Abstract
Conflicting evidence exists concerning the supplementation of vitamin D in knee osteoarthritis condition. This systematic literature review was done to explore the effects of vitamin D supplementation in the management of knee osteoarthritis. Electronic literature search was done in databases like PubMed®, Embase®, and Cochrane CENTRAL from inception to 6th July 2016. The quality of included Randomized Controlled Trials (RCTs) was assessed using Cochrane risk of bias tool. We considered change in Western Ontario and McMaster Universities (WOMAC) index, Visual Analog Scale (VAS) and Functional Pain Score (FPS) as the primary outcome measure. Change in tibial cartilage thickness, joint space width and safety profile was considered as secondary outcomes. Participants were randomized either to treatment or placebo group. Participants received cholecalciferol as an intervention through oral route in the dose range of 800–60,000 IU except in the one study where participants received ergocalciferol. All included RCTs showed a significant increase in serum vitamin D level in the treatment group compared to the placebo group at the end point. No significant reduction in pain and function was reported on WOMAC scale except in one study. No significant difference was reported for WOMAC stiffness in any study. VAS was assessed in three studies in which two showed statistically significant improvement in knee pain. Three of the RCTs reported safety data with one incidence of calculus ureteric and hip fracture found to be related to the drug. The study found evidence from RCTs to be insufficient to support the use of vitamin D supplementation for patients with knee osteoarthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knee osteoarthritis (knee OA) is a common, progressive and degenerative musculoskeletal disorder which accounts for 83% of all type of osteoarthritis [1]. It generally progresses with the age >50 years; however, it can occur in young people also [2]. Prevalence of knee OA is higher in females (13%) compared to males (10%) of age ≥60 years and with increasing life expectancy and aging global population, it is expected to rise further [3]. As per an estimate, there are >250 million people affected by knee OA worldwide [1]. Knee OA and its associated symptoms such as pain, swelling, and stiffness imparts a high toll on patients’ health-related quality of life (HRQOL) [4–6] and has substantial direct and indirect economic burden [7–12].
Osteoarthritis is characterized by progressive loss of cartilage, whereas vitamin D has been shown to reduce cartilage degradation [13, 14]. Observational studies have found low levels of vitamin D to be associated with higher prevalence of knee OA along with increased risk of disease progression [15–17]. Moreover, one small Randomized Controlled Trial (RCT) has reported significant clinical improvement in patients with knee OA receiving vitamin D treatment [18]. Findings of Korean National Health and Nutrition Examination Survey (KNHANES V) states that maintenance of sufficient levels of vitamin D may be important to prevent a decline in the HRQOL of elderly knee OA patient [19]. Wang et al. in an RCT have found the favorable effects of vitamin D supplementation in delaying the progression of effusion-synovitis in peoples with an inflammatory knee OA [20]. Findings of the Amsterdam osteoarthritis cohort study linked muscle weakness to the insufficient level of vitamin D in knee OA patients, however, the effect was attenuated once body mass index (BMI) was added to the model [21]. In Knee OA patients low level of vitamin D (<10 ng/ml) was also found to be responsible for the progression of medial femoro-tibial OA [22]. Thus, supplementing with vitamin D may potentially play a beneficial role in the prevention and progression of knee OA. However, few RCTs have reported contradicting findings [13, 23].
Amidst the varying evidence, no systematic review has been performed to compare the effects of vitamin D supplementation in the patients with knee OA till the time of our search. Hence, a systematic review was conducted to evaluate the effect of vitamin D supplementation in the patients with the knee OA. The therapeutic role of vitamin D supplementation in reducing structural progression and improving the management of knee OA was assessed.
Materials and methods
The methodology complies with our registered protocol at PROSPERO (registration No. CRD42015027920) [24] and with PRISMA 2009 checklist [25, 26] (Refer Supplementary Table 1).
Search strategy
We searched databases (PubMed®, Cochrane CENTRAL, Embase®), trial registries, and key websites up to 6th July 2016, followed by bibliographic hand searches and contacts with study authors. Databases were searched for articles related to vitamin D and knee OA with suitable keywords (Refer Supplementary Table 2 for the detailed search string).
