Abstract
Engineered nanomaterials (ENM) are anthropogenic materials with at least one dimension less than 100 nm. Their ubiquitous employment in biomedical and industrial applications in the absence of full toxicological assessments raises significant concerns over their safety on human health. This is a significant concern, especially for metal and metal oxide ENM as they may possess the greatest potential to impair human health. A large body of literature has developed that reflects adverse systemic effects associated with exposure to these materials, but an integrated mechanistic framework for how ENM exposure influences morbidity remains elusive. This may be due in large part to the tremendous diversity of existing ENM and the rate at which novel ENM are produced. In this review, the influence of specific ENM physicochemical characteristics and hemodynamic factors on cardiovascular toxicity is discussed. Additionally, the toxicity of metallic and metal oxide ENM is presented in the context of the cardiovascular system and its discrete anatomical and functional components. Finally, future directions and understudied topics are presented. While it is clear that the nanotechnology boom has increased our interest in ENM toxicity, it is also evident that the field of cardiovascular nanotoxicology remains in its infancy and continued, expansive research is necessary in order to determine the mechanisms via which ENM exposure contributes to cardiovascular morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demand for diverse materials with novel properties has effectively driven the birth and advancement of the nanotechnology field. The unique physicochemical characteristics that certain materials display at the nanoscale make them highly desirable and versatile. The National Nanotechnology Initiative defined the term Nanotechnology as “the understanding and control of matter at dimensions between approximately 1 and 100 nm, where unique phenomena enable novel applications” [1]. This definition, while widely accepted, constrains the scope and depth of the exponentially increasing number of engineered nanomaterials (ENM) used in science, engineering, medicine, and industry (e.g., pharmaceuticals, coatings, food). While it is apparent that nanotechnology holds tremendous potential benefits, it also carries significant risks to human activities as the biological effects caused by exposure to this almost limitless array of materials are not adequately understood. The literature consistently reflects the adverse effects of particle exposure on human health, particularly the cardiovascular system with several mechanisms being consistently proposed to account for the increase in morbidity including oxidative stress, systemic inflammation, endothelial dysfunction, thrombosis, and arrhythmia [2, 3].
While these studies indicate an impairment in overall cardiovascular function, this system is comprised of discrete segments that are fundamentally different in their function and structure. It is the goal of this review to discuss present toxicological findings from a novel perspective, namely that of the effect of ENM exposure on the specific levels of the vasculature, in an attempt to provide an organizational model for the diverse vascular effects associated with ENM toxicity.
ENM biocompatibility is dependent on specific chemical and physical parameters [4]. Chemical composition remains a primary factor for risk assessment of nanoparticles, with the classical example being metallic and metal oxide ENM where partial release of ions is associated with increased toxicity [5]. Carbonaceous ENM, due to their elongated shape, are unable to remain dispersed in the blood and therefore have a short plasma half-life. For this reason, our primary focus will be on the most prolific and commonly experienced metallic nanomaterials subdivided according to chemical composition: metals and metal oxides.
The Cardiovascular System
The cardiovascular system is essentially a central pump (the heart) and a closed system of conduits responsible largely for parallel transport and distribution of essential substances to tissues and the removal of metabolic waste products. The extraordinary heterogeneity of this network allows the cardiovascular system to perform these and other functions with an exquisite level of precision and control.
In attempting to predict the effects of ENM exposure on the cardiovascular system, several factors should be considered. First, ENM distribution within vascular segments depends upon not only the predominant physical forces acting in the specified vascular locus but also on the physicochemical characteristics of the nanomaterial. Second, the severity of the effects of ENM deposition is dictated by the unique function of each segment.
Macrocirculation
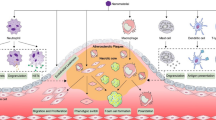
The macrocirculation refers to the conduit arteries which connect the heart with the systemic organs and also function as pressure reservoirs essential for cardiac cycles. The high pressures in this level of the vasculature contribute to the creation of turbulent flow and eddy currents (eddy current turbulence and impaction, Fig. 1) at bifurcations (arterial bifurcation and impaction, Fig. 1). Non-linear blood flow patterns increase resistance and decrease blood flow rates which may greatly affect particle deposition [6]. In this case, deposition rate of particles in the micron range increases linearly with particle diameter [7]. This effect becomes augmented at bifurcations or during pathological conditions where localized turbulence may develop and lead to ENM impaction within the adjacent vascular walls [8••].
Schematic representation of the cardiopulmonary system and identification of locations for ENM influences/activities. Black arrows: blood flow direction. Red represents oxygenated blood. Blue represents deoxygenated blood. (1) Extrapulmonary translocation to systemic circulation. (2) Arterial bifurcation and impaction. (3) Eddy current turbulence and impaction. (4) Segre-Silberg effect and plasma settling. (5) Systemic capillary translocation and tissue deposition. (6) Venular inflammatory signaling. (7) Venous settling. For simplicity, only one systemic tissue is depicted
Therefore, the pulsatile, high pressure in the macrocirculation favors non-laminar, turbulent blood flow which increases the likelihood for particle deposition in the vascular wall. ENM, as a result of their plasma suspension and mixing, tend to have a greater propensity to impact the macrovascular wall at bifurcations or at loci presenting eddy currents.
Microcirculation
The microcirculation is defined as all the vascular tissue within a given organ, which includes arterioles, the capillary network, and venules [9]. The essential function of the microcirculation is the maintenance of an ideal environment for the continuous exchange of nutrients and metabolic waste with the adjacent tissues. In order for this to occur efficiently, a high surface area and flow resistance are necessary. Arterioles are major regulators of blood flow and are fundamental players in the maintenance of blood pressure and flow distribution. The extent of this regulation is dependent upon the tissue type and is influenced by local factors as well as central input.
The local characteristics specific to the microcirculation may lead to a diffused distribution of ENM throughout specific tissues. Laminar flow predominates at a threshold vessel diameter of ∼300 μm [8••]. This, along with the slower flow and lower pressure, favors ENM settling within the microvascular compartment. In conditions of laminar flow, the Segre-Silberg effect is used to describe parabolic flow pattern of colloidal suspensions. Erythrocytes travelling at higher flow velocities remain in the flow centerline, while smaller particles and non-cellular elements are forced laterally towards the vascular wall (Segre-Silberg effect and plasma settling, Fig. 1) [10].
Capillaries are single-celled vessels composed solely of endothelial cells and represent the location wherein nutrient and waste exchange occur between tissues and blood. The relatively high flow resistance makes this compartment an ideal location for translocation and impaction of ENM (systemic capillary translocation and tissue deposition, Fig. 1).
Lastly, the venous compartment supports the return of blood to the heart and storage due to its high capacitance. A higher percentage of blood is habitually present in the veins; therefore, a significant proportion of ENM may exist here (venous settling, Fig. 1) [11].
ENM Exposure
ENM toxicity depends on intrinsic and extrinsic factors. First, the possible exposure route initially influences overall toxicity and the degree of ENM deposition, translocation, and interaction with cells, tissues, or specific intracellular components (extrapulmonary translocation to systemic circulation, Fig. 1). Historically, considering the ease with which ENM can be aerosolized and inhaled, the most frequently studied exposure route has been the pulmonary route; however, given recent developments in ENM-based therapies as well as their ubiquitous presence, other routes (e.g., ocular, gastric, dermal, injection, etc.) are becoming increasingly important.
