repeats within PREB demonstrate sequence similarity to a subset of proteins belonging to this family, which consists of proteins that act as gene regulators [3]. Unlike other WD-repeat proteins, PREB exerts transcriptional regulation, as it has been shown to stimulate gene expression by directly binding to DNA [1, 5]. Previously, we showed that PREB binds to the APN promoter and inhibits its activity in 3T3L1 cells. Native PREB binds to the APN promoter, whereas a mutant PREB-binding site abrogates the effect of not only PREB but also cyclic adenosine monophosphate (cAMP). These results suggest an alternative mechanism by which PREB may be involved in the cAMP-mediated inhibition of APN promoter activity. Additionally, a previous report suggested that PREB could mediate the protein kinase A (PKA)-induced stimulation of prolactin promoter activity [1], thereby suggesting a role for this protein in cAMP-mediated transcriptional responses. A possible model for this may involve the activation of PREB via PKA-mediated phosphorylation. Although it is not yet known whether PREB can serve as a PKA substrate, either in vitro or in vivo, the predicted sequence of this protein contains a number of motifs resembling consensus PKA phosphorylation sites [18]. Further studies are needed to determine the specific regulatory mechanisms that enable PREB regulation of APN gene transcription.
The concentration of PREB protein increases in response to glucose in a dose-dependent manner [18]. In patients with diabetes, APN levels are decreased [19]. In this way, patients with T2DM may have an increased risk of low-APN-mediated vascular inflammation, and subsequent damage, leading to the development of microvascular complications [20]. In the pancreas, PREB plays an important role in insulin gene expression in response to changes in glucose concentrations; however, in the adipose tissue, PREB inhibited the expression of APN in response to various glucose concentrations. In contrast, monocyte chemoattractant protein-1 (MCP1) is a potent chemotactic factor for monocytes and a key factor initiating the inflammatory process of atherogenesis. Previously, we have shown that PREB can function as a transcriptional regulator of the MCP1 promoter in response to cytokines. PREB binds to the MCP1 promoter, and in cells expressing PREB, MCP1 expression is increased. Together with this report, these results suggest that PREB might contribute to the atherosclerotic process.
Adipose tissue plays a critical role not only in energy homeostasis, but also in being a complex and highly active metabolic organ. APN, the most abundant and adipose-specific adipokine, is a collagen-like protein that is exclusively produced in the white adipose tissue. APN is stimulated during adipocyte differentiation and circulates at relatively high concentrations in the serum [21]. A decreased level of APN was considered a risk factor for the development of T2DM [22]. A previous study has indicated that the decreased levels of APN exacerbated insulin resistance by decreasing insulin sensitivity [23]. Previous reports have shown that a physiological dose of adiponectin improves insulin resistance in mouse models of obesity and T2DM [10]. Our results revealed that PREB mice displayed insulin resistance accompanied by a reduction in APN. Also, a decrease in the serum levels of leptin, which is an adipocytokine, improved insulin resistance. Transgenic overexpression of leptin has been shown to rescue insulin resistance and diabetes in a mouse model of lipoatrophic diabetes [24]. Insulin resistance in PREB mice might have been mediated by the decreased serum levels of both APN and leptin. Further studies are needed to clarify the mechanisms of insulin resistance in PREB mice. Figure 2 shows that PREB mice, fed with a chow diet, displayed glucose intolerance due to the insulin resistance. However, mice HFD for 2 months did not reveal any significant difference in glucose intolerance as compared to the control group. The reasons why the HFD did not induce the insulin resistance in PREB mouse remain unknown. Although the normal mice fed with HFD for 2 months develop fatty livers mediating insulin resistance, we did not observe a fatty liver in the HFD-fed PREB mice [K. Murao and H. Imachi, unpublished data]. Further studies are required to clarify the reasons why HFD did not induce insulin resistance in PREB mouse. As T2DM is in large part attributable to insulin resistance and/or hyperinsulinemia [25], PREB transgenic mice might serve as suitable models for studying adipose tissue dysfunction in diabetic patients.
The insulin-sensitizing action and benefits of TZDs, such as pioglitazone, which is an anti-diabetic drug acting as a PPARr agonist for patients with T2DM, have been very well elucidated. In the adipose tissue, negative regulation of PPARr signaling may possibly contribute to insulin resistance with decreased APN signaling, induced by activation of the liver X receptor (LXR) [26]. APN transcription levels are upregulated in adipocytes upon treatment with TZDs [27]. A previous clinical trial has demonstrated that a low dose of pioglitazone increases serum APN in diabetic patients [28]. Therefore, we hypothesized that the activation of PPARr would also lead to the activation of APN in PREB transgenic mice and reverse their insulin resistance. In this study, the treatment with low-dose pioglitazone for a period of 10 days reversed insulin resistance in PREB transgenic mice, suggesting that these mice responded to the PPARr activation. Further studies are necessary to determine the detailed mechanisms by which the overexpression of PREB induces insulin resistance in vivo.
Conclusion
In conclusion, we generated transgenic mice that overexpress the PREB gene. These results demonstrate that PREB may contribute to the regulation of APN gene expression in vivo. The PREB transgenic mice displayed insulin resistance, possibly due to the downregulation of the APN gene. Further investigation will help determine the possible physiological role of PREB in adipose tissue.
