Abstract
Aim
Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists have immunomodulatory and anti-inflammatory effects. The study investigated the autoimmune injuries of diabetic cardiomyopathy (DCM) and tested the hypothesis that PPAR-γ agonists suppress disordered immune responses in diabetic heart, thereby preventing evolution of DCM.
Methods
STZ-induced diabetic rats were assigned to five groups: DM group, given no treatment; INS group, given insulin (4U kg−1 d−1); PIL group, given low dose pioglitazone (4 mg kg−1 d−1); PIL/INS group, given both low dose pioglitazone and insulin; PIH group, given high dose pioglitazone (20 mg kg−1 d−1). Normal rats (CON group) were also monitored as control. The pathologic abnormalities of hearts were observed. The immunoglobulin deposition was examined by immunohistochemistry and immunofluorescence.
Results
At 16 weeks, interstitial fibrosis was shown in diabetic heart which was accompanied by plenty of inflammatory cells infiltrated. Pioglitazone therapy could ameliorate the cardiac injuries. Shown by immunohistochemistry, the difference of integrated optical density (IOD) of immunoglobulin deposition among each group had statistic significance. No obvious immunoglobulins were deposited in the intercellular substance of heart in CON group (IgA 290.8 ± 88.1, IgG 960.4 ± 316.0 and IgM 341.3 ± 67.9). But the deposition of immunoglobulins increased significantly in DM group (IgA 7,047.5 ± 1,328.3, P < 0.05; IgG 28,945.9 ± 5,160.7, P < 0.05 and IgM 8,580.8 ± 1,336.8, P < 0.05). Administration of pioglitazone greatly reduced the increased deposition in a dose-dependent fashion. Moreover, the statistical significance was the same with immunofluorescence analysis as with immunohistochemical examination.
Conclusions
The data suggest that disordered immune responses play an important role in the pathogenesis of DCM. Pioglitazone showed protective effects by inhibiting the immunoglobulin deposition on diabetic myocardium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic cardiomyopathy (DCM), a distinct cardiomyopathy specific to diabetes, is not uncommon and is associated with increased morbidity and mortality in diabetic patients. The pathogenesis of DCM is complex and remains unclear up till now. By biopsy, we have found deposition of immunoglobulin complexes on the skin, kidney and skeletal muscle in many patients with type 1 or type 2 diabetes [1, 2]. Using the streptozotocin (STZ)-induced diabetic rat model, our previous studies demonstrated that immunoglobulin deposition was remarkably increased on the heart, aorta, retina and kidney in diabetic rats [3, 4], and cardiovascular complications of diabetes could be prevented by the administration of cyclosporine A (CsA) [5, 6]. The data above imply that multi-organ immune injuries may exist in diabetes and besides some specific antibodies, e.g., ICA and GAD, a number of non-specific antibodies may be involved in the disease process. Moreover, immunosuppressive therapy may be effective. Nevertheless, immunosuppressive drugs have shown major side effects that preclude their clinical use in the long-term range.
To date, more and more studies indicate that the insulin-sensitizing thiazolidinediones (TZDs), which are currently believed to be the selective activators of the peroxisome proliferators activated receptor γ (PPAR-γ), have immunomodulatory and anti-inflammatory properties [7–10]. In monocytes and macrophages, PPARγ activation inhibits the expression of a number of proinflammatory mediators [11, 12] and attenuates the oxidative burst in macrophages [13]. In T-lymphocytes, PPARγ activation inhibits both Ag-specific and non-specific T-cell activation, T-cell proliferation, and the production of several proinflammatory cytokines [14–17]. Numerous studies have documented that oral administration of PPARγ agonists ameliorates the clinical course and histopathological features in several autoimmune and inflammatory diseases such as multiple sclerosis [18, 19], myocarditis [20, 21], psoriasis [22, 23] and ulcerative colitis [24, 25]. At the same time, the discovery that TZDs have immunomodulatory and anti-inflammatory effects has also led to the evaluation of their potential use in the treatment of diabetic complications [7]. However, little is known about whether TZDs can suppress disordered immune responses in the diabetic heart, thereby preventing evolution of DCM.
Therefore, the aim of the study was to analyze the effect of pioglitazone on cardiac immunoglobulin deposition in STZ-induced diabetic rats and to evaluate its protective efficacy against the development of DCM. As a secondary gain, we wished to compare the effects of pioglitazone at different doses.