ENM Properties
The physicochemical properties of ENM are fundamental determinants of their biodistribution and biokinetics [3, 12]. The nanoscale size of ENM results in a significantly greater surface area which augments chemical reactivity and differs from those of the corresponding bulk materials. Therefore, thorough material characterization is a priority in order to properly determine potential ENM toxic effects. This section will focus on what we consider to be the major physical and chemical determinants of biological responses triggered by ENM exposures (size, charge and surface chemistry, and shape).
Size
The most fundamental ENM physical property determining pulmonary deposition, translocation, and cellular uptake is size [13••, 14]. ENM particles show a great tendency to agglomerate into larger structures based on surface electrical potential and diameter [15]. This has a significant impact on ENM pulmonary deposition pattern [7]. Particle agglomerates ∼1–10 μm in diameter deposit preferentially in the upper airways by gravitational sedimentation and inertial impaction, while those below 0.1 μm diffuse throughout the entire airways and effectively penetrate to the tracheobronchial and alveolar regions. Once deposited, ENM may enter the systemic circulation and translocate to extrapulmonary tissues in a size-dependent manner [16]. Strong evidence exists that transcellular routes are mainly responsible for the translocation of ENM, with endocytosis consistently emerging as the key mode of cellular uptake [17]. Gold nanoparticles (AuNP) localized in membrane-bound vesicles within alveolar macrophages and rat alveolar type I lung cells [18], while titanium dioxide nanoparticles (TiO2-NP) were internalized via lamellar bodies and multivesicular structures in an immortalized epithelial cell line [19]. ENM <200 nm are predominantly taken up by cells via clathrin-coated pits, while ∼500-nm particles may be internalized through caveolae-mediated endocytosis [20, 21]. Other possible mechanisms may involve macropinocytosis, passive transport, and scavenger receptor-mediated endocytosis [22, 23]. Paracellular transport plays a minor role in healthy lungs but may become significant in inflammatory conditions which may severely compromise lung integrity [24].
The ability of ENM to enter the systemic circulation and translocate is intimately correlated with particle diameter [25]. ENM have extended circulating half-lives, weaker opsonization, and less phagocytic clearance than particles >100 nm. AuNP (10 nm) translocate from the blood to spleen, kidney, liver, testes, thymus, heart, and lung, while larger particles (50, 100, and 200 nm) were present predominantly in the liver and spleen [26]. Moreover, ENM smaller than serum albumin (40–50 kDa, approximate diameter 4–6 nm) are eliminated primarily through the kidneys [17, 27]. This phenomenon is due to the inherent selectivity of the glomerular filtration barrier that restricts the passage of molecules according to size, shape, and charge [28, 29]. ENM within the kidney may therefore avoid ultrafiltration, persist in the circulation, and translocate to other organs, such as the liver and spleen. These secondary destinations of ENM include predominantly the blood conditioning organs with major homeostatic functions (liver, kidney, spleen). While this may also be a direct consequence of the intrinsic ENM properties, the net accumulation in these organs may simply be due to the significant portion of the cardiac output these organs receive. Notwithstanding the plethora of work involving ENM kinetics, it is still unclear if they remain in the vasculature or whether they translocate to the cellular constituents of these secondary destinations.
Charge and Surface Chemistry
ENM surface properties are a direct product of their size. The decrease in atomic radius occurring in nanoscale materials increases the energy gap between electron transition states, resulting in an increased surface reactivity. This phenomenon, known as quantum confinement, accounts for the difference in chemical reactivity between ENM and bulk materials [30]. The surface properties of metals and metal oxides are influenced by the presence of defects in their crystalline structures, adsorbed ions, and functional groups. The increased reactivity may favor ENM with biological molecules, subcellular components, as well as the immune system.
Functional groups are specific moieties within molecules responsible for their chemical identity. The addition of these chemical groups (e.g., carboxylate, amines, etc.) can significantly modify ENM reactivity. This property becomes exceedingly important in a toxicological context as well as in ENM-based drug delivery systems, which often entails modification of their surface chemistry with specific biomolecules and chemical groups. Surface functionalization has been shown to influence biodistribution [12, 31], cellular uptake [32], and toxicity [33].
The cell membrane possesses a net negative charge resulting from the presence of anionic residues such as proteins, phospholipids, and glycoproteins. The plasma membrane also contains discrete microdomains of glycolipids and proteins known as lipid rafts. It is therefore reasonable to assume that the localized distribution of these negatively charged biomolecules may selectively dictate the interaction with ENM based on surface charge as well as chemical composition. For this reason, ENM with cationic chemical groups are frequently used to promote cellular adsorption and uptake [34]. Mesoporous silica nanoparticles (MSNP) coated with cationic polyethyleneimine (PEI) undergo greater cellular uptake than unmodified MSNP [35]. Moreover, intravenously administered positively charged AuNP adsorbed more significantly to the cell membrane of circulating red blood cells than neutral or negatively charged AuNP [36].
Surface chemistry may also determine ENM distribution to specific target organs and their inflammatory response associated with exposure. Intravenous administration of 2.8 nm AuNP coated with negatively charged thioglycolic acid accumulated preferentially within the liver compared to positively charged AuNP [36]. Sixty-nanometer polystyrene test particles (PSL) with positive surface charge resulted in increased thrombogenesis compared to control and groups exposed to carboxyl-coated PSL [37]. Charged (positive and negative) nanoparticles elicit a more robust response by the complement system, undergo greater opsonization, and activate phagocytosis by cells of the mononuclear phagocytic system [38]. Moreover, carbon nanotubes (CNT) functionalized with PEI also induced a greater NRLP3 inflammasome activation and IL-1β release in vitro by THP-1 cells than pristine and positively charged CNT [39]. Activation of immunological pathways may result in the systemic release of proinflammatory cytokines and oxidative stress, thus causing vascular dysfunction [40].
Addition of functional groups has been historically employed to increase ENM circulation time and concentrations for potential therapeutic use. Amphiphilic polymer coatings are effective in reducing first-pass metabolism by the liver [41]. Increasing surface hydrophobicity by coating liposomes with erythrocytic sialic acid moieties reduced opsonization and clearance by phagocytic cells [42]. Coating nanoparticles with polysaccharides such as dextran [43] as well as polyethylene glycol [44] increased plasma half-life due to a decreased activation of innate immunity. These findings highlight the effect of functionalization on ENM vascular concentrations and clearance by both the immune system and conditioning organs.
The osmolarity, pH, and charge of specific intracellular and extracellular compartments can also impact ENM surface chemistry [45]. When suspended in a liquid phase, ENM form a colloidal system. The stability of this system and the consequent interaction with cellular components can be assessed by measuring the zeta potential of ENM [46]. This is a key parameter governing the electrokinetic behavior of particles in solution and is a measure of the electrostatic attraction or repulsion between ENM particles [47]. An example of the effect of the liquid phase on ENM properties is metal oxides which show an increased agglomeration in pH environments approaching their isoelectric point [48]. Within the vasculature, ENM agglomeration may have significant implications as the increase in overall particle size could potentially decrease renal clearance and increase ENM plasma half-life, thus increasing the likelihood of deposition within the vascular wall.