Inclusion and exclusion criteria
We included RCTs that compared vitamin D (in any form and dose) with placebo in patients with knee OA. Only those articles published in the English language and full-text were included. Articles were first screened for inclusion by examining title and abstract followed by retrieving and assessing full-text of the potentially relevant reports by two reviewers (SH and AS) independently. Any disagreements about the inclusion were resolved by consensus. If consensus was not achieved, then the decision was made by consultation with the third reviewer (AKN).
Reviews, case series, case–control, cohort and cross-sectional studies were excluded because this review is limited only to RCTs. We also excluded animal study, genetic study and letter to the editor.
Data extraction
Data were collected independently by the two reviewers (SH and AS) from the selected study in the predesigned data extraction sheet. Details extracted were: (a) author name and year, (b) study design including single or multicentre, (c) participant characteristics, (d) intervention given and its duration, (e) intervention dose and route of administration, (e) follow-up period, (f) primary and secondary endpoint. Any discrepancies in the data collection were first tried to resolve by discussion, if not then only the third reviewer (AKN) consulted.
Assessment of risk of bias
The included articles were assessed for the methodological quality by two authors (SH and AS) independently using Cochrane Risk of Bias Tool (CRBT) [27] and the analysis was done using RevMan (v5.3) [28]. The criterion for the decision included sequence generation, allocation concealment, blinding of participant and personnel, blinding of the outcome, incomplete outcome data, selective reporting and other bias.
Summary measures and statistical analysis
We considered a change in Western Ontario and McMaster universities (WOMAC) index as the primary outcomes, which assess knee pain, stiffness, and function [29]. Reduction in Visual Analog Scale (VAS) and Functional Pain Score (FPS) were also considered under primary outcomes [18, 23]. Cartilage thickness, joint space width (JSW) and the safety profile were considered under secondary outcomes [13]. The RTCs included in the systematic review were not eligible for conducting a meta-analysis pertaining to the heterogeneity across included studies in terms of forms and doses of vitamin D used, duration of the follow-up, and patients population. Hence, the data were qualitatively analyzed and presented in the form of a narrative synthesis [30].
Results
Search output
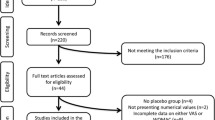
The literature search yielded 909 articles, after excluding the duplicates and irrelevant publication based on the screening of title and review of abstract, 5 articles were included in the final analysis (Fig. 1). Two of the included articles were reporting the same study published as full-text and as a pilot study by the same author, hence the pilot study was excluded and only the full-text study was included.
Study description
Intervention In all the studies participants were included on the basis of American College of Rheumatology (ACR) diagnostic criteria except in warner et al. [23] study. Participants were randomized either to treatment or placebo group. In the treatment group participants received cholecalciferol as an intervention through oral route in the dose range of 800–60,000 IU except in the warner et al. [23] study where participants received ergocalciferol.
Study design All the included studies were a single-center double-blind randomized controlled trial except Jin et al. [31] and Arden et al. [32] which was multicentric. The follow-up duration for the included RCTs ranged from 3 months to 36 months. Characteristics of the included RCTs are shown in Table 1.
Participants The included RCTs comprise of 1189 participants from five studies (597 in the treatment arm and 592 in the placebo arm) with age 45 years or older [13, 18, 23, 31, 32]. Females were higher in number in both the groups across all the included studies, furthermore, the patient population in Warner et al. [23] study included only females. A comparison was done between the baseline characteristics of the included studies (Table 2).
Risk of bias The risk of bias assessment of the five included studies is presented in Fig. 2. The majority of these studies were of low risk of bias based on selection bias and performance bias while the high risk of bias was observed in blinding of outcome assessment and selective reporting in Sanghi and Jin et al. [18, 31] study, respectively.
Effect of intervention All the studies presented the data in the form of tables. The comparison was made in all the included studies between the vitamin D group and placebo group after the scheduled treatment period (Table 3).
Primary outcome measure
Assessment of knee pain was done in all the studies. Measures taken into consideration for the assessment were serum 25-hydroxy vitamin D level, WOMAC index, VAS score and FPS score. The WOMAC index was assessed as the primary outcomes in all the studies except in Arden et al. [32] which assessed it under secondary outcomes.
