Abstract
The purpose of this study was to investigate the effect of gold nanoparticles on the signaling cascade related to angiogenesis and vascular permeability induced by Vascular Endothelial Growth Factor (VEGF) in Bovine retinal endothelial cells (BRECs). The effect of VEGF and gold nanoparticles on cell viability, migration and tubule formation was assessed. PP2 (Src Tyrosine Kinase inhibitor) was used as the positive control and the inhibitor assay was performed to compare the effect of AuNPs on VEGF induced angiogenesis. The transient transfection assay was performed to study the VEGFR2/Src activity during experimental conditions and was confirmed using western blot analysis. Treatment of BRECs with VEGF significantly increased the cell proliferation, migration and tube formation. Furthermore, gold nanoparticles (500 nM) significantly inhibited the proliferation, migration and tube formation, in the presence of VEGF in BRECs. The gold nanoparticles also inhibited VEGF induced Src phosphorylation through which their mode of action in inhibiting angiogenic pathways is revealed. The fate of the gold nanoparticles within the cells is being analyzed using the TEM images obtained. The potential of AuNPs to inhibit the VEGF165-induced VEGFR-2 phosphorylation is also being confirmed through the receptor assay which elucidates one of the possible mechanism by which AuNPs inhibit VEGF induced angiogenesis. These results indicate that gold nanoparticles can block VEGF activation of important signaling pathways, specifically Src in BRECs and hence modulation of these pathways may contribute to gold nanoparticles ability to block VEGF-induced retinal neovascularization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis, a physiological process involved in the growth of new blood vessels from pre-existing vessels, plays a key role in development of ocular complications including diabetic retinopathy, choroid neovascularization etc. Several clinical trials have reported the dominance or importance of VEGF in the development of this ocular pathogenesis [1]. Growth factors such as VEGF and fibroblast growth factor (FGF) are considered the major angiogenic factors that play a crucial role in normal and pathological angiogenesis which exert their effects via specific binding to cell surface-expressed receptors equipped with tyrosine kinase activity [2]. Consequently, VEGF and FGF (Fibroblast Growth Factor) are current targets of intense efforts to inhibit deregulated blood vessel formation in diseases such as cancer. Neovascularization which is one of the serious consequences related to pathogenesis of proliferative diabetic retinopathy is regulated by various growth factors and cytokines especially by hypoxia-inducible VEGF, that mediates endothelial cell (EC) dysfunction, proliferation, migration, permeability through adhesive contacts of ECs with the extracellular matrix [3, 4].
The role of VEGF in tumour angiogenesis [5] is being confirmed through in vivo experiments that clearly elucidated that VEGF treatment elicits EC proliferation, migration, and new vessel growth [6]. A unique feature of VEGF compared with other growth factors involved in the regulation of new vessel formation, is the high specificity of VEGF for the vascular ECs [7]. The biological effects of VEGF on ECs are mediated through the activation of two receptor tyrosine kinases: fms-like tyrosine kinase-1 (Flt-1) and fetal liver kinase-1/kinase insert domain-containing receptor (Flk-1/KDR). Gene knockout experiments have confirmed that Flk-1 play a critical role in angiogenesis [8], and Flk-1 knockout embryos are unable to form blood islands and to generate hematopoietic precursors [9].
In this scenario the need for VEGF targeting drugs that inhibit the complications mediated by its respective signaling cascades has arisen a great deal of interest in exploring the various potential therapeutic molecules. Most therapeutic agents developed against ocular neovascularization that include bevacizumab (Avastin, Genentech, South San Francisco, CA, USA), a monoclonal antibody with high affinity binding to human VEGF; Pegaptanib sodium (Macugen, Eyetech Pharmaceuticals and Pfizer, New York, NY, USA), an anti-VEGF aptamer that specifically blocks the 165 isoform of VEGF are all known for their pharmacological efficiency against VEGF mediated pathogenesis [10, 11]. Moreover, many products, such as alpha-defensins, VEGF165b (an endogenous C-terminal splice variant of VEGF), ephrin A1, and vasohibin, have been reported to even suppress retinal angiogenesis [12–14]. The matter of fact lies on the verity that these pharmacological drugs are highly cost effective for their various methods involved in production and development [15]. Therefore, therapies developed by targeting molecular mechanisms underlying VEGF mediated retinal angiogenesis may provide better treatment results with meager side effects to retinal neovascularization [16].
The recent emergence of nanotechnology has provided a new therapeutic modality in gold nanoparticles for serving mankind. For instance efforts are being made to investigate the therapeutic potential of nanoparticles where they could serve as an active component or just could be a physical support for functional moieties. The distinct properties of gold nanoparticles (AuNPs) such as low cytotoxic, capability to undergo easy surface modification with thiol-containing molecules, immobilization to wide range of biomolecules such as amino acids, proteins/enzymes and DNA, high optical extinction coefficients etc. have made them highly preferable for their potential in the use for nanomedicine. The anti-angiogenic properties of gold nanoparticles explicate their effective role in treatment against progression of tumor models including ovarian cancer [17]. These NPs have also recently emerged as an attractive candidate for delivery of various payloads into their targets [18, 19]. Thus the therapeutic strategies of gold nanoparticles in specifically targeting diabetic retinopathy and analyzing the signaling cascades through which they influence the control over the progression of the disease is still the need of the era.
In the present study, the molecular mechanism of AuNPs on VEGF-induced angiogenesis and vascular permeability in bovine retinal endothelial cells is been investigated. In addition to this, the status of phosphorylation of AuNPs on VEGF-induced VEGFR2/Src phosphorylation is also been examined. The results obtained from this study may provide better understanding over the molecular mechanism by which the gold nanoparticles influence the VEGF-induced angiogenesis and permeability in general and this elucidation might lead to the emergence of gold nanoparticle as a potential therapeutic molecule to inhibit the angiogenesis-related diseases such as ocular neovascularization and tumor progressions.
Materials and methods
Chemicals and reagents
Recombinant human VEGF165 was purchased from R&D Systems (Minneapolis, MN, USA). Rabbit polyclonal antibody against Flk-1/KDR (VEGF receptor 2; VEGFR2) was purchased from Cell Signaling Technology (Beverly, MA, USA). Streptomycin and bovine serum albumin (BSA) were purchased from Calbiochem (LA Jolla, CA). Fetal bovine serum (FBS) was purchased from Sera Laboratories International (USA, CA). MTT assay kit was purchased from Roche Diagnostics (Mannheim, Germany). Iscove’s Modified Dulbecco’s Medium (IMDM) was purchased from Sigma (St. Louis, MO, USA). Tissue culture dishes and 96 well plates were purchased from FalconR (BD Labware, Franklin Lakes, NJ, USA). Other chemicals were purchased from Sigma (St. Louis, MO, USA). All the media, chemicals and reagents used in the following experiments were purchased from Sigma until specified.