When ENM penetrate physiological compartments, a complex mixture of proteins and biomolecules may bind non-specifically to their surface. The composition of this adsorbed coat, also known as protein corona, is influenced by ENM surface chemistry [33, 49, 50]. The dynamics of this interaction is also defined by ENM physicochemical properties such as size, surface charge, and composition [51]. The acquisition of a distinct protein corona may in turn influence the “biological identity,” cellular uptake, and the immunogenicity of ENM [52, 53]. In a recent study, the adsorption of serum proteins to AuNP was inversely dependent on size [53]. Similarly, plasma IgG showed a higher affinity for 100 and 50 nm PSL nanoparticles and little binding to carboxyl- and amine-modified 50-nm PSL nanoparticles [33]. While the formation of a protein corona has been shown to increase macrophage phagocytic activity [53] and elicit a more pronounced innate immune response, it may also enhance ENM biocompatibility [33] and therefore modulate the impact of ENM exposure on the cardiovascular system.
Shape
Recent studies have highlighted the importance of ENM morphology in the interaction with biological systems. ENM with a large width-to-height ratio or aspect ratio (nanorods, nanobelts, nanowires, and nanotubes) possess the potential to pierce cellular membranes and disrupt the integrity of anatomical barriers. ENM with high curvature angles resist uptake by phagocytic cells, particularly macrophages [54]. CNT with a low aspect ratio (220 nm length and 25 nm diameter) administered subcutaneously in rats persisted within the macrophage cytosol for >4 weeks, while high aspect ratio CNT (825 nm length and 25 nm diameter) were not phagocytized and triggered a robust inflammatory response [55]. Ellipsoid particles are more readily phagocytized by immune cells than spherical particles [56]. Gold nanorods of greater aspect ratio are not only internalized slower than shorter ones but are also more toxic to human HeCaT keratinocytes [57]. In addition to the effect of shape on the toxic potential of ENM, the presence of surface defects may also affect clearance [17]. The inability to efficiently clear ENM by the immune system may result in frustrated phagocytosis and pro-fibrotic cellular responses [58] as well as the systemic release of proinflammatory chemokines that may lead to vascular consequences.
ENM aspect ratio must be considered when determining their potential vascular effects. ENM with high aspect ratios are more likely to interrupt laminar flow, deposit in the vascular wall, and damage endothelial or vascular smooth muscle cells plasma membrane under conditions of turbulent flow or in bifurcations. Moreover, high aspect ratio fibers may occlude microvessels and trigger thrombus formation leading to ischemic events.
Examples of ENM Vascular Toxicity
Metallic ENM
Gold Nanoparticles
AuNP have been historically used in medicine as therapeutic agents and are also utilized in biological imaging and as drug delivery systems. Their bioinert character coupled with a high biocompatibility has fostered significant interest in their implementation as therapeutic and diagnostic (theranostic) agents. Their optical properties, characterized by enhanced absorption and scattering at specific electromagnetic wavelengths, have contributed to the application of AuNP for cancer imaging or as tumor-targeting systems [59].
Within the vasculature, the effects of AuNP exposure remain to be fully determined. Recent toxicological studies with cell cultures have shown the ability of AuNP to decrease cell viability and proliferation of microvascular endothelial cells [60]. Similarly, human umbilical vein endothelial cells exposed to AuNP presented decreased levels of vascular endothelium growth factor (VEGF) [61]. VEGF, a major angiogenic factor, also promotes endothelial cell migration and proliferation and may initiate the signaling cascade leading to nitric oxide (NO) production [62]. Therefore, VEGF reduction may adversely affect vascular reactivity.
In a porcine model of the blood–brain barrier (BBB), brain microvascular endothelial cells treated with 3 and 5 nm AuNP displayed augmented permeability but no change in systemic levels of the proinflammatory cytokines TNF-α and IL-1β [63]. Wistar–Kyoto rats infused with different sizes (10–100 nm) of AuNP presented hepatic leukocytic infiltration and disruption of the venous intima in a size-dependent manner [64]. Venous wall damage may impair capacitance, decrease blood return, and/or enhance inflammatory signaling (venular inflammatory signaling, Fig. 1).
Gold nanorods functionalized with polyelectrolytes induced aortic endothelial cytotoxicity and impaired endothelium-dependent relaxation of aortic rings [65]. In a separate study, 5 nm AuNP hyperpolarized aortic vascular smooth muscle via activation of BKCa potassium channels and influx of potassium ions, leading to vascular relaxation in an endothelium-independent manner [66].
In summary, there is evidence supporting cardiovascular toxicity via damage to endothelial cells, excitotoxicity, and/or inflammatory activation. The mechanisms through which this dysfunction may occur remain to be fully clarified, and substantial work exists linking AuNP exposure with the systemic release of specific inflammatory mediators. The disruption in endothelium-dependent dilation could also occur via inhibition of VEGF-mediated NO production. Endothelial dysfunction may impair the ability of resistance vessels, to adequately respond to local and central stimuli, leading to inadequate blood flow regulation.
Silver Nanoparticles
Perhaps the most utilized metallic nanoparticle, silver nanoparticles (AgNP) are an integral component in the health industry and food storage. The strong antibacterial properties exhibited by AgNP have led to their wide application from home appliances to water treatment [67]. Silver ions released by AgNP collapse the proton motive force in Vibrio cholera, resulting in cell death [68]. Further, silver ions depress the activity of bacterial respiratory chain dehydrogenases and damage bacterial cell membrane structural integrity [69]. Cotton fibers containing AgNP display potent antibacterial activity against major human pathogens, including Staphylococcus aureus [69].
AgNP also display enhanced absorption and scattering intensities from the oscillations of conduction electrons at an excitation wavelength of ∼385 nm [70]. The wavelength of the emitted light is dependent on both particle size and surface refractive index. This optical characteristic as well as the inherent catalytic property of AgNP allow them to be potentially useful as nanoscale sensors for the detection of environmental pollutants or in laboratory testing [71].
Similar to AuNP, AgNP exposure has been associated with antiangiogenic effects, suggesting a shared mechanism of vascular toxicity by metal nanoparticles. VEGF-induced cell proliferation, migration, and capillary formation in bovine retinal endothelial cells were markedly reduced following exposure via PI3K/Akt inhibition [72].
In an in vitro BBB model, isolated rat brain microvascular endothelial cells treated with increasing AgNP sizes (25, 40, and 80 nm) displayed cytotoxic and immunogenic in both a size- and time- (4 and 8 h) dependent manner [73]. In this experiment, larger AgNP resulted in less severe inflammation and microvascular cytotoxicity. The time dependence of the cytotoxic effects of AgNP was also visible in human microvascular endothelial cells and endothelial colony-forming cells. At high concentrations, AgNP degrade membrane integrity, initiate DNA damage, and stimulate the formation of free radicals. Interestingly, at sublethal concentrations, microvascular cells remained viable but showed a significant impairment in proliferation [74]. AgNP exposure resulted in inhibition of NO-mediated relaxation in isolated aortic rings [75]. AgNP were preferentially internalized by human umbilical vein endothelial cells, resulting in reactive oxygen species (ROS) production, cytoskeletal actin rearrangement, and disruption of intercellular endothelial integrity. Balb/c mice exposed intravenously to AgNP also presented increased vascular permeability, leading to the systemic release of AgNP and peripheral inflammation in the liver, lungs and kidneys [76]. Endothelial cell injury, dysfunction, and oxidative stress induced by exposure to AgNP may occur due to IKK/NF-κB activation [77].