Materials and methods
Animals
Seventy-two male Sprague–Dawley rats (mean body weight 250 g, 8 weeks of age at the beginning of experiments) were kept in a barrier system with regulated temperature and humidity and on a 12/12-h light–dark cycle. During the whole experimental process, rats were fed with certified standard rat diet. All animal treatments were strictly in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental design and sample collection
Sixty rats were randomly selected and administered a single intravenous injection of 50 mg/kg STZ (Sigma, NY) freshly resolved in citrate buffer (0.10 mol L−1, pH 4.5), whereas the remaining animals were taken as a control group (CON group) and injected with the same volume of vehicle (saline). Three days after STZ injection, hyperglycemia was documented by measuring the glucose content of tail vein blood with glucometer (One Touch Ultra, Lifescan). Rats with random blood glucose concentrations ≥16.7 mmol L−1 were considered to be diabetic. Then, diabetic rats were randomly allotted to five groups of 12 animals: DM group, given no treatment; INS group, given insulin glargine (Sanofi-Aventis Pharma) by subcutaneous injection at the dosage of 4 U kg−1 d−1; PIL group, given pioglitazone (Takeda Pharma, Japan) in aqueous solution by gavage at the low dosage of 4 mg kg−1 d−1; PIL/INS group, given simultaneous pioglitazone and insulin glargine treatments, receiving the same dosage and schedule as the PIL and INS groups; PIH group, given pioglitazone at the high dosage of 20 mg kg−1 d−1. Body weight and blood glucose were measured at regular intervals. Maintenance of a diabetic state was confirmed by weekly tail vein blood glucose measurements. Samples for blood glucose were usually taken at 8:00 a.m. during light period. At the end of 16-week treatment, 24 h urine samples were collected in metabolic cages to determine urine albumin. Then, the rats were sacrificed and blood was collected to determine serum glutamic-pyruvic transaminase (SGPT), total bilirubin (TBIL) and creatinine. Some sections of heart were fixed in 10 % formalin for pathological examination and immunohistological staining. Other sections of heart were embedded in optimal cutting temperature compound (OCT) and stored at −80 °C for immunofluorescence staining. Clinical chemistry analysis of serum and urine samples was carried out using appropriate commercial kits (Biosino Biotechnology & Science Inc, China).
Cardiac histological examination
Paraffin tissue slices (5-μm-thick) were used for hematoxylin-eosin (HE) and Masson trichrome staining. Representative regions were photographed under bright field optics using a Leica DMRB light microscope (Leica, Wetzlar, Germany) equipped with digital image acquisition. Immunohistochemical detection for IgA, IgG and IgM was performed by standard procedures. For the negative control, the primary antibody was replaced by PB evaluation. The deposition of IgG, IgA, or IgM was determined by the brown-colored area and was analyzed by integrated optical density (IOD) using the Image-Pro Plus 6.0 software (Media Cybernetics, United States). Briefly, five images were taken randomly from every slice and the average values of IOD were analyzed in a blinded manner.
Frozen tissue slices (5-μm-thick) were fixed in cold acetone for 5 min. The antibodies labeled with fluorochrome were goat anti-rat antibodies against IgG (1:200 working solution), IgA (1:80 working solution), and IgM (1:80 working solution). Samples are observed by fluorescence microscope. The deposition of IgG, IgA, or IgM was determined by the green fluorescence area and was analyzed by IOD in the same way.
Statistical analysis
Data were presented as mean ± standard deviation (SD) and analyzed by one-way ANOVA followed by Bonferroni’s post hoc test using SPSS 11.5 software. P values <0.05 were considered statistically significant.
Results
Animal biochemical data
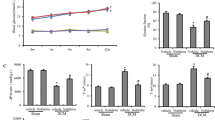
At the end of 16 weeks of treatment, body weight of CON rats significantly exceeded that of diabetic rats (Table 1). The treated groups showed a significant decrease in levels of mean blood glucose as compared to untreated diabetic rats (Table 1). Serum creatinine was higher in diabetic rats than in CON rats, and both insulin and pioglitazone treatment similarly reduced serum creatinine in diabetic rats (Table 2). Compared with the CON group, 24-h urinary albumin excretion was significantly higher in the DM group, and pioglitazone treatment significantly reduced albuminuria (Table 2). There was no significant difference with respect to liver function among the six groups at the time of the terminal study (P > 0.05; Table 3).