Synthesis of gold nanoparticles
Synthesis of gold nanoparticles was carried out using B. licheniformis as described in the method earlier with slight modifications [20, 21]. The culture was maintained at 4°C in nutrient agar plates. It was sub-cultured every 14 days in the nutrient medium. The reaction flask was kept in the shaker at room temperature for 24 h at 200 rpm. After 24 h incubation, the cultures were centrifuged at 15,000 rpm for 15 min. The pellets obtained about 1 g of wet weight were re-suspended in 100 ml of 1 mM aqueous HAuCl4 solution and incubated for 48 h.
Purification of gold nanoparticles
The cells from each Erlenmeyer flasks were washed twice with 50 mM phosphate buffer (pH 7.0) and re-suspended in 5 ml of the same buffer. Ultrasonic disruption of cells was carried out using an ultrasonic processor (Sonics Vibra Cell VC-505/220, Newtown, USA) over three 15 s periods, and with an interval of 45 s between periods. The sonicated samples were taken and the resulting solution was filtered through a 0.22 μm filter (Millipore, USA) to remove cell-debris. The sonicated samples were centrifuged at 15,000 rpm for 30 min at 37°C.
Characterization of gold nanoparticles
Characterization of synthesized gold nanoparticles (AuNPs) was carried out according to methods described previously [22]. UV/Vis spectroscopy measurements of AuNP samples were carried out on a UV–Visible spectrophotometer (Shimadzu Model 9200, Japan) operating at a resolution of 0.72 nm. The samples was subjected Transmission Electron Microscopy JEOL 6701 and to a XDL 3000 powder X-ray diffractometer. Lattice parameters were calculated using high-angle reflections of XRD. The Full Widths at Half Maximum (FWHM) values of X-ray diffractions were used to calculate particle sizes using the Debye–Scherrer formula.
Determination of concentration of the gold nanoparticles
The concentration of gold nanoparticles was determined by the method which has been previously reported. The calculation is as follows [23].
To determine the average number of atoms per nanoparticles
where, N = number of atoms per nanoparticles, π = 3.14, ρ = density of face centered, cubic (fcc) gold = 19.3 g/cm3, D = average diameter of nanoparticles = 50 nm = 50 × 10−7 cm, M = atomic mass of gold = 197 g, N A = number of atoms per mole (Avogadro’s Number) = 6.023 × 1023
Determine the molar concentration of the nanoparticles solution using the following formula:
where, C = molar concentration of nanoparticle solution, N T = Total number of gold atoms added as AuCl4 − = 1 M, N = number of atoms per nanoparticle (from calculation1), V = volume of the reaction solution in l, N A = Avogadro’s Number = 6.023 × 1023
Further, the required concentration were made out from the obtained values
Endotoxin assay
The Millipore H2O, used in all the experiments in our research, was tested for endotoxins using the Gel clot method according to manufacturer’s instructions (Lal endotoxin assay kit). Formation of gel-clot when sample treated according to the kit manufacturer indicated the presence of endotoxin in a sample analyzed. Similarly, prior to treatment in mice, the nanoparticles suspension in deionized water was checked for possible endotoxin contamination.
Cell culture
Bovine retinal endothelial cells (BRECs) were isolated and cultured as described earlier [24]. Briefly, freshly isolated retinas were washed and cut into 3 mm segments and transferred to a tube containing 4 ml of an enzyme cocktail (1 ml/retina). The enzyme cocktail is a mixture of 500 μg/ml collagenase type IV, 200 μg/ml DNase and 200 μg/ml pronase in 10 mM phosphate buffered saline containing 0.5% BSA (Bovine Serum Albumin) at 37°C for 30 min. The resultant enzyme digest was passed through 53 μm steel mesh (W.S Tyler, UK). The trapped blood vessels were washed three times with cold MEM by centrifugation at 400 × g for 5 min. The pellet containing microvessel fragments were finally suspended in IMDM with growth supplements on 35 × 10 mm culture dish coated with 1.5% gelatin type A and incubated at 37°C with 5% CO2. Characterization of cultured BRECs was carried out based on morphological analysis in phase-contrast microscope and confirmation was carried out using endothelial cell specific markers (Chemicon International, USA) and also (Carl Zeiss Axio Vision Product Suite CD 29, US).
AuNPs (100–1,000 nM) were prepared in sterile distilled water and diluted to the required concentrations using the cell culture medium. Appropriate amounts of Au-NPs stock solution were added to the cultures to obtain respective concentration of AuNPs and incubated for 24 h. The cells were starved for 12 h in all the experiments unless specified.
Growth factor treatment
Once the confluency reached about 70%, the cells were rendered quiescent for 12 h in serum-free IMDM before treatment with 25 ng/ml VEGF either in the presence or absence of 500 nM of AuNPs at 37°C for indicated times. The cells exposed to medium alone acted as a control group.
In vitro angiogenesis assays
The influence of gold nanoparticles over the angiogenesis in bovine retinal endothelial cells was examined quantitatively using in vitro proliferation, migration, and tubule formation models, as described previously [25].
Cell proliferation assay
Cell proliferation assay was performed according to method described earlier [20]. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide dye reduction assay using 96-well microtiter plates was performed according to the manufacturer’s instructions (Roche Diagnostics, Mannheim, Germany). The assay depends on the reduction of MTT by the mitochondrial dehydrogenases of viable cells to a blue formazan product, which can be measured using a scanning multiwell spectrophotometer (Biorad, Model 680, and Japan). BRECs (2 × 103), in a total volume of 100 μl of IMDM with 10% FBS, were incubated in each well at 37°C with 5% CO2 for 48 h. To determine the effect of various concentrations of Au-NPs alone or AuNPs along with VEGF on the proliferation of BRECs, the grown medium was replaced with IMDM medium containing 0.5% FBS at various concentrations of VEGF or Au-NPs as and then incubated for 24 h.
To examine the effect of gold nanoparticles on VEGF-induced endothelial cell proliferation, BRECs were treated with a combination of 500 nM of AuNPs and VEGF (25 ng/ml), where Au-NPs were added to the cells 30 min before the treatment of VEGF, which was still incubated for 24 h. After 24 h of incubation (37°C, 5% CO2 in a humid atmosphere), 10 μl of MTT (5 mg/ml in PBS) was added to each well, and the plate was incubated for a further 4 h (at 37°C). The resulting formazan was dissolved in 100 μl of dissolving buffer (provided as part of the kit) and absorbance of the solution was read at 595 nm. All determinations were carried out in triplicate. Concentrations of Au-NPs showing 50% reductions in cell viability (i.e., IC50 values) were then calculated.
Cell migration assay
In vitro scratched wounds were created by scraping the cell monolayer with a sterile disposable cell scraper (BD Falcon, Bedford USA). After injury of the monolayer, the cells were gently washed. The wound closure after treatment with various concentrations of AuNP and the effect of AuNP (500 nM) on VEGF treated cells were monitored after 24 h under the phase-contrast microscope and photographed using a digital camera (Canon power shot A 640, Japan). The assays were performed in the presence of 5-flurouracil (1 mM, Sigma, St. Louis, MO) to prevent migration of the cell monolayer at the wound edges. For quantitative representation of the results, the percentage total distance migrated from the edge of the monolayer was determined by using Axiovision software (Zeiss, US) at five different positions (every 5 mm).