Altogether, AgNP exposure reduces VEGF levels which may lead to decreased endothelial proliferation and NO synthase activity. The physiological repercussions may include a decreased total vascular density and impaired NO-dependent vasodilation which could significantly impair tissue perfusion. Activation of inflammatory pathways. ROS generation and disruption of cytoskeletal components may cause an increase in plasma filtration and decreased reabsorption of nutrients.
Copper
Copper nanoparticles (CuNP) have been successfully used as antimicrobial agents, particularly in textiles, plastics, and as additives for coatings. Their antimicrobial activity is associated with ROS generation, nucleic acid damage, and lipid and protein oxidation [78]. The high thermal conductivity and catalytic activity of CuNP make them efficient as sintering additives and catalysts for chemical reactions such as in the synthesis of methanol and glycol. Notwithstanding these industrial uses, CuNP are defined as a class 3 (moderately toxic) agent with cytotoxic and proinflammatory effects being reported in vitro [63]. The direct toxic effects of CuNP on the different components of the cardiovascular system are less well understood, and additional research is warranted. Porcine brain microvessel endothelial cells treated with 15 μg/ml CuNP of different sizes (40 and 60 nm) increased cellular permeability in a size-dependent manner. The increased permeability has been linked with the proinflammatory cytokines TNF-α, IL-1β, and prostaglandins [63]. CuNP are linked with ROS generation and inflammation in mouse pulmonary epithelial cells and human cardiac microvascular endothelial cells [79].
These effects indicate that CuNP may have a significant effect on vascular function via the release of proinflammatory cytokines. In a different study utilizing a chick embryo model, CuNP exposure resulted in marked proangiogenic and proliferative effect via the upregulation of VEGF and other angiogenic factors [80, 81]. It is noteworthy that modulation of VEGF signaling is a recurring theme in the antiangiogenic effects of metallic ENM. Increasing evidence suggests that this effect may occur due to inhibition of phosphorylation of associated downstream effector molecules including Src [82] and Akt [61], which could lead to increased endothelial cells apoptosis [83], impaired hematopoietic cell development, survival, differentiation, and migration [84–86] as well as aberrant vasculogenesis and heart development in prenatally exposed subjects [86].
The higher in vivo and ex vivo chemical reactivity of copper compared to noble metals such as silver and gold may account for the more significant inflammatory response associated with CuNP exposure. Copper’s electron configuration allows it to function as an efficient electron donor, resulting in greater ROS generation and systemic levels of inflammatory chemokines which may lead to significant vascular dysfunction.
Metal Oxides
Titanium Dioxide Nanoparticles
TiO2-NP is among the most widely used nanomaterials. Its pervasive use and toxicity have caused TiO2-NP to be classified as a potential occupational carcinogen to humans [87]. Its ability to function as a high-energy electron donor following treatment with UV light makes TiO2-NP a versatile ENM with applications in consumer products such as topical sunscreens and cosmetics, as well as industrial components including paints [88].
Pulmonary TiO2-NP exposure has been associated with systemic inflammation in vivo [89–91]. Leukocyte activation, the release of proinflammatory mediators, oxidative stress, and DNA damage [92] may lead to endothelium-dependent dysfunction. In isolated subepicardial arterioles of rats exposed to TiO2-NP (primary particle diameter ∼21 nm), a significant impairment in endothelium-dependent vasodilation was noted [93]. Similar findings were reported in other vascular beds including the uterus [94] and in skeletal muscle [95••]. This endothelium-dependent microvascular dysfunction has been linked with a reduced NO bioavailability triggered by the generation of reactive oxygen species following exposure or via the initiation of inflammatory mechanisms in the lung, resulting in the systemic release of cytokines, or within the vascular wall itself [96, 97]. In a similar study, acute (24 h) TiO2-NP exposure reduced microvascular NO bioavailability and altered cyclooxygenase-mediated vasoreactivity. TiO2-NP may also enhance adrenergic sensitivity via upregulation of α-adrenergic receptor density or increased release of neurotransmitters such as norepinephrine and neuropeptide Y at neurovascular junctions [98]. It is reasonable to speculate that an augmented sympathetic tone could have a more pronounced effect on veins, as they are more responsive to α-adrenergic stimulation [99]. The increase in venous tone may result in greater capillary filtration or reduced reabsorption, reduced plasma volume, and increased hematocrit.
The mechanisms underlying the effects of TiO2-NP exposure on the vasculature are fairly well documented. TiO2-NP exposure may lead to an impaired vascular function via ROS. These highly reactive free radicals react with endothelial NO, decreasing its bioavailablity [96]. Pulmonary TiO2-NP exposure is associated with the systemic release of proinflammatory cytokines and increased leukocyte activation. Lastly, an increase in sympathetic activation and altered cyclooxygenase signaling caused by TiO2-NP affects vascular reactivity [98]. Inhalation of TiO2-NP increases sympathetic tone via activation of the transient receptor potential channel in pulmonary C-fiber sensory neurons. Increased C-fiber activity augments neuronal function in the nodose ganglion and consequently affects autonomic regulation by the medullary cardiovascular regulatory center [100].
At the microvascular level, an increase in sympathetic tone results in higher total peripheral resistance and afterload, both factors associated with decreases in stroke volume and tissue perfusion. While these effects may be initially mitigated by an increase in cardiac contractility, chronic increases in afterload result in cardiac hypertrophy and pathological cardiac remodeling [101].
Cerium Dioxide Nanoparticles
The ability of cerium dioxide nanoparticles (CeO2-NP) to catalyze oxidation reactions by lowering the activation energy at which these reactions occur has allowed them to be implemented for public use as a diesel fuel additive [102] to increase combustion efficiency. The inherent capacity of CeO2-NP to shift between two valence states (Ce3+ and Ce4+) by reacting with highly reactive free radicals such as superoxide has encouraged its potential use as an antioxidant agent [103].
Intravenous CeO2-NP infusion results in time-dependent increases in oxidative stress and granuloma formation in the liver and spleen [104, 105]. The long-term biopersistence and adverse effects associated with CeO2-NP exposure raise significant concerns about its therapeutic potential as an antioxidant. Its impact on cardiovascular function is poorly understood but recent studies with human aortic endothelial cells exposed acutely (4 h) to CeO2-NP at different concentrations (0.001–50 μg/ml) showed an elevated albeit a modest effect of the inflammatory markers intercellular adhesion molecule 1 (ICAM-1), monocytic chemotactic protein 1 (MCP-1), and interleukin 8 (IL-8) [106]. While this study shows a modest effect on the macrocirculation, previous work conducted by our laboratory has shown that pulmonary CeO2-NP exposure impairs coronary and microvascular endothelium-dependent and endothelium-independent dilation in a dose- and route-dependent manner [107–109]. The microvascular dysfunction seen was linked to ROS production, decreased NO production, and elevated inflammatory cytokine levels [109].