Cardiac pathological alterations in the diabetic rats
As shown by HE stain, heart from group DM revealed varied degree of changes from degeneration to necrosis of myocytes. Meanwhile, the interstitial and perivascular infiltration of inflammatory cells and the thickening of vessel wall were evident in diabetic heart. In Masson staining, collagen fibers take on green color. The DM group presented the interstitial and perivascular fibrosis, and the formation of microaneurysms in small capillary vessels (Fig. 1). Pioglitazone therapy could ameliorate, to some extent, the cardiac injuries mentioned above.
Immunohistological evaluation after treatment
As shown by immunohistochemistry, the difference of IOD of immunoglobulins deposition among each group had statistic significance (Table 4; Figs. 2, 3, 4). Compared with CON group, DM group had significantly increased deposition of IgA, IgG and IgM in the intercellular substance of heart. Administration of insulin or pioglitazone greatly reduced the increased deposition. Pioglitazone treatment was more effective than insulin treatment. Combination of pioglitazone and insulin reduced the increased deposition more than pioglitazone or insulin alone. Furthermore, high dose pioglitazone had a greater effect compared with low dose pioglitazone.
The outcome of immunofluorescence also demonstrated that there were statistic differences in the immunoglobulins deposition among each group (Table 5; Fig. 5). In control rats, no obvious cardiac immunofluorescence was detected. In DM group, there was more intense cardiac immunofluorescence of IgA, IgG or IgM than in the treatment groups. Pioglitazone treatment was more effective than insulin treatment. The statistical significance was the same with immunofluorescence analysis as with immunohistochemical examination.
Immunofluorescence examination for IgA in diabetic rats treated with pioglitazone and/or insulin (original magnification ×400). a CON group. b DM group. c INS group. d PIL group. e PIL/INS group. f PIH group. Arrows indicate the positive deposition on myocardial vascular intima. The deposition was strongly positive in DM group, moderately positive in INS group and poorly positive in PIH group. It might suggest administration of insulin or pioglitazone simultaneously reduce the increased deposition on myocardial vascular intima
Discussion
The pathogenesis of DCM is incompletely understood and several mechanisms have been implicated. Its pathological substrate is characterized by the presence of myocardial damage, reactive hypertrophy, interstitial and perivascular fibrosis. In this study, the observations of cardiac myocyte hypertrophy, degeneration and necrosis, as well as myocardial interstitial fibrosis in rats of STZ diabetes, are in accordance with the pathological characterizations of DCM. Simultaneously, the study showed infiltration of lymphocytes and deposition of immunoglobulin between cardiac myofibers from group DM, whereas heart from group CON did not reveal the above abnormalities. These results confirmed that there was disordered immunity (including both cellular and humoral immunity) which could lead to pathological changes of DCM. The involvement of disordered immune function in the causation of type 1 diabetes is well known. There is accumulating evidence that an ongoing cytokine-induced acute-phase response (sometimes called low-grade inflammation, but part of a widespread activation of the innate immune system) is closely involved in the pathogenesis of type 2 diabetes and associated complications [26]. Immunologic abnormality and inflammatory mediators seem to trigger a common pathway leading to type 1 and 2 diabetes [27]. Recently, declassifying diabetes has been pointed out owing to the more realistic possibility that both immune-mediated and non-immune-mediated processes might act in synergy, especially in later onset cases of diabetes [28]. To date, data suggesting an immunoglobulin-mediated injury in the heart of diabetic animal model are scarce, thus requiring a better elucidation of mechanisms responsible for DCM. Previous studies have confirmed that proteins modified by oxidation or glycation could induce pathogenic antibodies in a variety of diseases including diabetes mellitus, systemic lupus erythematosus and rheumatoid arthritis [29–33]. Accordingly, it is reasonable to assume that diabetic cardiac injury is caused by some components (like proteins) of cells modified by oxidation or glycation under a long-term hyperglycemic state. Oxidized or glycated proteins induce pathogenic antibodies. The binding of antibodies to antigen activates the complement system, forming the membrane attack complex (MAC) [34]. On one hand, binding to the cell membrane, the MAC disrupts the phospholipid bilayer of target cells and causes swelling and death, leading to cardiac injury. On the other hand, insertion of the MAC into endothelial cell membranes causes the release of growth factors such as basic fibroblast growth factor and platelet-derived growth factor that stimulate proliferation of fibroblasts and smooth muscle, mesangial, and other cells [34, 35]. Thus, the MAC possibly in part contributes to thickening of vascular wall and myocardial fibrosis. In short, DCM may be the result of disordered immune responses in vivo.