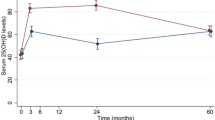
Serum 25-hydroxy vitamin D Vitamin D deficiency was defined as serum 25-hydroxyvitamin D levels below <20 ng/ml in all the studies [13, 18, 23, 31, 32]. Participants were vitamin D deficient in each of the included studies at baseline. A significant increase was observed in serum vitamin D level in the treatment group as compared to placebo group post-treatment in all the studies (P = <0.001). Target to achieve vitamin D level was varying as per the study. For instance, vitamin D target level was set at ≥20 ng/ml in Warner et al. [23]; >50 nmol/L in Sanghi et al. [18]; 36–100 ng/ml in McAlindon et al. [13]; and greater than 60 nmol/L in Jin et al. study [31]. In Warner et al. [23] 98 and 50% patient achieve the target in the treatment and placebo group, respectively. In McAlindon et al. [13] 61.3 and 8.3% patient achieve the target in the treatment and placebo group, respectively. In Jin et al. [31] 79 and 43% patient achieve the target in the treatment and placebo group, respectively.
WOMAC index WOMAC index is a scale which measures the pain, stiffness, and function in a patient with knee OA.
WOMAC pain It was assessed in all the studies except Warner et al. [23] study. WOMAC pain expressed in the range of 0–20 in McAlindon et al. [13] and Sanghi et al. [18], while in Jin et al. [31] it is expressed in the range of 0–500. In Sanghi et al. [18] study pain was reduced by 0.55 unit (95% CI −0.07 to 1.02) in the vitamin D group in contrary to this it increased in the placebo group by 1.16 units (95% CI 0.82–1.49). Significant differences were observed between the groups, −1.70 on WOMAC pain (95% CI −2.28 to 1.12) at P < 0.001. Knee pain was reduced by two units in both the treatment group and in the placebo group in McAlindon study [13]. The treatment effect was non-significant in reducing pain after treatment [−2.31 for the vitamin D group vs −1.46 for the placebo group; between-group difference, −0.87 (95% CI (−2.12 to 0.38)); P = 0.17]. In Jin et al. [31] study total WOMAC pain was reduced in both the group after treatment, but no significant difference was observed in treatment and placebo groups [−49.9 for the vitamin D group vs −35.1 for the placebo group; between-group difference, −14.8 (95% CI −32.5 to 2.9); P = 0.10]. Arden et al. [32] study reported an increase in WOMAC pain in the placebo group and the small decrease was noticed in the treatment group. No significant reduction in pain was reported (P = Not reported).
WOMAC stiffness This parameter was reported by three studies namely Sanghi et al., Jin et al. and Arden et al. [18, 31, 32]. No significant change was noted in stiffness score post vitamin D intervention in the study findings of Sanghi et al. [18] [0.15 for the vitamin D group vs 0.09 for the placebo group; between-group difference, 0.06 (95% CI −0.15 to 0.26); P = 0.58]. Similar (non-significant) findings were reported by Jin et al. [31] in post hoc analysis [−0.19.7 for the vitamin D group vs −15.4 for the placebo group; between-group difference, −4.2 (95% CI −12.5 to 4.0); P = 0.31]. In Arden et al. [32] findings, reduction in WOMAC stiffness was reported for both the group. No significant improvement was reported (P = Not reported).
WOMAC function Except warner et al. [23] all the studies have reported WOMAC function score [13, 18, 31, 32]. At baseline, knee function was poor in the vitamin D group, but no significant change was noticed post-treatment [−6.97 for the vitamin D group vs −3.82 for the placebo group; between-group difference, −3.11 (95% CI −6.52 to 0.30); P = 0.07] in findings reported by McAlindon et al. [13]. In Sanghi et al. [18] study, Knee function score was reduced by 1.4 units while an increment of 0.7 units was noted in the placebo group. Significant improvement in knee function score was observed in the study [−1.36 for the vitamin D group vs 0.69 for the placebo group; between-group difference, −2.05 (95% CI −2.92 to −1.19); P = <0.001]. Improvement in knee function was also reported by Jin et al. [31] in the post hoc analysis. In Arden et al. [32] study increase in WOMAC function was reported for both the groups, but no statistical significance was observed (P = Not reported).