Tube formation assay
Matrigel (10 mg/ml) was applied on a 0.5 ml/35 mm cell-culture dish and incubated at 37°C for at least 30 min for hardening. BRECs treated with Au-NPs (500 nM), PP2 (10 μM) and VEGF (25 ng/ml) for 16 h were prepared by trypsinization, washed once with growth medium, and re-suspended in 1.5 × 105 cells per ml in endothelial cell growth medium. Cells (2 ml) were gently added to the Matrigel-coated plates, incubated at 37°C, monitored at 10× magnification for 24 h (Carl Zeiss Axio Vision Product Suite CD 29, Germany) and photographed with a digital camera (Canon Power shot A 640, Japan). Tubular length was measured using Axiovision FRET, Rel 4.6 software (Zeiss, Germany).
Transwell monolayer permeability assay
The permeability of endothelial cells was analyzed quantitatively using in vitro RITC-dextran assay as described previously [26]. To measure solute flux across endothelial cells, retinal endothelial cells were seeded onto 12-mm diameter transwell filter inserts with a 0.4 μm pore size (Corning Inc); the inserts were placed into 12-well tissue culture plates. In some experiments, cells were first transfected with mutant Src constructs and then transferred to chambers. Chambers were examined microscopically for confluence, integrity, and uniformity of endothelial cell monolayer. 10 μM of rhodamine isothiocyanate (RITC)-dextran (70-kDa) (Sigma St Louis, MO) were applied to the apical chamber of the transwell inserts with a confluent endothelial cell monolayer.
The effect of AuNPs in a dose dependent manner over the permeability of endothelial cells was investigated. In some experiments, PP2 (Src inhibitor) and Au-NPs were added to endothelial cell cultures 30 min prior to VEGF treatment. The media volumes used equalized fluid heights in the apical and basolateral chambers, so that only diffusive forces were involved in solute permeability. At the indicated times after cytokine treatment, 100 μl samples were taken from the basolateral chamber and placed in a 96-well plate. A sample was taken from the apical chamber at the last time point; the amount of fluorescence in this chamber did not change significantly over the course of the experiment. Aliquots were quantified using a fluorescence multiwell plate reader (Biotek, Vermount, USA)
Plasmid constructs and transient transfection assay
The mutants at Lys295 (Kinase-deficient HA-Src KD K295M) and Tyr527 (constitutive active HA-Src-CA Y527F) were used accordingly as described earlier [26]. BRECs were transiently transfected using nucleofection technique (Amaxa Biosystems, Koeln, Germany) and grown to 80% confluence in IMDM medium. Briefly, cells were harvested by trypsinization and centrifuged at 3,000 rpm for 10 min. The pellet was resuspended in the nucleofector solution (Basic nucleofector kit, Amaxa Inc, Germany) to a final concentration of 4–5 × 105 cells/100 μl. At the time of transfection, 1–3 μg of DNA encoding green fluorescent protein (pmaxGFP), constitutively active Src or dominant negative Src was added along with nucleofector solution and then subjected to electroporation using a nucleofector device-II (Amaxa Biosystems, Koeln, Germany: Program M-003) according to manufacturer’s instructions. After electroporation, transfected cells were resuspended in 35 × 15 mm gelatin coated dishes containing 1 ml of prewarmed IMDM media and incubated in 5% CO2 at 37°C. The transfection efficiency was about 80–90% which was determined using pmaxGFP plasmid (Amaxa Biosystems) and cell viability determined by trypan blue exclusion was about 90%.
Preparation of cell lysate for kinase phosphorylation assays
BRECs in serum-free IMDM were treated with or without AuNPs for 1 h and then with 25 ng/ml VEGF for 10 min. Then the medium was removed by aspiration and the cells were washed once with cold PBS. Thereafter, the cells were harvested using 0.5 ml of cell lysis buffer (20 mM Tris–HCl; pH 7.4), 150 mM NaCl, 1 mM Na2EDTA (Disodium ethylenediamine tetraacetate),1 mM EGTA (ethylene glycol tetraacetic acid), 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 mM phenylmethylsulfonylfluoride (PMSF) and incubated on ice. After freezing and thawing, cell lysates were sonicated on ice for 15 s and then centrifuged at 25,000 × g for 20 min at 4°C to precipitate cell debris. The supernatant was transferred to new microtubes and the protein concentration was determined using the Bradford protein assay (Bio-Rad, Hercules, CA, USA) and stored at −80°C until assayed for VEGFR2 phosphorylation. For immunoprecipitation, cell lysates were incubated with Flk-1antibody for 16 h at 4°C and then incubated with 25 μl of protein A/G plus Agarose (Santa Cruz Biotechnology, Inc.) for 2 h on a roller system at 4°C. The beads were then washed three times with 500 μl of lysis buffer. Beads were then collected by centrifugation, washed, and resuspended in 25 μl of 2 X SDS loading buffer and subjected to western blotting analysis. In order to detect protein expression of VEGFR2, blots were probed with a rabbit antibody raised against VEGFR2 (1:1,000; Santa Cruz Biotechnology, Inc, Santa Cruz CA). In order to detect activated VEGFR2, blots were probed with a mouse antibody that specifically recognizes phosphotyrosine (1:1,000; Santa Cruz Biotechnology, Inc, Santa Cruz CA) and immunoreactive bands were detected as described in the protocol for western blotting.
Quantification of phospho-Src Y419 in cell lysate
Concentrations of phospho-Src were quantified by using a human phosphor-Src (Y419) ELISA kit based upon peptide competitive analysis (R & D systems, Minneapolis, MN) as per manufacturer’s instructions. Briefly, 1 × 107 cells were seeded in a 60 mm tissue-culture dish and grown for 24 h. After the cells get attached and grow to confluence, the monolayer was starved for 6 h in IMDM with 0.5% FBS. After various treatments, cells were washed with 1X PBS (centrifuged at 2,000 × g, 10 min) and lysed using lysis buffer containing 1 mM EDTA, 0.05% Triton X-100, 5 mM NaF, 6 M Urea, 5 mM PMSF, 1 mM Na3VO4, 2.5 mM sodium pyrophosphate and a protease inhibitors (Sigma St. Louis, MO). After centrifugation at 2,000 × g for 10 min at 4°C, the supernatant containing proteins was removed and sixfold dilution was made with buffer containing 1 mM EDTA, 0.5% Triton X-100, 1 M urea in 1X PBS. 100 μl of samples was added to each well of 96-well microplate coated with phospho-Src (Y419) capture antibody and incubated for 2 h at room temperature. After incubation, the plates were washed twice with PBS and incubated in blocking solution for 30 min. Following another wash with PBS, cells were incubated with the phospho-Src (Y419) detection antibody for 2 h at room temperature. After washing, 100 μl of streptavidin-HRP was added into each well and incubated for 20 min and then, 100 μl tetramethylbenzidine/H2O2 was added to the plates followed by the addition of 50 μl of stop solution. Colour formation was measured at an absorbance of 450 nm using a plate reader, which is directly proportional to the concentration of phospho-Src in the samples. The concentration of phospho-Src was determined using a calibration curve by generating a four parameter logistic curve fit.