PPAR-γ is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors that are related to retinoid and steroid hormone receptors. It has been known to affect not only glucose homeostasis, but also immune responses. It is expressed in different cell types of the immune system, e.g., macrophages, microglia, dendritic cells, and lymphocytes [36]. Recent studies have documented that PPAR-γ activators suppress the T-cell proliferative response and inhibit inflammatory cytokine production by cells of the monocyte-macrophage lineage [9, 37]. They may exert anti-inflammatory effects via direct transcription regulation, by inhibition of the activity of transcription factors such as AP-1 and NF-kB [38–40]. It has been proposed that this may occur through both PPARγ receptor–mediated [41] and receptor–independent mechanisms [42, 43].
So far, more and more studies have demonstrated that TZDs exhibit beneficial pleiotropic effects on the cardiovascular system. Extensive data from ex vivo experiments and animal models suggest that TZDs inhibit or reverse cardiac hypertrophy and improve parameters of systolic and diastolic performance [44, 45]. Further evidence for potentially beneficial effects of TZDs on myocardial function comes from animal models of acute myocardial ischemia. In both murine and canine models of ischemia and reperfusion, PPARγ agonism with TZDs resulted in dose-dependent decreases in myocardial infarct size and improved parameters of cardiac performance [46, 47]. In accordance with these laboratory studies, an interesting study on 5,000 patients receiving pioglitazone for a period of 2.5 years revealed a good safety profile of pioglitazone. The results showed that patients treated with pioglitazone had a lower death rate, nonfatal myocardial infarction, stroke, leg amputation, and acute coronary syndrome [48]. Other studies also reported that pioglitazone reduced vascular risk and inflammatory markers, and improved carotid intima media thickness independent of its glycemic effect, suggesting that this drug would be good for diabetic patients with cardiovascular disease. When compared with rosiglitazone, pioglitazone is associated with a reduction in the risk of hospitalization for acute myocardial infarction [49]. Despite the potential reduction in risk of cardiovascular disease, the use of TZDs is associated with water retention which can be harmful to patients with heart failure. However, no specific human data suggest that drugs in this class have a direct negative effect on cardiac function. By restricting its indication for type 2 diabetic patients without heart failure, until now no study has been reported on the increase of cardiovascular events in patients taking pioglitazone.
A particularly novel finding of this study was that the increased deposition of IgA, IgG and IgM in DM group could be reduced significantly by insulin or pioglitazone treatment and the latter was more effective. This finding indicates that pioglitazone, as a PPAR-γ agonist, may protect against the development of DCM through its immunomodulatory action per se, independent of glycemic control. Meanwhile, a dose–response was apparent, as results were consistently better as doses of pioglitazone were increased in the treatment. In addition, pioglitazone and insulin combination therapy had a greater protective effect.
Elucidation of the mechanisms responsible for DCM will further motivate the generation of novel therapies tailored to decrease the cardiovascular morbidity and mortality in diabetes. The data presented in this manuscript support the involvement of disordered cellular immunity and humoral immunity in the causation of DCM and suggest protective effects of pioglitazone against DCM as a class of immunotherapeutic drugs. However, systolic and diastolic function (e.g., obtained with echocardiography) in diabetic rat hearts was not examined in this study due to financial consideration. Immunoglobulin deposition between cardiac myofibers only indicates the immune-mediated injury of the heart, but cannot represent the change of its function. In addition, it should be also mentioned that the results of the present study were obtained with pioglitazone, thus cannot be extended to other molecules of TZDs.
In summary, the utility of TZDs in treating cardiovascular, autoimmune and inflammatory diseases is of immense clinical potential. Much more research needs to be done to seek for additional safe and effective drugs in this class.