Total WOMAC Total WOMAC was presented in three studies in 993 participants [18, 31, 32]. Total WOMAC was expressed in the range of 0–96 in Sanghi et al. [18] and 0–2400 in Jin et al. [31] study. WOMAC scores were significantly reduced by 2 units in vitamin D group and 1.5 units in the placebo group; −2.12 in the vitamin D group vs 1.41 in the placebo group with a between-group difference of −3.53 (95% CI −4.39 to −2.71) at P value <0.001 in Sanghi et al. [18]. In Jin et al. [31] study, a significant reduction in WOMAC score was reported; post hoc analysis result showed that −239.2 in the vitamin D group vs −147.8 in the placebo group with a between-group difference of −91.4 (95% CI −165.1 to −17.7) at P value 0.02. No significant improvement in total WOMAC score was observed in Arden et al. [32] findings (P = Not reported).
VAS VAS represents the intensity of pain. VAS score was assessed in 569 patients in three studies [18, 23, 31] except for McAlindon [13] and Arden et al. [32]. The increase in VAS score represents worsening of knee pain. No significant difference was observed on VAS score in Warner et al. [23] study as compared to placebo and no positive association was observed between vitamin D level and VAS score (r = 0.038). A significant reduction was observed in knee pain as demonstrated on VAS score by Sanghi et al. [18] (P = 0.020) and Jin et al. [31] (P = 0.05).
FPS FPS score represents the effect of pain on daily activities. Higher the score more severe the problem. FPS score was reported only by Warner et al. [23] among 50 patients. A significant reduction was reported on FPS score (P = 0.05), but it supports the placebo and no positive correlation was observed for pain on FPS score. So, overall no significant reduction was observed during the treatment period. In the subgroup of patients having vitamin D ≤ 20 ng/mL significant increase in FPS score was observed (P = 0.04).
Secondary outcome measures
Tibial cartilage volume There was no significant difference reported in tibial cartilage volume over the treatment period in McAlindon et al. [13] and Jin et al. [31] study in comparison to treatment and placebo groups.
JSW JSW is a parameter for determining the cartilage thickness and helpful in assessing knee cartilage disease. JSW is reported among 192 patients by McAlindon et al. [13] and Arden et al. [32]. No significant difference was reported between the treatment and the placebo groups in both the studies.
Adverse event Safety profile was assessed only in three studies. In McAlindon et al. [13] study a total of 16 patients experienced adverse events in treatment as well as in placebo group. No drug-related adverse event was reported except hip fracture in one patient. Endocrine (6 vs 1) and musculoskeletal (41 vs 30) event were higher in the treatment group as compared to placebo group. In Jin et al. [31] study, 27 and 18% patient in the treatment group and placebo group experienced at least one adverse event. Serious Adverse Event (SAEs) were reported among 11 patients in vitamin D group and 7 patients in the placebo group. In Arden et al. [32] study no significant difference was observed in terms of the SAEs between treatment and placebo group. None of SAEs were reported to be drug-related except one calculus ureteric in vitamin D group and pancreatitis in the placebo group.
Discussion
Osteoarthritis is the most common form of joint diseases and the knee is most commonly affected joint. Osteoarthritis of knee is a degenerative musculoskeletal disorder usually, manifests after the 45 years of age. Studies have demonstrated the negative impact of vitamin D deficiency in many disease conditions including musculoskeletal disorder ranging from knee OA to back pain [33, 34]. In present systematic review, we identified five RCTs evaluating the role of vitamin D supplementation in patients with knee OA. The result demonstrated no significant improvement in the patients with knee OA receiving vitamin D supplementation.
All included RCTs showed a significant increase in serum vitamin D level in the treatment group compared to the placebo group at endpoint. WOMAC pain was assessed among 1139 patients and found no significant reduction in pain post-treatment in all the included studies [13, 23, 31, 32] which assessed this parameter except in Sanghi et al. which shows the significant effects of vitamin D in reducing pain [18]. One possible reason for this difference could be the short duration of follow-up and presence of high concentration of vitamin D level at the baseline in Sanghi et al. study [18]. No significant difference was reported by Sanghi et al. [18], Jin et al. [31], and Arden et al. [32] for WOMAC stiffness score as compared to pre and post vitamin D treatment. No significant difference was reported for WOMAC stiffness in any of the studies. WOMAC function which was assessed in all the studies [13, 18, 31, 32] except warner et al. [23], and was improved in almost all the studies but none of the study showed statistically significant improvement post-treatment except Sanghi et al. [18]. WOMAC total score was significantly reduced in Sanghi et al. [18] and Jin et al. study [31] while no significant reduction was reported by Arden et al. [32]. The treatment effects of vitamin D supplementation compared to placebo on WOMAC total, pain, physical function, and stiffness were statistically non-significant and unlikely to be clinically relevant. Though, WOMAC total, pain, and function scores showed a slight improvement in one of the studies included [18].