References
Fliss MS, Hinkle PM, Bancroft C (1999) Expression cloning and characterization of PREB (prolactin regulatory element binding), a novel WD motif DNA-binding protein with a capacity to regulate prolactin promoter activity. Mol Endocrinol 13(4):644–657. doi:10.1210/mend.13.4.0260
Taylor Clelland CL, Craciun L, Bancroft C, Lufkin T (2000) Mapping and developmental expression analysis of the WD-repeat gene Preb. Genomics 63(3):391–399. doi:10.1006/geno.1999.6089
Neer EJ, Schmidt CJ, Nambudripad R, Smith TF (1994) The ancient regulatory-protein family of WD-repeat proteins. Nature 371(6495):297–300. doi:10.1038/371297a0
Taylor Clelland CL, Levy B, McKie JM, Duncan AM, Hirschhorn K, Bancroft C (2000) Cloning and characterization of human PREB; a gene that maps to a genomic region associated with trisomy 2p syndrome. Mamm Genome 11(8):675–681
Ohtsuka S, Murao K, Imachi H, Cao WM, Yu X, Li J, Iwama H, Wong NC, Bancroft C, Ishida T (2006) Prolactin regulatory element binding protein as a potential transcriptional factor for the insulin gene in response to glucose stimulation. Diabetologia 49(7):1599–1607. doi:10.1007/s00125-006-0255-y
Murao K, Imachi H, Yu X, Cao WM, Muraoka T, Dobashi H, Hosomi N, Haba R, Iwama H, Ishida T (2008) The transcriptional factor prolactin regulatory element-binding protein mediates the gene transcription of adrenal scavenger receptor class B type I via 3′,5′-cyclic adenosine 5′-monophosphate. Endocrinology 149(12):6103–6112. doi:10.1210/en.2008-0380
Imachi H, Murao K, Cao WM, Muraoka T, Nishiuchi T, Dobashi H, Hosomi N, Iwama H, Ishida T (2008) The prolactin regulatory element-binding regulates of the 11β-hydroxylase gene. Biochem Biophys Res Commun 376(3):531–535. doi:10.1016/j.bbrc.2008.09.027
Li J, Murao K, Imachi H, Yu X, Muraoka T, Kim JB, Ishida T (2010) Prolactin regulatory element-binding protein involved in cAMP-mediated suppression of adiponectin gene. J Cell Mol Med 14(6A):1294–1302. doi:10.1111/j.1582-4934.2009.00752.x
Matsuzawa Y (2006) Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med 3(1):35–42. doi:10.1038/ncpcardio0380
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7(8):941–946. doi:10.1038/90984
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7(8):947–953. doi:10.1038/90992
Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J (2002) Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 360(9326):57–58. doi:10.1016/S0140-6736(02)09335-2
Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R (2003) Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 301(4):1045–1050
Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I (2003) Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52(7):1655–1663
Delporte ML, Funahashi T, Takahashi M, Matsuzawa Y, Brichard SM (2002) Pre- and post-translational negative effect of beta-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem J 367(Pt 3):677–685. doi:10.1042/BJ20020610
Pajvani UB, Scherer PE (2003) Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep 3(3):207–213
Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF (2012) Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci USA 109(36):14568–14573. doi:10.1073/pnas.1211611109
Kennelly PJ, Krebs EG (1991) Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 266(24):15555–15558
Goldstein BJ, Scalia RG, Ma XL (2009) Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med 6(1):27–35. doi:10.1038/ncpcardio1398
Niswender KD, Magnuson MA (2007) Obesity and the beta cell: lessons from leptin. J Clin Investig 117(10):2753–2756. doi:10.1172/JCI33528
Chandran M, Phillips SA, Ciaraldi T, Henry RR (2003) Adiponectin: more than just another fat cell hormone? Diabetes Care 26(8):2442–2450
Daimon M, Oizumi T, Kato T (2012) Decreased serum levels of adiponectin as a risk for development of type 2 diabetes, and impaired glucose tolerance as a risk for stroke–the Funagata study. Nihon Rinsho Jpn J Clin Med 70(Suppl 3):256–259
Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8(7):731–737. doi:10.1038/nm724
Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, Nakao K (2001) Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes 50(6):1440–1448
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86(5):1930–1935. doi:10.1210/jcem.86.5.7463
Zheng F, Zhang S, Lu W, Wu F, Yin X, Yu D, Pan Q, Li H (2014) Regulation of insulin resistance and adiponectin signaling in adipose tissue by liver X receptor activation highlights a cross-talk with PPARgamma. PLoS ONE 9(6):e101269. doi:10.1371/journal.pone.0101269
Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM (2001) Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 86(8):3815–3819. doi:10.1210/jcem.86.8.7741
Aso Y, Hara K, Ozeki N, Yatsuka C, Nakano T, Matsumoto S, Suetsugu M, Nakamachi T, Takebayashi K, Haruki K, Inukai T (2009) Low-dose pioglitazone increases serum high molecular weight adiponectin and improves glycemic control in Japanese patients with poorly controlled type 2 diabetes. Diabetes Res Clin Pract 85(2):147–152. doi:10.1016/j.diabres.2009.05.015
Acknowledgements
We thank Miss. Azusa Sugimoto and Dr. Yu Guan for their technical assistance. This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan. (to H.I, K.M. 24591352, 15K09415).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Procedures for the maintenance and use of animals were approved by the Ethics Review Board of Kagawa University, Kagawa, Japan, and all applicable institutional and governmental guidelines concerning the ethical use of animals were followed.
Informed consent
No informed consent.
Rights and permissions
About this article
Cite this article
Zhang, X.Z., Imachi, H., Lyu, J.Y. et al. Prolactin regulatory element-binding protein is involved in suppression of the adiponectin gene in vivo. J Endocrinol Invest 40, 437–445 (2017). https://doi.org/10.1007/s40618-016-0589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-016-0589-3