Transmission electron microscopy (TEM) analysis
TEM sample preparation involving cells, however, was performed by treating cells with Au-NPs and/or VEGF for 6 h with under serum-free conditions. After the incubation, BRECs were centrifuged initially at 2,500 × g for 10 min. The resultant cell pellets were then washed thrice with PBS, and fixed in Trump’s fixative (1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer, pH 7.2). Thin section (90 nm) of samples for transmission electron microscopy (TEM) analysis were prepared on carbon-coated copper TEM grids and stained with lead citrate. TEM measurements were performed on a JEOL model 1200EX instrument operated at an accelerating voltage of 120 kV.
Statistical analysis
All the data were expressed as the Means ± Standard Deviation (SD). One-tailed Student’s t tests were also used for evaluations of pairs of means, and to establish which groups differed from the control group. A significance level of P < 0.05 was considered to be statistically significant (Graph Pad, San Diego, CA).
Results
Biosynthesis and characterization of gold nanoparticles
The biosynthesis of Au-nanoparticles from the biomass of B. licheniformis was carried out. An aqueous HAuCl4 ion was reduced to AuNPs when added to the biomass of B. licheniformis [22]. Prior to the study of anti-angiogenic effect of AuNPs, characterization of biologically synthesized AuNPs was performed as described earlier [20–22]. During the visual observation, biomass incubated with 0.1 g/l of HAuCl4 showed a color change from pale yellow to dark purple whereas no color change could be observed in biomass which lacked HAuCl4. The dark purple color remained stable and which indicated the formation of Au-NPs [27]. The UV–Vis spectra results obtained showed absorption maximum at about 540 nm which is attributable to the surface plasmon resonance band (SPR) of the gold nanoparticles (data not shown). Various metal nanoparticles with sizes ranging from 2 to 100 nm are being assigned to their respective surface plasmon [28]. The crystalline nature of the synthesized particles was carried out using X-ray diffraction analysis.
The diffraction peaks at 2θ = 38.31°, 44.46°, 64.67° and 77.45° obtained are identical with those reported for the standard gold metal (Au0) (Joint Committee on Powder Diffraction Standards-JCPDS,USA) (data not shown). No other diffraction peaks were observed, which solely confirms the synthesis of naked gold nanoparticles [27]. Finally the confirmation of the biologically synthesized Au-NPs was carried out using transmission electron microscopy analysis, which confirmed the formation of gold nanoparticles at the size of 50 nm of spherical shape (Fig. 1). The endotoxin assay revealed that the gold nanoparticles synthesized were endotoxin free.
TEM images obtained from purified fractions of Au-NPs synthesized using B. licheniformis. Purified Au-NPs from B. licheniformis were examined by electron microscopy. Several fields were photographed and were used to determine the diameter of nanoparticles. The range of observed diameters was around 50 nm
Cell proliferation assay
To determine the intensity of the cytotoxicity of AuNPs over the endothelial cells, BRECs were exposed to various concentrations of AuNPs for 24 h. The results obtained showed a dose-dependent response increase in the cytotoxicity of AuNPs on the endothelial cells. Exposure of the cells to concentrations greater than 500 nM of AuNPs caused significant cell death. Exposure of BREC cells to any dose from 0 to 500 nM did not induce any cytotoxic effects Fig. 2a. Therefore, in order to study the effects other than cytotoxicity of Au-NPs on BRECs, a non-toxic dose 500 nM of Au-NP was used in the subsequent experiments. Following cytotoxicity the effect of Au-NPs over endothelial cell proliferation was examined which revealed the possible inhibitory effect of Au-NPs on VEGF-induced endothelial cell proliferation. A dose-dependent increase in cell proliferation over time through VEGF treatment was observed. After 24 h incubation, VEGF (25 ng/ml) significantly induced the cell proliferation as compared to control (Fig. 2b). Au-NPs (500 nM) treatment significantly blocked the VEGF-induced proliferation, as like PP2 (10 μM). Thus the above result makes clear that Au-NPs and PP2 significantly blocks the VEGF-induced endothelial cell proliferation in BRECs.
Effect of AuNPs on the VEGF induced proliferation of BRECs. a Dose dependent effect of AuNPs on proliferation of BRECs. Cells at the density of 1 × 103 cells/ml were treated with AuNPs at various concentrations from 100 to 600 nM and the dose dependent effect of AuNPs over the cell proliferation was studied. b Effect of AuNPs on VEGF-induced proliferation of bovine retinal endothelial cells (BRECs). Cells at the density of 1 × 103 cells/ml were treated with or without Au-NPs (500 nM) or PP2 in the presence of VEGF for 24 h and the restorative effect of AuNPs over the VEGF induced proliferation were studied. The AuNPs added along with the VEGF significantly reduced the VEGF induced proliferation in BRECs compared to the VEGF control. The PP2 Src inhibitor was used as the positive control to study the potential of AuNPs to inhibit the proliferation in BRECs. Values are mean ± SE of six experiments. Significant differences from control group were observed (P < 0.05)
Migration assay
Cell migration plays a crucial role in the formation of new blood vessels in angiogenesis and consequently vital for tumor growth and metastasis. It was reported that Src plays an important role in regulating VEGF-induced endothelial cell migration and changes in permeability [29–31], and therefore the ability of Au-NPs to inhibit VEGF-induced BREC migration and thereby it’s potential in controlling angiogenesis was examined. This was carried out based on the wound-healing migration assay that revealed the chemotactic motility of AuNPs in BRECs.
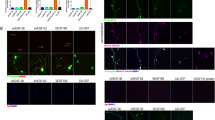
The dose dependent effect of Au-NPs on the chemotactic motility of BRECs was assessed and the effect of CA-Src on AuNPs added migration of the cells was assessed shown in Fig. 3. During the experimental conditions, as expected, exogenous VEGF stimulated microvascular endothelial cell migration was up to 95% compared to the untreated cells. The maximum effect of gold nanoparticles in inhibiting migration was obtained in concentration of 500 nM Au-NPs. At this concentration a significant reduction in VEGF-induced endothelial cell proliferation was obtained. The ability of Au-NPs to impede cell migration was also tested and BRECs migration was prevented by the treatment with Au-NPs. Enhanced migration of endothelial cells and complete wound closure by 24 h was observed in VEGF-treated plates (Fig. 4) while a significant area of the wound remained uncovered in Au-NPs treated ones compared to the control. The latter phenomenon (wound remaining uncovered) was also observed in plates treated with a combination of VEGF (25 ng/ml) and Au-NP (500 nM). Therefore, Au-NPs were able to significantly block the VEGF-induced endothelial cell migration thereby controlling the progression of the pathogenesis.