References
Gao H, Qiu M (2005) More research work should be done on the pathogenesis of autoimmune injuries to the multiple organs in some T2 diabetes Mellitus. Natl Med J China 85:793–795
Qiu M, Meng C (2006) The role of complement activation in the pathogenesis of diabetic complications. Chin J Endocrinol Metab 22:303–305
Meng C, Gao H, Qiu M et al (2006) Immunological injury on the retina in streptozotocin-induced diabetic rats. Chin J Endocrinol Metab 22:588–589
Zhang X, Cui J, Qiu M et al (2008) The effects of cyclosporine A on immunoglobulins deposition in retina of streptozotocin-induced diabetic rats. Chin J Intern Med 47:125–128
Cui J, Zhang P, Qiu M et al (2010) Preventive effects of cyclosporine A on immunoglobulins deposition on heart of STZ-induced diabetic rats. Chin J Diabetes Mellitus 2:142–147
Cui J, Qiu M, Li D et al (2010) The protective effects of cyclosporine A on aortic immunological injuries in STZ-induced diabetic rats. Chin J Cardiol 38:440–444
Pershadsingh HA (2004) Peroxisome proliferator-activated receptor-gamma: therapeutic target for diseases beyond diabetes: quo vadis? Expert Opin Investig Drugs 13:215–228
Lena S, Samir NP, Kodjo A et al (2009) Rosiglitazone modulates innate immune responses to Plasmodium falciparum and improves outcome in experimental cerebral malaria. J Infect Dis 199:1536–1545
Cuzzocrea S, Pisano B, Dugo L et al (2004) Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur J Pharmacol 483:79–93
Antonelli A, Ferrari SM, Frascerra S et al (2010) CXCL9 and CXCL11 chemokines modulation by peroxisome proliferator-activated receptor-alpha agonists secretion in Graves’ and normal thyrocytes. J Clin Endocrinol Metab 95:E413–E420
Klotz L, Schmidt M, Giese T et al (2005) Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J Immunol 175:4948–4955
Von Knethen A, Brune B (2002) Activation of peroxisome proliferator activated receptor γ by nitric oxide in monocytes/macrophages down-regulates p47phox and attenuates the respiratory burst. J Immunol 169:2619–2626
Faine LA, Rudnicki M, César FA et al (2011) Anti-inflammatory and antioxidant properties of a new arylidene-thiazolidinedione in macrophages. Curr Med Chem 18:3351–3360
Choi JM, Bothwell AL (2012) The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol Cells 33:217–222
Cunard R, Eto Y, Muljadi JT et al (2004) Repression of IFN-γ expression by peroxisome proliferator-activated receptor γ. J Immunol 172:7530–7536
Housley WJ, Adams CO, Vang AG et al (2011) Peroxisome proliferator-activated receptor gamma is required for CD4 + T cell-mediated lymphopenia-associated autoimmunity. J Immunol 187:4161–4169
Yanagita M, Kobayashi R, Kojima Y et al (2012) Nicotine modulates the immunological function of dendritic cells through peroxisome proliferator-activated receptor-γ upregulation. Cell Immunol 274:26–33
Niino M (2007) Peroxisome proliferator-activated receptor agonists as potential therapeutic agents in multiple sclerosis. Mini Rev Med Chem 7:1129–1135
Peiris M, Monteith GR, Roberts-Thomson SJ et al (2007) A model of experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice for the characterisation of intervention therapies. J Neurosci Methods 163:245–254
Hasegawa H, Takano H, Zou Y et al (2005) Pioglitazone, a peroxisome proliferator-activated receptor gamma activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance. J Mol Cell Cardiol 38:257–265
Yuan Z, Liu Y, Zhang J et al (2005) Cardioprotective effects of peroxisome proliferator activated receptor c activators on acute myocarditis: anti-inflammatory actions associated with nuclear factor κB blockade. Heart 91:1203–1208
Demerjian M, Man MQ, Choi EH et al (2006) Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol 15:154–160
Pershadsingh HA, Benson SC, Ellis CN (2005) Improvement in psoriasis with rosiglitazone in a diabetic and a nondiabetic patient. Skin med 4:386–390
Schaefer KL, Denevich S, Ma C et al (2005) Intestinal antiinflammatory effects of thiazolidinedione peroxisome proliferator-activated receptor-gamma ligands on T helper type 1 chemokine regulation include nontranscriptional control mechanisms. Inflamm Bowel Dis 11:244–252
Buckingham RE (2005) Thiazolidinediones: pleiotropic drugs with potent anti-inflammatory properties for tissue protection. Hepatol Res 33:167–170
Pickup JC (2004) Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27:813–823
Donath MY, Storling J, Maedler K et al (2003) Inflammatory mediators and islet beta-cell failure. A link between type 1 and type 2 diabetes. J Mol Med 81:455–470