Similar to TiO2-NP, CeO2-NP exposure may trigger ROS generation, via NO synthase uncoupling and mitochondrial damage [109], thus affecting endothelium-derived NO signaling as well as vascular smooth muscle function. Inflammatory responses to CeO2-NP exposure may play a role in these vascular effects, as corroborated by increased expression of inflammatory markers [109]. Vascular inflammation and oxidative stress may significantly impair vascular permeability and endothelial function.
Iron Oxide Nanoparticles
Investigations with various types of iron oxide nanoparticles (FeO-NP) have been carried out in order to identify potential biomedical applications, mostly for maghemite, ү-FeeO3, and magnetite, FeeO4 [110]. Based on their thermal and electrical conductivity and magnetic properties, FeO-NP have found applications as targeted drug delivery systems, in magnetic resonance imaging, and as a contrasting agent [110].
Despite these clinical applications, FeO-NP use has come under intense scrutiny due to its pronounced cytotoxic effects. Previous occupational studies have shown that FeO-NP exposure induces severe complications such as siderosis and hemochromatosis due to iron overload [111, 112]. Additionally, recent research has also established an effect of FeO-NP on endothelium viability with cytotoxic effects resulting due to the catalytic generation of ROS through Fenton chemistry [113–116]. The generation of free radicals was also seen in dextran stabilized FeO-NP in vitro with human umbilical endothelial cells [117]. FeO-NP exposure of human aortic endothelial cells resulted in upregulation of ICAM-1 and IL-8. Particle phagocytosis by monocytes induced significant oxidative stress and resulted in severe endothelial toxicity [116]. In mice, intravenous injection of FeO-NP (0.4, 2, and 10 μg/kg) caused significant prothrombotic effects in pial arterioles and venules as early as 1-h post-exposure, along with increased cardiac levels of markers of oxidative stress, including lipid peroxidation, ROS levels, and increased superoxide dismutase activity [118].
The predominant mechanism through which FeO-NP exposure leads to vascular dysfunction is through Fenton chemistry and oxidative stress. The ability to generate highly reactive ROS may also account for the inflammatory and cardiovascular effects caused by FeO-NP [118]. Moreover, the release of ions within the plasma has also been linked to severe conditions such as hemochromatosis associated with iron overload.
Zinc Oxide Nanoparticles
Zinc oxide nanoparticles (ZnO-NP) have been historically used as an anti-bacterial and in cosmetics such as sunscreen lotions due to their UV-blocking ability. However, severe systemic and cytotoxic effects have been linked with ZnO-NP exposure. DNA fragmentation, mitochondrial damage, and toxicity through ROS generation have been reported in diverse in vitro studies [79, 119] and in vivo exposure models [120].
In vitro ZnO-NP exposure had significant cytotoxic effects on human cardiac microvascular endothelial cells and elicited a strong inflammatory response. This deleterious effect was associated with the dissolution of ZnO-NP and the release of highly reactive ionic groups [121]. Additionally, ZnO-NP internalized by human aortic endothelial cells showed pronounced inflammatory consequences and cell death [122]. Similar in vitro effects of exposure were reported in human umbilical vein endothelial cells [123, 124].
Taken together, there is increasing evidence that ZnO-NP are cytotoxic and may affect endothelial cell function through ROS generation and damage to intracellular organelles. The release of zinc ions may also initiate an inflammatory response and further lead to vascular dysfunction. Aortic inflammation may potentially lead to a decrease in vascular compliance, causing an increase in afterload which may induce cardiac hypertrophy. Damage to veins may significantly decrease capacitance and venous return as well as preload. Both arterial and venous effects of ZnO-NP exposure could result in a drastic reduction in cardiac output.
Discussion
Evidence has accumulated establishing that ENM exposure negatively and diversely affects cardiovascular health. Cardiovascular endpoints associated with pulmonary ENM exposure however do not depend solely on translocation to the systemic circulation. Dysfunction may occur via activation of the immune system, resulting in the release of inflammatory mediators in the systemic circulation or via dysregulation of the autonomic nervous system [2]. Therefore, in investigating the cardiovascular impact of ENM, it is critical to bear in mind that all these mechanisms may be at play, at differing intensities over time. The severity and location of these effects are heavily dependent upon the physicochemical properties of the ENM in question as well as the inherent physiological conditions of the cardiovascular continuum. A major target of ENM exposure is the endothelium, which is responsible for the secretion of and response to paracrine, autocrine, and endocrine substances in the vascular environment. ENM exposure impairs endothelium-dependent dilation due to decreased NO bioavailability resulting from ROS generation or via inflammatory mechanisms.
Cardiovascular toxicity has been shown to be inversely correlated to particle size, most likely due to the increased ability of smaller particles to deposit and penetrate deep within the site of entry, traverse biological barriers, and access the systemic circulation. Even though systemic translocation of ENM from the site of entry may not be significant [125], intentional introduction of ENM or modification of ENM properties may increase significantly the proportion entering the circulation [126] and accumulating at a target tissue [13••]. Alternatively, these observations may be due to the high number of particles or the large surface area available for interactions after an exposure. Future studies focused on the most appropriate dose metric for these issues are warranted.
ENM size favors an increased reactivity, a phenomenon which becomes exceedingly important when considering metal oxides ENM and their catalytic ability to generate ROS via Fenton chemistry. Increased reactivity may also increase the likelihood for ENM to interact with biomolecules, enhancing their potential immunogenicity and overall toxicity.
ENM shape is also a key determinant of cytotoxicity. ENM with longer aspect ratios have a greater tendency to settle out of centerline blood flow and deposit within the vascular wall compared to their spherical counterparts. Continued research is warranted to better elucidate the relationship between shape, hemodynamics, and systemic vascular toxicity.
Lastly, surface charge and chemistry can also influence the biological effects of ENM. Clearance by the kidneys, particle agglomeration, and the interaction with both immune and cardiovascular components are impacted by ENM surface properties. Charged particles elicit a more significant inflammatory response via activation of both the mononuclear phagocytic system and the caspase-inflammasome axis. ENM functionalization may not only affect ENM biopersistence and half-life but may greatly dictate the interaction with physiological biomolecules and therefore the formation of a protein corona. Within the vasculature, changes in ENM surface chemistry can affect ENM agglomeration which in turn may increase ENM plasma half-life and vascular deposition. It is logical to hypothesize that an extended plasma half-life may result in not only an increase in ENM concentration over time but may allow for greater adsorption of host proteins to the particles’ surface and enhance their immunogenicity.
The impacts of ENM exposure on the systemic circulation and cardiovascular health are tissue specific. The vessels comprising the macrocirculation function as blood conduits and pressure reservoirs. While these functions are fundamental for cardiovascular health, they do not depend on significant degrees of vasomotion and regulation by central and local mechanisms. Therefore, ENM deposition in this compartment may not necessarily cause significant deficits to downstream tissue perfusion but could exacerbate preexisting cardiovascular conditions such as atherosclerosis. Damage to the macrocirculation leads to a decreased vascular compliance and consequently an increase in afterload. A prolonged increase in afterload and vascular resistance may lead to increased heart work and eventually cardiac hypertrophy.