Dose dependent effect of AuNPs on BRECs migration. BRECs were wounded with micropipette tip and were treated with AuNPs at various concentrations from 100 to 600 nM and the dose dependent effect of AuNPs over the cell migration was studied. Experiments were performed thrice with similar results and significant differences from control group were observed (P < 0.05). The effect of CA-Src on the AuNPs induced migration was also studied
Effect of AuNPs on VEGF induced BRECs migration. BRECs were wounded with pipette and treated with VEGF (50 ng/ml) in the presence or absence of either 500 nM of AuNPs or PP2 (10 μM). After incubation, the migrated cells were quantified by manual counting. These experiments were performed thrice with similar results and significant differences from control group were observed (P < 0.05)
Tube formation assay
Another important step that involves in the cascades responsible for angiogenesis is the formation of tubules [32]. To examine the potential effects of Au-NPs on the tubular structure formation in endothelial cells, we investigated the effect of Au-NPs on VEGF-induced tube formation based on the two-dimensional matrigel assay. When BRECs were placed on the VEGF-reduced matrigel, elongated and robust tube-like structures were formed after incubation in the presence of VEGF (Fig. 5). The length of the tubules formed was calculated using the inverted phase contrast microscopy which directly revealed the ability of endothelial cells to form tubular structures. As shown in Fig. 4, 500 nM Au-NPs treated with VEGF (25 ng/ml) inhibited 80% tube formation of BRECs on Matrigel. These results elucidate that Au-NPs could block VEGF-induced in vitro angiogenesis by inhibiting cell migration, invasion, and tube formation. These results hence reveal the potential and significant role of Au-NPs in preventing VEGF-induced tube formation.
AuNPs and PP2 inhibit VEGF-induced tube formation of endothelial cells. BRECs (1 × 105 cells) were inoculated on the surface of the Matrigel, and treated with VEGF (50 ng/ml) in the presence or absence of either 500 nM of AuNPs or PP2. The morphological changes of the cells and tubes formed were observed under a microscope and photographed at 200× magnification. Tube formation was quantified by counting the number of connected cells in randomly selected fields at 200× magnification (Carl Zeiss, Chester, VA, USA), and dividing that number by the total number of cells in the field. Column shows the quantitative measurement of tube length. These experiments were performed thrice with similar results and significant differences from control group were observed (P < 0.05)
RITC-Dextran permeability assay
Numerous vision-threatening ocular conditions including diabetic macular edema, retinal vein occlusions, retinopathy of prematurity and exudative age-related macular degeneration are mediated by a key pathophysiological role i.e., Retinal vascular leakage [33]. Thus the possible inhibitory effect of Au-NPs on VEGF-induced endothelial cell permeability was further examined. Initially the dose dependent effect of AuNPs (100–500 nM) over the VEGF induced permeability of BRECs was assessed (Fig. 6a). Then in the experiment part, Au-NPs were added 30 min prior to VEGF treatment. Au-NPs inhibition of VEGF-induced permeability occurred in a dose-dependent fashion; 500 nM Au-NPs was sufficient to inhibit VEGF-induced dextran permeability to the level of control (Fig. 6b). The result obtained suggests that Au-NPs completely abrogated the VEGF-induced dextran permeability.
Effect of AuNPs on VEGF induced endothelial cell permeability. a BRECs were grown to confluent monolayers on porous membranes and were incubated with AuNPs of various concentrations from 100 to 500 nM and the flux of RITC-dextran from the upper to the lower chamber was measured 12 h after the treatment. The dose dependent effect of AuNPs on VEGF induced permeability of endothelial cells was assessed. Experiments were carried out in triplicate and the **P < 0.01. b AuNPs and PP2 inhibit the VEGF-induced endothelial cell permeability. BRECs were grown to confluent monolayers on porous membranes and were incubated with Au-NPs (500 nM) or PP2 (10 μM) with either 25 ng/ml VEGF, and the flux of RITC-dextran from the upper to the lower chamber was measured 12 h after the treatment. AuNPs and PP2 were added 30 min prior to VEGF treatment. Pre-treatment with AuNPs or PP2 reduced the VEGF-induced permeability to the level of control (0.5% serum).Values are expressed in relative fluorescence counts (RFCs) as means ± SEM, with each condition performed at least in triplicate where **P < 0.01
Transfection assay
To elucidate the role of Src in endothelial cell proliferation induced by VEGF, BRECs were transfected with DN Src and CA Src, and the effect of VEGF and Au-NPs on cell proliferation was analyzed as just described. The major cascades during angiogenesis such as cell differentiation, proliferation, and motility are known to be mediated by Src which is a non-receptor tyrosine kinase [34]. The assay showed that overexpression of DN Src blocked VEGF-induced cell proliferation where as overexpression of CA Src significantly increases proliferation of endothelial cells in the absence or presence of VEGF in BRECs (Fig. 7a). In addition, over-expression of CA Src mutant totally counteracted the inhibitory effect of gold nanoparticles on VEGF-induced proliferation, bringing endothelial cell proliferation in the presence of gold nanoparticles back to the level of CA Src alone (Fig. 7b). Taken together, these results suggest that VEGF promotes the endothelial cell proliferation, gold nanoparticles could counteract this VEGF-induced cell migration and that these effects are dependent on Src pathway.
AuNPs inhibit the VEGF-induced cell proliferation via Src pathway. BRECs were transiently transfected with DNA dominant negative Src (HA-Src KD K295M) and constitutive active Src (HA-Src-CA Y527F). a Transfected BRECs were treated AuNPs and PP2 in the presence or absence of VEGF for 24 h at 37°C Over expression of DN Src mutant significantly blocks the VEGF-induced proliferation to the level of control where as over expression of CA Src had a additive effect after the growth factor treatment; b CA Src mutant confers resistance to inhibitory effect of AuNPs and PP2 in cell proliferation when compared to wild type PRECs. Treatment with combination of VEGF + AuNP and VEGF +PP2 were significantly induced cell proliferation in CA Src transfected cells when compared to control (*P < 0.05 vs. control). Data are mean ± SEM representing the identical results of three independent experiments
Src kinase assay
To support the contention that effects of Au-NPs and PP2 inhibitor on VEGF-induced angiogenesis and permeability were specifically directed through the Src pathway, we performed phospho-Src peptide competition immunoassay to measure the status of Src phosphorylation at Y419. Treatment with VEGF significantly increased the levels of phosphorylated Src (Y419) protein in the cell extracts whereas the treatment of Au-NP in the VEGF induced cells decreased Src phosphorylation in BRECs to a greater extent in comparison with control.