Gale EAM (2006) Declassifying diabetes. Diabetologia 49:1989–1995
Kurien BT, Scofield RH (2008) Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev 7:567–573
Kurien BT, Hensley K, Bachmann M et al (2006) Oxidatively modified autoantigens in autoimmune diseases. Free Radic Biol Med 41:549–556
Ahmed N, Babaei-Jadidi R, Howell SK et al (2005) Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia 48:1590–1603
Wang G, Li H, Firoze Khan M (2012) Differential oxidative modification of proteins in MRL+/+ and MRL/lpr mice: increased formation of lipid peroxidation-derived aldehyde-protein adducts may contribute to accelerated onset of autoimmune response. Free Radic Res 46:1472–1481
Tam LS, Shang Q, Li EK et al (2013) Serum soluble receptor for advanced glycation end products levels and aortic augmentation index in early rheumatoid arthritis-a prospective study. Semin Arthritis Rheum 42:333–345
Speidl WS, Kastl SP, Huber K et al (2011) Complement in atherosclerosis: friend or foe? J Thromb Haemost 9:428–440
Fosbrink M, Niculescu F, Rus V et al (2006) C5b-9-induced endothelial cell proliferation and migration are dependent on Akt inactivation of forkhead transcription factor FOXO1. J Biol Chem 281:19009–19018
Raquel H, William TH, Montse C et al (2011) Immunoregulatory mechanisms of macrophage PPAR γ in mice with experimental inflammatory bowel disease. Mucosal Immunol 4:304–313
Zhang W, Eric AS, Paska AP et al (2008) Pioglitazone inhibits the expression of inflammatory cytokines from both monocytes and lymphocytes in patients with impaired glucose tolerance. Arterioscler Thromb Vasc Biol 28:2312–2318
Gao M, Hu Z, Zheng Y et al (2011) Peroxisome proliferator-activated receptor γ agonist troglitazone inhibits high mobility group box 1 expression in endothelial cells via suppressing transcriptional activity of nuclear factor κB and activator protein 1. Shock 36:228–234
Chen J, Mehta JL (2006) Angiotensin II-mediated oxidative stress and procollagen-1 expression in cardiac fibroblasts: blockade by pravastatin and pioglitazone. Am J Physiol Heart Circ Physiol 291:H1738–H1745
Ti Y, Hao MX, Li CB et al (2011) Rosiglitazone attenuates myocardial remodeling in spontaneously hypertensive rats. Hypertens Res 34:354–360
Straus DS, Glass CK (2007) Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol 28:551–558
Chawla A, Barak Y, Nagy L et al (2001) PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 7:48–52
Lee JH, Woo JH, Woo SU et al (2008) The 15-deoxy-delta 12,14-prostaglandin J2 suppresses monocyte chemoattractant protein-1 expression in IFN-gamma-stimulated astrocytes through induction of MAPK phosphatase-1. J Immunol 181:8642–8649
Asakawa M, Takano H, Nagai T et al (2002) Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation 105:1240–1246
Tsuji T, Mizushige K, Noma T et al (2001) Pioglitazone improves left ventricular diastolic function and decreases collagen accumulation in prediabetic stage of a type II diabetic rat. J Cardiovasc Pharmacol 38:868–874
Khandoudi N, Delerive P, Berrebi-Bertrand I, Buckingham RE, Staels B, Bril A (2002) Rosiglitazone, a peroxisome proliferator-activated receptorgamma, inhibits the Jun NH(2)-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes 51:1507–1514
Liu HR, Tao L, Gao E et al (2004) Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc Res 62:135–144
Dormandy JA, Charbonnel B, Eckland DJA et al (2005) PRO active investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitazone clinical trial in macro vascular events): a randomised controlled trial. Lancet 366:1279–1289
Derosa G (2010) Efficacy and tolerability of pioglitazone in patients with type 2 diabetes mellitus: comparison with other oral antihyperglycaemic agents. Drugs 70:1945–1961
Acknowledgments
Grant support: Key Endocrine Laboratory of General Hospital and Animal Laboratory of Health Department of Tianjin Medical University. We thank Mr. WANG Yongming and Mrs. ZHANG Xinshi for helping with the care of the animals and technical aspects of this study.
Conflict of interest
M. Yuan, M. Qiu, J. Cui, X. Zhang, P. Zhang declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, M., Qiu, M., Cui, J. et al. Protective effects of pioglitazone against immunoglobulin deposition on heart of streptozotocin-induced diabetic rats. J Endocrinol Invest 37, 375–384 (2014). https://doi.org/10.1007/s40618-013-0046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-013-0046-5