Knee pain assessed on VAS score significantly improved in Sanghi et al. and Jin et al. study [18, 31], but no significant reduction in pain was observed in Warner et al. study [23]. Reduction in FPS score was also found to be non-significant in warner et al. findings [23]. The possible reason behind this could be due to the limited sample size and short duration of follow-up (3 months) and female gender. No significant reduction was observed in tibial cartilage volume and JSW in any study. Moreover, other outcomes such as: knee pain as measured on VAS, tibial cartilage volume, and JSW also did not result in significant improvement as compared to placebo.
Safety assessment was done in three studies involving 1033 patients [13, 31, 32]. No drug-related SAEs were reported except calculus ureteric in vitamin D group and pancreatitis in the placebo group in Arden et al. study and hip fracture in McAlindon et al. study [13, 32].
Consistent with findings of this systematic review, most of the published clinical (RCTs and observational studies) that evaluated vitamin D in patients with knee OA have reported no or little benefits in FPS, improvement in knee pain, JSW and change in cartilage volume [13, 23, 35–39]. However, few studies confirmed the improvement in WOMAC total score, WOMAC function, and VAS Score [18, 31, 33] in patients with knee OA receiving vitamin D supplementation. Notably, these studies comprise small sample size, short follow-up period and lack of patients’ reported outcomes.
The strengths of the present systematic review include an exhaustive search of published trials; inclusion of all the primary outcome data reported in the included trials for evaluation; and transparent evaluation of the quality of evidence. The main weakness of this systematic review is that we were not able to retrieve all of the existing gray-literature and unpublished information since literature search was only performed in PubMed, EMBASE, and Cochrane CENTRAL.
High-quality evidence from well-designed, RCTs with longer follow-up duration and large sample size is needed to further clarify on the present findings.
In conclusion, this systematic review suggests the lack of evidence to support Vitamin D supplementation for reducing structural disease progression and improving the management of knee OA. Hence these findings do not support the use of vitamin D supplementation for patients with knee OA. Few of the existing guidelines recommend vitamin D as a medication for this condition; however, these results call for a reconsideration of these recommendations.
References
Musculoskeletal conditions—the second greatest cause of disability. Available at: http://bjdonline.org/musculoskeletal-conditions-the-second-greatest-cause-of-disability-2/. Accessed 2 Oct 16
March L, Smith EU, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F et al (2014) Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol 28(3):353–366
Zhang Y, Jordan JM (2010) Epidemiology of osteoarthritis. Clin Geriatr Med 26(3):355–369
Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT et al (2006) The burden of disease and injury in the United States 1996. Popul Health Metr 4:11
Ackerman IN, Bucknill A, Page RS, Broughton NS, Roberts C, Cavka B et al (2015) The substantial personal burden experienced by younger people with hip or knee osteoarthritis. Osteoarthr Cartil 23(8):1276–1284
Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L et al (2008) Understanding the pain experience in hip and knee osteoarthritis—an OARSI/OMERACT initiative. Osteoarthr Cartil 16(4):415–422
Agaliotis M, Mackey MG, Jan S, Fransen M (2014) Burden of reduced work productivity among people with chronic knee pain: a systematic review. Occup Environ Med 71(9):651–659
Leardini G, Salaffi F, Caporali R, Canesi B, Rovati L, Montanelli R et al (2004) Direct and indirect costs of osteoarthritis of the knee. Clin Exp Rheumatol 22(6):699–706
Ruiz D, Koenig L, Dall TM, Gallo P, Narzikul A, Parvizi J et al (2013) The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Jt Surg Am 95(16):1473–1480
Maetzel A, Li LC, Pencharz J, Tomlinson G, Bombardier C (2004) The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann Rheum Dis 63(4):395–401
Murphy L, Helmick CG (2012) The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs 112(3 Suppl 1):S13–S19
Buckwalter JA, Saltzman C, Brown T (2004) The impact of osteoarthritis: implications for research. Clin Orthop Relat Res 427:S6–S15
McAlindon T, LaValley M, Schneider E, Nuite M, Lee JY, Price LL et al (2013) Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA 309(2):155–162
Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81(3):353–373
McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B et al (1996) Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med 125(5):353–359
Ding C, Cicuttini F, Parameswaran V, Burgess J, Quinn S, Jones G (2009) Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum 60(5):1381–1389
Bergink AP, Uitterlinden AG, Van Leeuwen JPTM, Buurman CJ, Hofman A, Verhaar JAN et al (2009) Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: the Rotterdam Study. J Clin Rheumatol 15(5):230–237
Sanghi D, Mishra A, Sharma AC, Singh A, Natu SM, Agarwal S et al (2013) Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clin Orthop Relat Res 471(11):3556–3562
Kim HJ, Lee JY, Kim TJ, Lee JW (2015) Association between serum vitamin D status and health-related quality of life (HRQOL) in an older Korean population with radiographic knee osteoarthritis: data from the Korean national health and nutrition examination survey (2010–2011). Health Qual Life Outcomes 13:48
Wang X, Cicuttini F, Jin X, Wluka AE, Han W, Zhu Z et al (2017) Knee effusion-synovitis volume measurement and effects of vitamin D supplementation in patients with knee osteoarthritis. Osteoarthr Cartil. doi:10.1016/j.joca.2017.02.804
Koeckhoven E, van der Leeden M, Roorda LD, van Schoor NM, Lips P, de Zwart A et al (2016) The association between serum 25-hydroxy vitamin D level and upper leg strength in patients with knee osteoarthritis: results of the Amsterdam Osteoarthritis Cohort. J Rheumatol 43(7):1400–1405
Brennan-Speranza TC, Mor D, Mason RS, Bartlett JR, Duque G, Levinger I et al (2017) Skeletal muscle vitamin D in patients with end stage osteoarthritis of the knee. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2017.01.022
Warner AE, Arnspiger SA (2008) Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J Clin Rheumatol 14(1):12–16
Hussain S, Singh A, Najmi AK. Vitamin D for the management of knee osteoarthritis: a systematic review of randomized controlled trials. 2015; PROSPERO:CRD42015027920
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349:g7647
Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Seida JK et al (2009) Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ 339:b4012
Review Manager (RevMan) [Computer program] (2014). Version 53 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration
Cao Y, Jones G, Cicuttini F, Winzenberg T, Wluka A, Sharman J et al (2012) Vitamin D supplementation in the management of knee osteoarthritis: study protocol for a randomized controlled trial. Trials 6(13):131
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version.1:b92. 2006
Jin X, Jones G, Cicuttini F, Wluka A, Zhu Z, Han W et al (2016) Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA 315(10):1005–1013
Arden NK, Cro S, Sheard S, Doré CJ, Bara A, Tebbs SA et al (2016) The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthr Cartil 24(11):1858–1866
Heidari B, Heidari P, Samari E, Ramzannia Jalali M (2014) Frequency of vitamin D deficiency in common musculoskeletal conditions. J Babol Univ Med Sci 16(7–15):28
Goula T, Kouskoukis A, Drosos G, Tselepis AS, Ververidis A, Valkanis C et al (2015) Vitamin D status in patients with knee or hip osteoarthritis in a Mediterranean country. J Orthop Traumatol 16(1):35–39
Muraki S, Dennison E, Jameson K, Boucher BJ, Akune T, Yoshimura N et al (2011) Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthr Cartil 19(11):1301–1306
Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A et al (2007) Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum 56(1):129–136
Konstari S, Paananen M, Heliövaara M, Knekt P, Marniemi J, Impivaara O et al (2012) Association of 25-hydroxyvitamin D with the incidence of knee and hip osteoarthritis: a 22-year follow-up study. Scand J Rheumatol 41(2):124–131
Reginster JY, Pelousse F (2013) Supplementation with vitamin D did not reduce cartilage volume loss or pain in knee osteoarthritis. Ann Intern Med 158(8):9
Alkan SE, Karagoz A, Genc H, Cakit BD, Taskin S, Erdem HR (2014) THU0197 The effects of vitamin D levels on pain intensity, muscle strength and functional status of the elderly patients with knee osteoarthritis. Ann Rheum Dis 73(Suppl 2):249–250
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hussain, S., Singh, A., Akhtar, M. et al. Vitamin D supplementation for the management of knee osteoarthritis: a systematic review of randomized controlled trials. Rheumatol Int 37, 1489–1498 (2017). https://doi.org/10.1007/s00296-017-3719-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3719-0