The microcirculation is the major regulatory functional component of the cardiovascular system. Its fundamental role in the generation of total peripheral resistance, immune response, and waste exchange is well known. ENM deposition in arterioles may impair vascular reactivity, thus affecting the ability of these vessels to generate appreciable resistance to blood flow. This could have devastating effects on tissue perfusion and nutrient-waste exchange in capillaries. ENM deposition within capillaries may severely impact vascular permeability by damaging endothelial cells. The release of ions by ENM could affect the subtle balance between capillary and interstitial fluid colloid and oncotic pressures. Lastly, localization of ENM within venules may promote leukocytic infiltration and trigger an innate immune response resulting in the release of proinflammatory mediators. Damage to venules may increase capillary filtration and hematocrit, decreasing overall blood volume and cardiac output while increasing the likelihood of thrombotic events.
Conclusion
The field of cardiovascular nanotoxicology is still in its relative infancy, as corroborated by the heterogeneous and sometimes contradictory nature of the results reported in this review. With the increasing presence of ENM in everyday consumer products, the risk of unintentional occupational and domestic exposure remains very high. The use of in vitro models, different exposure conditions, and ENM characteristics may partly account for the incongruity in the toxicological research. However, the advent of new and more powerful experimental tools such as high throughput screening may prove beneficial for the assessment of the vascular effects of ENM exposures.
If nanotechnology is to make its greatest contribution to human health, the regional heterogeneity and unique ENM properties as well as their interactions must be directly addressed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
National Nanotechnology Coordination Office. National Science and Technology Council Committee on Technology Subcommittee on Nanoscale Science Engineering and Technology; National Science and Technology Council, editor. The National Nanotechnology Initiative, Supplement to the President’s FY2012 Budget. 1–50. 2012. Ref Type: Report
Newby DE, Mannucci PM, Tell GS, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36(2):83–93b.
Zhu M, Nie G, Meng H, et al. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc Chem Res. 2013;46(3):622.
Kirchner C, Liedl T, Kudera S, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5(2):331–8.
Bystrzejewska-Piotrowska G, Golimowski J, Urban PL. Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag. 2009;29(9):2587–95.
Tsuda A, Butler JP, Fredberg JJ. Effects of alveolated duct structure on aerosol kinetics. I. Diffusional deposition in the absence of gravity. J Appl Physiol (1985). 1994;76(6):2497–509.
Tsuda A, Henry FS, Butler JP. Particle transport and deposition: basic physics of particle kinetics. Compr Physiol. 2013;3(4):1437–71.
Stapleton PA, Nurkiewicz TR. Vascular distribution of nanomaterials. Wiley. Interdiscip. Rev. Nanomed Nanobiotechnol. 2014;6(4):338–48 This work describes ENM distribution within the vasculature and the adverse effects in each distinct vascular segment.
Pries AR, Secomb TW. Rheology of the microcirculation. Clin Hemorheol Microcirc. 2003;29(3–4):143–8.
Popel AS, Johnson PC. Microcirculation and hemorheology. Annu Rev Fluid Mech. 2005:3743–69.
Kolanjiyil AV, Kleinstreuer C. Nanoparticle mass transfer from lung airways to systemic regions—part I: whole-lung aerosol dynamics. J Biomech Eng. 2013;135(12):121003.
Kreyling WG, Semmler-Behnke M, Moller W. Ultrafine particle-lung interactions: does size matter? J Aerosol Med. 2006;19(1):74–83.
Kreyling WG, Semmler-Behnke M, Seitz J, et al. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;(21 Suppl):155–60 This study establishes the ability of inhaled nanoparticles to translocate to extrapulmonary compartments and tissues.
Palombo M, Deshmukh M, Myers D, et al. Pharmaceutical and toxicological properties of engineered nanomaterials for drug delivery. Annu. Rev. Pharmacol. Toxicol. 2014:54581–98.
Liu HH, Surawanvijit S, Rallo R, et al. Analysis of nanoparticle agglomeration in aqueous suspensions via constant-number Monte Carlo simulation. Environ Sci Technol. 2011;45(21):9284–92.
Sadauskas E, Jacobsen NR, Danscher G, et al. Biodistribution of gold nanoparticles in mouse lung following intratracheal instillation. Chem. Cent. J. 2009; 316.
Bertrand N, Leroux JC. The journey of a drug-carrier in the body: an anatomo-physiological perspective. J Control Release. 2012;161(2):152–63.
Takenaka S, Karg E, Kreyling WG, et al. Distribution pattern of inhaled ultrafine gold particles in the rat lung. Inhal Toxicol. 2006;18(10):733–40.
Singh S, Shi T, Duffin R, et al. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol Appl Pharmacol. 2007;222:141–51.
Rejman J, Oberle V, Zuhorn IS, et al. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377(Pt 1):159–69.
Conner SD, Schmid SL. Differential requirements for AP-2 in clathrin-mediated endocytosis. J Cell Biol. 2003;162(5):773–9.
Arredouani MS, Franco F, Imrich A, et al. Scavenger receptors SR-AI/II and MARCO limit pulmonary dendritic cell migration and allergic airway inflammation. J Immunol. 2007;178(9):5912–20.
Kanno Y, Miyama Y, Takane Y, et al. Identification of intracellular localization signals and of mechanisms underlining the nucleocytoplasmic shuttling of human aryl hydrocarbon receptor repressor. Biochem Biophys Res Commun. 2007;364(4):1026–31.
Muhlfeld C, Gehr P, Rothen-Rutishauser B. Translocation and cellular entering mechanisms of nanoparticles in the respiratory tract. Swiss Med Wkly. 2008;138(27–28):387–91.
Kreyling WG, Hirn S, Moller W, et al. Air-blood barrier translocation of tracheally instilled gold nanoparticles inversely depends on particle size. ACS Nano. 2014;8(1):222–33.
De Jong WH, Hagens WI, Krystek P, et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–9.
Deshmukh M, Kutscher HL, Gao D, et al. Biodistribution and renal clearance of biocompatible lung targeted poly (ethylene glycol) (PEG) nanogel aggregates. J Control Release. 2012;164(1):65–73.
Brenner BM, Hostetter TH, Humes HD. Glomerular permselectivity: barrier function based on discrimination of molecular size and charge. Am J Phys. 1978;234(6):F455–60.
Brenner BM, Hostetter TH, Humes HD. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978;298(15):826–33.
Yoffe AD. Low-dimensional systems-quantum-size effects and electronic-properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-2-dimensional systems. Adv Phys. 1993;42(2):173–266.
Shubayev VI, Pisanic TR, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61(6):467–77.
Chithrani DB. Intracellular uptake, transport, and processing of gold nanostructures. Mol Membr Biol. 2010;27(7):299–311.
Lundqvist M, Stigler J, Elia G, et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105(38):14265–70.
Chithrani DB, Dunne M, Stewart J, et al. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomedicine. 2010;6(1):161–9.
Xia T, Kovochich M, Liong M, et al. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano. 2009;3(10):3273–86.
Hirn S, Semmler-Behnke M, Schleh C, et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur J Pharm Biopharm. 2011;77(3):407–16.
Nemmar A, Hoet PH, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–4.
Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146(12):4234–41.
Li R, Wang X, Ji Z, et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano. 2013;7(3):2352–68.