The increased phospho-Src (Y419) form after VEGF (25 ng/ml) treatment was significantly decreased by the pre-incubation of 500 nM Au-NP (Fig. 8a). In addition, significant changes of phosphorylated Src were observed in VEGF treated with PP2 (Src inhibitor). These data indicate that Au-NP inhibit VEGF-induced endothelial cell permeability through the inhibition of phospho-Src (Y419) activation similar to that of PP2.
a Effect of AuNPs and PP2 on VEGF-induced Src phosphorylation. BRECs were treated with VEGF in presence and absence of 500 nM AuNP or 10 μM PP2 for 1 h. Level of Src phosphorylation (Y419) in cell lysate (1 × 107 cells) was checked by sandwich ELISA. VEGF (25 ng/ml) treatment significantly increase the Src phosphorylation compared to the control. Both AuNP and PP2 significantly decreased the VEGF-induced Src phosphorylation in BRECs. Data are means ± SEM representing similar results was obtained in three independent experiments (n = 3, *P < 0.05 vs. control, **P < 0.01 vs. control). b Src modulates the inhibitory action of AuNPs on VEGF-induced Src phosphorylation (Y419). Illustrates the effect of Src mutants on VEGF-induced Src phosphorylation. DN Src mutant significantly blocks the VEGF-induced Src phosphorylation whereas CA Src had an additive effect on Src phosphorylation. c AuNPs and PP2 rescue the inhibitory effect of Src phosphorylation in CA Src mutant. CA Src mutant confers resistance to Au-NP blocking effect on VEGF-induced Src phosphorylation at Y419. Data are means ± SEM representing similar results was obtained in three independent experiments (n = 3, *P < 0.05 vs. control, **P < 0.01 vs. control)
The central role of Src pathway as a target for the anti-angiogenic and anti-permeability effect is further confirmed. BRECs were transfected with a plasmid encoding DN Src and CA Src, followed by a treatment with VEGF in the presence or absence of Au-NP and the level of Src phosphorylation were quantified by ELISA. Overexpression of DN Src reduced VEGF-mediated Src phosphorylation similar to that as Y419 does to the level of control, whereas substantial increase in the Src phosphorylation was obtained due to over expression of CA Src.
An additive effect over the Src phosphorylation at Y419 was obtained when stimulation of these cells with VEGF treatment was carried out (Fig. 8b). Overexpression of the CA Src completely counteracted the inhibitory effect of Au-NP on VEGF-induced Src phosphorylation (Y419) (Fig. 8c). Therefore, our result concludes that Au-NPs directly blocks VEGF-induced Src phosphorylation on BRECs and controls cellular permeability through the inhibition of Src activation.
VEGF cell surface receptor assay
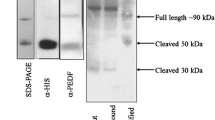
The mechanism behind the inhibitory effect of gold nanoparticles was further investigated by analyzing the effect of gold nanoparticles on receptor level recognition such as VEGFR2 phosphorylation. Au-NPs (500 nM) significantly inhibited the VEGF165-induced VEGFR-2 phosphorylation (Fig. 9a, b) as like as VEGFR-2 specific inhibitor CT322 and GSK1363089. Thus, our result of receptor level phosphorylation clearly suggests that gold nanoparticles may bind directly to VEGF165 and inhibit its interaction with cell surface receptor hence inhibiting phosphorylation. Hence the above result suggests that Au-NPs significantly blocks VEGF-induced Src activation via VEGFR2 by inhibiting the phosphorylation status in BRECs.
AuNPs inhibit VEGF-induced phosphorylation of VEGFR2. Serum-starved BRECs were treated with VEGF (25 ng/ml) in the presence or absence of AuNPs for 15 min. Equal amounts of total cell lysates were analyzed by Western blot analysis for phospho-VEGFR2 and VEGFR2. Densitometric normalization of phosphorylated-VEGFR2 against VEGFR2. Band density is shown in columns. All Western blot analyses were performed triplicates. Equal amounts of total cell lysate were analyzed by Western blot analysis for phospho-VEGFR2 and VEGFR2. Densitometric normalization of phospho-VEGFR2 and VEGFR2. Band density is shown in columns. These experiments were performed thrice with similar results and significant differences from control group were observed (P < 0.05)
TEM analysis
In order to analyze the fate of the nanoparticles after the treatment whether the particles get internalized within the cells or they remain bound to the cell in endothelial cells, we have performed TEM analysis in BRECs samples treated with Au-NP in presence and absence of VEGF and the specific location of the nanoparticles during the treatment has been checked through various time intervals under a transmission electron microscope. After 6 h, BRECs samples treated with Au-NPs and VEGF, the maximum of Au-NPs get bound to the cellular membrane and a fewer particles were detected within the endocytic compartments namely endosomes (Fig. 10a), whereas in Au-NPs treated BRECs samples at the same time elapse, most of Au-NPs were detected peripherally, in the early endosomes and also a major amount of the particles where being engulfed within the multi-vesicular bodies of the cells (Fig. 10b). Thus, this result reveals that the nanoparticles of size ~50 nm were internalized in endothelial cells during the VEGF and Au-NPs treatments.
TEM analysis of AuNPs in BRECs. a BRECs treated with gold nanoparticles along with VEGF. The transmission electron microscopy image revealed that, gold nanoparticles when treated along with VEGF in BRECs for an incubation period of about 6 h, most of the particles get bound to the cell membrane and only a few are internalized within the endosomes in the cells. b BRECs treated with gold nanoparticles alone: The transmission electron microscopy image revealed that, gold nanoparticles when treated alone in BRECs for an incubation period of about 6 h, nanoparticles get internalized within the multi-vesicular bodies of the cells
Discussion
The findings of the present study demonstrate that the gold nanoparticles significantly inhibited VEGF-induced cell proliferation, migration and tube formation in BRECs. In addition, our findings have shown the inhibitory effect of gold nanoparticles on VEGF-induced VEGFR2/Src phosphorylation thereby suggesting their possible role in triggering the downstream signaling pathway, elucidating a clear mechanism through which they act. This potential role of AuNPs in inhibiting the disease progression for VEGF mediated ocular complications being confirmed, are similar to the role of TG100801 owned by TargeGen which is a Src kinase inhibitor under Phase trial I for neovascularization.
Recently, gold nanoparticles were reported to inhibit VEGF165-induced proliferation of HUVEC cells by binding the sulfur/amines present in the heparin binding domains of VEGF165 [35]. Similarly the anti-angiogenic effects of various carbon materials on vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF2)-induced angiogenesis has been already evaluated in the chick chorioallantoic membrane (CAM) model [36].
It has been reported that endothelial cell proliferation and migration in response to VEGF play an important role in angiogenesis, which is essential for the tumors to enlarge and metastasize. Thus, efforts to reduce the growth and spread of neoplasm’s have recently focused on suppressing angiogenesis [37, 38]. Our results demonstrated that gold nanoparticles significantly inhibited VEGF-induced BREC proliferation, migration and tube formation. These findings are consistent with previous reports [35] that demonstrated gold nanoparticles significant inhibition of VEGF-induced HUVEC proliferation and migration. The involvement of Src in cell proliferation and migration and its important role in cancers were investigated [39] which reported the activation of Src kinase in response to cellular signaling promoted proliferation, survival, motility, and invasiveness. Moreover, evidence from in vivo models of metastasis showed that Src inhibition markedly reduced the rate of lymph and liver metastasis [40, 41]. In agreement with this, we found that Src inhibitor PP2 inhibits VEGF stimulated angiogenesis in vitro.