Rajagopalan S, Brook RD. The indoor-outdoor air-pollution continuum and the burden of cardiovascular disease: an opportunity for improving global health. Glob Heart. 2012;7(3):207–13.
Illum SL, Davis SS. Effect of the nonionic surfactant poloxamer 338 on the fate and deposition of polystyrene microspheres following intravenous administration. J Pharm Sci. 1983;72(9):1086–9.
Allen TM, Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987;223(1):42–6.
Moore A, Marecos E, Bogdanov Jr A, et al. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology. 2000;214(2):568–74.
Klibanov AL, Maruyama K, Beckerleg AM, et al. Activity of amphipathic poly (ethylene glycol) 5000 to prolong the circulation time of liposomes depends on the liposome size and is unfavorable for immunoliposome binding to target. Biochim Biophys Acta. 1991;1062(2):142–8.
Honary S, Ebrahimi P, Hadianamrei R. Optimization of size and encapsulation efficiency of 5-FU loaded chitosan nanoparticles by response surface methodology. Curr Drug Deliv. 2013;10(6):742–52.
Zhang H, Cui H. Synthesis and characterization of functionalized ionic liquid-stabilized metal (gold and platinum) nanoparticles and metal nanoparticle/carbon nanotube hybrids. Langmuir. 2009;25(5):2604–12.
Dougherty GM, Rose KA, Tok JB, et al. The zeta potential of surface-functionalized metallic nanorod particles in aqueous solution. Electrophoresis. 2008;29(5):1131–9.
Rittermeier A, Miao S, MK S, et al. The formation of colloidal copper nanoparticles stabilized by zinc stearate: one-pot single-step synthesis and characterization of the core-shell particles. Phys Chem ChemPhys. 2009;11(37):8358–66.
Alkilany AM, Thompson LB, Boulos SP, et al. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64(2):190–9.
Podila R, Chen R, Ke PC, et al. Effects of surface functional groups on the formation of nanoparticle-protein corona. Appl Phys Lett. 2012;101(26):263701.
Shannahan JH, Podila R, Aldossari AA, et al. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol Sci. 2015;143(1):136–46.
Dobrovolskaia MA, Patri AK, Zheng J, et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. 2009;5(2):106–17.
Walkey CD, Olsen JB, Guo H, et al. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134(4):2139–47.
Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25(8):1815–21.
Sato Y, Yokoyama A, Shibata K, et al. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. Mol BioSyst. 2005;1(2):176–82.
Sharma J, Tai Y, Imae T. Biomodulation approach for gold nanoparticles: synthesis of anisotropic to luminescent particles. Chem Asian J. 2010;5(1):70–3.
Wang Y, Guo S, Chen H, et al. Facile fabrication of large area of aggregated gold nanorods film for efficient surface-enhanced Raman scattering. J Colloid Interface Sci. 2008;318(1):82–7.
Wang J, Byrne JD, Napier ME, et al. More effective nanomedicines through particle design. Small. 2011;7(14):1919–31.
Mody VV, Siwale R, Singh A, et al. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2(4):282–9.
Freese C, Uboldi C, Gibson MI, et al. Uptake and cytotoxicity of citrate-coated gold nanospheres: comparative studies on human endothelial and epithelial cells. Part Fibre. Toxicol. 2012; 923.
Pan Y, Wu Q, Qin L, et al. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the Akt pathway. Biomed ResInt. 2014:2014418624.
Dulak J, Jozkowicz A. Regulation of vascular endothelial growth factor synthesis by nitric oxide: facts and controversies. Antioxid Redox Signal. 2003;5(1):123–32.
Trickler WJ. Lantz-McPeak SM, Robinson BL, et al. Porcine brain microvessel endothelial cells show pro-inflammatory response to the size and composition of metallic nanoparticles. Drug Metab Rev. 2014;46(2):224–31.
Abdelhalim MA, Jarrar BM. Gold nanoparticles administration induced prominent inflammatory, central vein intima disruption, fatty change and Kupffer cells hyperplasia. Lipids Health Dis. 2011; 10133.
Alkilany AM, Shatanawi A, Kurtz T, et al. Toxicity and cellular uptake of gold nanorods in vascular endothelium and smooth muscles of isolated rat blood vessel: importance of surface modification. Small. 2012;8(8):1270–8.
Soloviev A, Zholos A, Ivanova I, et al. Plasmonic gold nanoparticles possess the ability to open potassium channels in rat thoracic aorta smooth muscles in a remote control manner. Vasc Pharmacol. 2015:72190–6.
Bosetti M, Masse A, Tobin E, et al. Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomaterials. 2002;23(3):887–92.
Dibrov P, Dzioba J, Gosink KK, et al. Chemiosmotic mechanism of antimicrobial activity of Ag(+) in Vibrio cholerae. Antimicrob Agents Chemother. 2002;46(8):2668–70.
Li WR, Xie XB, Shi QS, et al. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals. 2011;24(1):135–41.
DD Jr E, Chumanov G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem. 2005;6(7):1221–31.
Berciaud S, Cognet L, Lounis B. Photothermal absorption spectroscopy of individual semiconductor nanocrystals. Nano Lett. 2005;5(11):2160–3.
Gurunathan S, Lee KJ, Kalishwaralal K, et al. Antiangiogenic properties of silver nanoparticles. Biomaterials. 2009;30(31):6341–50.
Trickler WJ, Lantz SM, Murdock RC, et al. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol Sci. 2010;118(1):160–70.
Castiglioni S, Caspani C, Cazzaniga A, et al. Short- and long-term effects of silver nanoparticles on human microvascular endothelial cells. World J Biol Chem. 2014;5(4):457–64.
Gonzalez C, Rosas-Hernandez H, Ramirez-Lee MA, et al. Role of silver nanoparticles (AgNPs) on the cardiovascular system. Arch Toxicol. 2016;90(3):493–511.
Guo H, Zhang J, Boudreau M, et al. Intravenous administration of silver nanoparticles causes organ toxicity through intracellular ROS-related loss of inter-endothelial junction. Part Fibre. Toxicol. 2016; 1321.
Shi J, Sun X, Lin Y, et al. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-kappa B pathways. Biomaterials. 2014;35(24):6657–66.
Chatterjee AK, Chakraborty R, Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 2014;25(13):135101.
Sun J, Wang S, Zhao D, et al. Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells: cytotoxicity, permeability, and inflammation of metal oxide nanoparticles. Cell Biol Toxicol. 2011;27(5):333–42.
Mroczek-Sosnowska N, Lukasiewicz M, Wnuk A, et al. In ovo administration of copper nanoparticles and copper sulfate positively influences chicken performance. J. Sci. Food Agric. 2015.
Mroczek-Sosnowska N, Sawosz E, Vadalasetty KP, et al. Nanoparticles of copper stimulate angiogenesis at systemic and molecular level. Int J Mol Sci. 2015;16(3):4838–49.
Kalishwaralal K, Sheikpranbabu S. Barath Mani Kanth S, et al. Gold nanoparticles inhibit vascular endothelial growth factor-induced angiogenesis and vascular permeability via Src dependent pathway in retinal endothelial cells. Angiogenesis. 2011;14(1):29–45.
Gerber HP, Hillan KJ, Ryan AM, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–59.