Thus the role of gold nanoparticles that inhibit angiogenesis has been analyzed for their mode of action and been confirmed for their effective role in blocking VEGF-induced Src phosphorylation on BRECs and controlled cellular permeability through the inhibition of Src activation.
Characterization of new molecules with anti-angiogenic properties and elucidation of their mechanism of action could facilitate new approaches for their treatments. Many molecules are under consideration for new therapeutic strategies aimed at reducing excessive vasopermeability which is one of the major problems in angiogenesis [33], similar to Angiopoietin 1, for instance, which is known for its impressive effect in blocking blood vessel leakage in vivo [42].
Our present study shows that gold nanoparticles exerted anti-vasopermeability effects by counteracting the biological effects of VEGF induced permeability. In previous studies, it was shown that 5-nm nano gold could inhibit the activity of VEGF165 through its interaction with the sulfur/amines present in the heparin-binding domain of VEGF165, whereas it was unable to inhibit the activity of VEGF121, presumably due to its lack of a heparin-binding domain [35]. Nevertheless, our results indicate that 50 nm nanoparticles inhibits VEGF-induced VEGFR2 phosphorylation. VEGF receptor 2 (Flk-1) has been reported to be the main receptor through which VEGF mediates its biological effects in ECs. Eliceiri et al. [29] showed that VEGF treatment resulted in a phosphorylation of Flk-1, which recruited and phosphorylated Src, which was reported to be an important molecule in the VEGF triggered angiogenic pathway and He et al. [43] reported that VEGF treatment stimulated the formation of a complex between Src and Flk-1 in ECs. In agreement with previous findings, we observed a significant inhibitory effect of gold nanoparticles on VEGF-induced VEGFR2 phosphorylation. These findings suggest that VEGFR2 may be the starting point that evokes VEGF-triggered downstream signaling pathways in BRECs.
Although VEGF-triggered angiogenic pathways in ECs are not fully understood, groups of signaling molecules such as Src, Akt, and mitogen-activated protein kinases (MAPKs) have been reported to be involved in VEGF signaling cascades. Among these signaling molecules, Src family tyrosine kinase has been strongly suggested to be involved in VEGF-induced angiogenesis. Src is a cytoplasmic protein tyrosine kinase whose activation and recruitment to peri-membranal signaling complexes hold important implications for cellular fate. It was reported that Src protein levels and Src kinase activity were significantly elevated in human breast, colon, and pancreatic cancers [44]. Furthermore, the promotion of cell proliferation and migration may be carried out through the activation of Src by growth factors and growth factor receptors [45] which are also well known for its role in endothelial cell proliferation and cell survival thereby playing a key role in angiogenesis.
To investigate whether Au-NPs could suppress the activation of Src cascade in angiogenesis, we examined the phosphorylation and activation of the tyrosine kinases in signaling pathway. The Src family of tyrosine kinases (SFKs) consists of eight members among which Src, Fyn and Yes are ubiquitously expressed while Fgr, Lyn, Lck, Hck and Blk have more tissue-restricted expression [39, 46] SFKs are activated in response to stimulation of a variety of cell surface receptors such as integrin receptors, tyrosine kinase receptors, and G-protein coupled receptors, and by cellular stress [47]. This is consistent with the previous results that gold nanoparticles bind to heparin-binding growth factor receptors through its heparin-binding domain and inhibit its interaction with cell surface receptors [34].
Cellular functions regulated by SFKs include adhesion, spreading, migration, proliferation, apoptosis, and differentiation [36, 39]. We then focused on the downstream signal transduction pathways that are associated with VEGF triggered BRECs proliferation and migration. As we expected, gold nanoparticles significantly inhibited VEGF-induced Src phosphorylation. These findings suggest that Src is a downstream effector of VEGFR2 in the VEGF-triggered angiogenic pathway in ECs. When gold nanoparticles were treated along with AbVF in primary CLL-B cells after 1 h incubation, internalization of Au-AbVF were found within the periphery i.e., the uncoated tubules and vacuoles, whereas when the Au alone were treated along in the primary CLL-B cells for the same time elapse of incubation, higher magnification showed that the particles were internalized within the multi-vesicular bodies of the primary CLL-B cells [48]. Our findings in Fig. 10 also proves that the gold nanoparticles get internalized within the multi-vesicular bodies of bovine retinal endothelial cells when treated alone and when treated along with VEGF most of the gold nanoparticles get bound to the cell membrane of the BRECs and a few get internalized within the endosomes of the cells.
Hence this study over the mechanism by which the Au-NPs inhibit the Src mediated pathway leading to angiogenesis relating to the internalization of the nanoparticles within the vesicular bodies of the cells, may provide a clear trafficking mechanism by which gold nanoparticles act within the cell, thereby affirming the futuristic potential of gold nanoparticles an economic remedy to neovascularization.
Conclusion
In conclusion, we have demonstrated Au-NPs to be a potent anti-angiogenic and anti-permeability molecule that inhibits angiogenesis and permeability in VEGF-induced BRECs through the inhibition of src pathway. Furthermore, we demonstrated that gold nanoparticles significantly inhibited VEGF-induced VEGFR2 and Src phosphorylation, which is an essential event for evoking the activation of the downstream signaling pathway. Finally, we showed the involvement of Src, in the VEGF-triggered angiogenic pathway in BRECs by using specific inhibitors against them. Although the exact mechanism of the beneficial effect by which gold nanoparticles inhibit VEGF-induced angiogenesis remains to be elucidated, our findings suggest that the action of gold nanoparticles in BRECs is mediated partly through the inhibition of VEGFR2 and Src tyrosine kinase activities. The findings in the present study may shed light on the pharmacological basis for the clinical application of gold nanoparticles for suppression of angiogenesis mediated complications. Further more the study provides a mechanism to account for the inhibition of Au-NPs on VEGF-mediated angiogenesis and also suggest that Au-NPs could be used as a therapeutic molecule for the treatment of diabetic retinopathy and other eye related neovascular diseases.