Kubo H, Alitalo K. The bloody fate of endothelial stem cells. Genes Dev. 2003;17(3):322–9.
Schatteman GC, Awad O. Hemangioblasts, angioblasts, and adult endothelial cell progenitors. Anat. Rec. A Discov. Mol. Cell Evol Biol. 2004;276(1):13–21.
Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127(18):3941–6.
NIOSH, DHHS. Current intelligence. Bulletin 63—occupational exposure to titanium dioxide. 2011.
Silva RM, Teesy C, Franzi L, et al. Biological response to nano-scale titanium dioxide (TiO2): role of particle dose, shape, and retention. J. Toxicol. Environ. Health A. 2013;76(16):953–72.
Nurkiewicz TR, Porter DW, Barger M, et al. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect. 2006;114(3):412–9.
Hougaard KS, Jackson P, Jensen KA, et al. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part Fibre. Toxicol. 2010; 716.
Warheit DB, Webb TR, Reed KL, et al. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology. 2007;230(1):90–104.
Cui Y, Liu H, Ze Y, et al. Gene expression in liver injury caused by long-term exposure to titanium dioxide nanoparticles in mice. Toxicol Sci. 2012;128(1):171–85.
LeBlanc AJ, Cumpston JL, Chen BT, et al. Nanoparticle inhalation impairs endothelium-dependent vasodilation in subepicardial arterioles. J Toxicol Environ Health A. 2009;72(24):1576–84.
Stapleton PA, McBride CR, Yi J, et al. Uterine microvascular sensitivity to nanomaterial inhalation: an in vivo assessment. Toxicol Appl Pharmacol. 2015;288(3):420–8.
Nurkiewicz, TR, Porter, DW, Hubbs, AF, et al. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre. Toxicol. 2008; 51. This study indicates that nanoparticle inhalation impairs microvascular function more significantly than exposure to micron-sized particles of similar elemental composition.
Nurkiewicz TR, Porter DW, Hubbs AF, et al. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol Sci. 2009;110(1):191–203.
LeBlanc AJ, Moseley AM, Chen BT, et al. Nanoparticle inhalation impairs coronary microvascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovasc Toxicol. 2010;10(1):27–36.
Knuckles TL, Yi J, Frazer DG, et al. Nanoparticle inhalation alters systemic arteriolar vasoreactivity through sympathetic and cyclooxygenase-mediated pathways. Nanotoxicology. 2012;6(7):724–35.
Abboud FM. The sympathetic nervous system and alpha adrenergic blocking agents in shock. Med Clin North Am. 1968;52(5):1049–60.
Kan H, Wu Z, Lin YC, et al. The role of nodose ganglia in the regulation of cardiovascular function following pulmonary exposure to ultrafine titanium dioxide. Nanotoxicology. 2014;8(4):447–54.
Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102(4):470–9.
Cassee FR, van Balen EC, Singh C, et al. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol. 2011;41(3):213–29.
Colon J, Herrera L, Smith J, et al. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5(2):225–31.
Yokel RA, Tseng MT, Dan M, et al. Biodistribution and biopersistence of ceria engineered nanomaterials: size dependence. Nanomedicine. 2013;9(3):398–407.
Yokel RA, TC A, MacPhail R, et al. Distribution, elimination, and biopersistence to 90 days of a systemically introduced 30 nm ceria-engineered nanomaterial in rats. Toxicol Sci. 2012;127(1):256–68.
Gojova A, Lee JT, Jung HS, et al. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhal Toxicol. 2009;21:1123–30.
Minarchick VC, Stapleton PA, Porter DW, et al. Pulmonary cerium dioxide nanoparticle exposure differentially impairs coronary and mesenteric arteriolar reactivity. Cardiovasc Toxicol. 2013;13(4):323–37.
Minarchick VC, Stapleton PA, Sabolsky EM, et al. Cerium dioxide nanoparticle exposure improves microvascular dysfunction and reduces oxidative stress in spontaneously hypertensive rats. Front Physiol 2015; 6339.
Minarchick VC, Stapleton PA, Fix NR, et al. Intravenous and gastric cerium dioxide nanoparticle exposure disrupts microvascular smooth muscle signaling. Toxicol Sci. 2015;144(1):77–89.
Gupta AK, Curtis AS. Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials. 2004;25(15):3029–40.
Brittenham GM. New advances in iron metabolism, iron deficiency, and iron overload. Curr Opin Hematol. 1994;1(2):101–6.
Horwitz LD, Rosenthal EA. Iron-mediated cardiovascular injury. Vasc Med. 1999;4(2):93–9.
Buyukhatipoglu K, Clyne AM. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J Biomed Mater Res A. 2011;96(1):186–95.
Buyukhatipoglu K, Miller TA, Clyne AM. Flame synthesis and in vitro biocompatibility assessment of superparamagnetic iron oxide nanoparticles: cellular uptake, toxicity and proliferation studies. J Nanosci Nanotechnol. 2009;9(12):6834–43.
Apopa PL, Qian Y, Shao R, et al. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part Fibre. Toxicol. 2009; 61.
Zhu MT, Wang Y, Feng WY, et al. Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J Nanosci Nanotechnol. 2010;10(12):8584–90.
Wu X, Tan Y, Mao H, et al. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int J Nanomedicine. 2010:5385–99.
Nemmar A, Beegam S, Yuvaraju P, et al. Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part fibre. Toxicol. 2016;13(1):22.
Sharma V, Singh P, Pandey AK, et al. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat Res. 2012;745(1–2):84–91.
Kim YH, Fazlollahi F, Kennedy IM, et al. Alveolar epithelial cell injury due to zinc oxide nanoparticle exposure. Am. J. Respir. Crit Care Med. 2010;182(11):1398–409.
Xia T, Kovochich M, Liong M, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–34.
Gojova A, Guo B, Kota RS, et al. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115(3):403–9.
Paszek E, Czyz J, Woznicka O, et al. Zinc oxide nanoparticles impair the integrity of human umbilical vein endothelial cell monolayer in vitro. J Biomed Nanotechnol. 2012;8(6):957–67.
Tsou TC, Yeh SC, Tsai FY, et al. Zinc oxide particles induce inflammatory responses in vascular endothelial cells via NF-kappa B signaling. J Hazard Mater. 2010;183(1–3):182–8.
Moller W, Felten K, Sommerer K, et al. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit Care Med. 2008;177(4):426–32.
Oberdorster G, Maynard A, Donaldson K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre. Toxicol. 2005; 28.
Acknowledgments
The authors would like to thank Caroll McBride for his expert technical support during the completion of this review and Elizabeth Dalton for her assistance in the development of Fig. 1. This work was supported by the following sources: National Institutes of Health R01-ES015022 (TRN) and K99-ES024783 (PAS) and the National Science Foundation Cooperative Agreement-DGE-1144676 (TRN, ABA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alaeddin B. Abukabda, Phoebe A. Stapleton, and Timothy R. Nurkiewicz declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Metals and Health
Rights and permissions
About this article
Cite this article
Abukabda, A.B., Stapleton, P.A. & Nurkiewicz, T.R. Metal Nanomaterial Toxicity Variations Within the Vascular System. Curr Envir Health Rpt 3, 379–391 (2016). https://doi.org/10.1007/s40572-016-0112-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-016-0112-1