References
Carmeliet P (2000) VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med 6:1102–1103
Cross MJ, Claesson-Welsh L (2001) FGF and VEGF function in angiogenesis: signaling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 22:201–207
Aiello LP, Avery RL, Arrigg PG, Bruce AK, Henry DJ, Sabera TS (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Hata Y, Nakagawa K, Ishibashi T, Inomata H, Ueno H, Sueishi K (1995) Hypoxia-induced expression of vascular endothelial growth factor by retinal glial cells promotes in vitro angiogenesis. Virchows Arch 426:479–486
Ferrara N (2002) VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2:795–803
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25
Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W (1993) High-affinity VEGF binding and developmental expression suggests Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72:835–846
Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62–66
Shen BQ, Lee DY, Zioncheck TF (1999) Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. J Biol Chem 274:33057–33063
Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN (2006) Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol 142:1–9
Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR (2004) VEGF inhibition study in ocular neovascularization clinical trial group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351:2805–2816
Economopoulou M, Bdeir K, Cines DB, Franz F, Yasmina B (2005) Inhibition of pathologic retinal neovascularization by alpha-defensins. Blood 106:3831–3838
Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA (2006) VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis 12:626–632
Ojima T, Takagi H, Suzuma K, Oh H, Suzuma I, Ohashi H (2006) Ephrin A1 inhibits vascular endothelial growth factor-induced intracellular signaling and suppresses retinal neovascularization and blood-retinal barrier breakdown. Am J Pathol 168:331–339
Shen J, Yang X, Xiao WH, Hackett SF, Sato Y, Campochiaro PA (2006) Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J 20:723–725
Xia X, Xiong S, Song W, Luo J, Wang Y, Zhou R (2008) Inhibition of retinal neovascularization by siRNA targeting VEGF165. Mol Vis 14:1965–1973
Ghosh P, Han G, Mrinmoy De, Kim CK, Rotello VM (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60:1307–1315
Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L (2004) Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv 11:169–183
Davda J, Labhasetwar V (2002) Characterization of nanoparticle uptake by endothelial cells. Int J Pharm 233:51–59
Kalishwaralal K, Banumathi E, Pandian SRK, Deepak V, Muniyandi J, Eom SH, Gurunathan S (2009) Silver nanoparticles inhibit VEGF-induced cell proliferation and migration in bovine retinal endothelial cells. Colloid Surf B 73:51–57
Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S (2008) Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B 65:150–153
Kalishwaralal K, Deepak V, Ram Kumar Pandian S, Gurunathan S (2009) Biological synthesis of gold nanocubes from Bacillus licheniformis. Bioresour Technol 100:5356–5358
Liu X, Atwater M, Wang J, Huo Q (2007) Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf B 58:3–7
Banumathi E, Haribalaganesh R, Babu SSP, Kumar NS, Sangiliyandi G (2009) High-yielding enzymatic method for isolation and culture of microvascular endothelial cells from bovine retinal blood vessels. Microvasc Res 77:377–381
Gurunathan S, Lee KJ, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH (2009) Antiangiogenic properties of silver nanoparticles. Biomaterials 31:6341–6350
Sheikpranbabu S, Kalishwaralal K, Venkataraman D, Eom SH, Park J, Gurunathan S (2009) Silver nanoparticles inhibit VEGF-and IL-1β-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. J Nanobiotechnol 7:8
Kumar SA, Peter YA, Nadeau JL (2008) Facile biosynthesis, separation and conjugation of gold nanoparticles to doxorubicin. Nanotechnology 19:495101
Sastry M, Patil V, Sainkar SRJ (1998) Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine films. Phys Chem B 102:1404–1410
Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA et al (1999) Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4:915–924
Liu F, Verin AD, Wang P, Day R, Wersto RP, Chrest FJ et al (2001) Differential regulation of sphingosine-1-phosphate and VEGF-induced endothelial cell chemotaxis: involvement of G (ialpha2)-linked Rho kinase activity. Am J Respir Cell Mol Biol 24:711–719
Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL (2001) Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med 7:222–227
Patan S (2004) Vasculogenesis and angiogenesis. Cancer Treat Res 117:3–32
Harhaj NS, Antonetti DA (2004) Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 36:1206–1237
Parsons JT, Parsons SJ (1997) Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol 9:187–192
Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA (2005) Antiangiogenic properties of gold nanoparticles. Clin Cancer Res 11:3530–3534
Murugesan S, Mousa SA, O’Connor LJ, Lincoln DW II, Linhardt RJ (2007) Carbon inhibits vascular endothelial growth factor- and fibroblast growth factor promoted angiogenesis. FEBS Lett 581:1157–1160
Nakashio A, Fujita N, Tsuruo T (2002) Topotecan inhibits VEGF- and bFGF-induced vascular endothelial cell migration via downregulation of the PI3 K-Akt signaling pathway. Int J Cancer 98:36–41
Favot L, Keravis T, Holl V, Le Bec A, Lugnier C (2003) VEGF-induced HUVEC migration and proliferation are decreased by PDE2 and PDE4 inhibitors. Thromb Haemost 90:334–343
Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13:513–609
Nam JS, Ino Y, Sakamoto M, Hirohashi S (2002) Src family kinase inhibitor PP2 restores the E cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res 8:2430–2436
Boyer B, Bourgeois Y, Poupon MF (2002) Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene 21:2347–2356
Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM (1999) Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286:2511–2514
He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB (1999) Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem 274:25130–25135
Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W (1999) Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet 21:187–190
Simeonova PP, Wang S, Hulderman T, Luster MI (2002) c-Src-dependent activation of the epidermal growth factor receptor and mitogen-activated protein kinase pathway by arsenic: role in carcinogenesis. J Biol Chem 277:2945–2950
Brown MT, Cooper JA (1996) Regulation, substrates and functions of src. Biochi Biophys Acta 1287:121–149
Abram CL, Courtneidge SA (2000) Src family tyrosine kinases and growth factor signaling. Exp Cell Res 254:1–13
Priyabrata M, Resham B, Nancy B, Yean KL, Chitta Ranjan P, Shanfeng W, Lichun L, Charla S, Pataki CB, Michael JY, Neil EK (2007) Potential therapeutic application of gold nanoparticles in B-chronic lymphocytic leukemia (BCLL): enhancing apoptosis. J Nanobiotechnol 5:4
Acknowledgments
The authors gratefully acknowledge Kyung Jin Lee, Department of Life Science, Cell Dynamics Research Centre, Gwangju Institute of Science and Technology, Gwangju 500-712, Republic of Korea for performing western blot analysis. The authors also wish to acknowledge the support of Dr. Puspha Viswanathan, Professor, Cancer Institute (WIA), Chennai, who helped us with TEM analysis. Prof G. Sangiliyandi was supported by grants from Council of Scientific and Industrial Research (CSIR), New Delhi (Project No. 37/0347) and Department of Science and Technology (DST), New Delhi (Project No. SR/NM/NS-31/2010), India. Mr. Kalishwaralal was supported by the grant for Senior Research Fellowship from Council of Scientific and Industrial Research (CSIR), New Delhi (Grant No. 9/1012(0003)2K10EMR-I), India.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted at the request of the editors in chief as the authors have manipulated images included in the article with the result being an altered representation of the data.
The authors Kalimuthu Kalishwaralal and Sardarpasha Sheikpranbabu contributed equally to this manuscript.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10456-011-9225-1
About this article
Cite this article
Kalishwaralal, K., Sheikpranbabu, S., BarathManiKanth, S. et al. RETRACTED ARTICLE: Gold nanoparticles inhibit vascular endothelial growth factor-induced angiogenesis and vascular permeability via Src dependent pathway in retinal endothelial cells. Angiogenesis 14, 29–45 (2011). https://doi.org/10.1007/s10456-010-9193-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-010-9193-x