Abstract
Background
Italy is expected to experience the largest growth in persons ≥65 years (>20 % by 2020). This demographic shift allows for geriatric research on predictive clinical and biological markers of outcomes related to frailty, re-hospitalization and mortality.
Aims
To describe rationale and methods of the Report-AGE study project of acute care patients in Italian National Research Center on Aging (INRCA) research hospitals.
Methods
Report-AGE study is a large observational study on health conditions and outcomes of hospitalized elderly patients (≥65 years). The primary objective of the study is to create a high-level data resource of demographics, comprehensive geriatric assessments, clinical and diagnostic information, as well as biological and molecular markers in all older patients admitted to INRCA Hospitals. Assessments in physical and nutritional parameters, co-morbid health conditions, and associations with frailty parameters are ongoing in older hospitalized adults following an acute event. Study collection began in September 2011.
Results
Up to date, there are 3479 patients ≥65 years (mean age: 85 ± 7years) with 1543 men and 1936 women enrolled. Data have been recorded regarding functional and clinical parameters before, during hospital admission and at discharge. Data collection for primary outcome analyses related to re-hospitalization and mortality is estimated for September 2016.
Discussion
This study aims at collecting precise clinical data, comprehensive geriatric assessment, risk factors, and biological data from acute care patients. Data will also be used to identify mechanisms underlying frailty in this specific population.
Conclusion
This study provides a descriptive epidemiological collection of the health conditions of older in-patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global demographics have underlined that persons over 65 years of age will double from 7.4 to 14.7 % between 2010 and 2040 [1] and those over the age of 80 (the “oldest-old”) are projected to grow more rapidly than the older population itself. In 2002, 12 % of the over 65 population accounted for 50 % of all hospitalizations [2]. Hospital admissions of this growing population are expected to rise to 21 % by 2030 [3]. Italy is expected to experience the largest growth in the 65 and older group over the next years, with this population projected to comprise an estimated 22.1 % by the year 2020 [2]. Italy holds the greatest potential as “the largest living laboratory for geriatric and gerontological research” [3] to identify and treat precise clinical and biological markers on negative outcomes in older patients.
Up to now, numerous and large community-dwelling prospective studies in older populations have only identified predictive markers of disability, frailty, mortality and hospitalization [4–6], while fewer and more heterogeneous studies have been dedicated to testing for predictive markers on negative outcomes in older hospitalized adults [7, 8]. Older persons are at an increased risk for hospital admission due to an age-related reduction of functional reserve in physiological systems, resulting in increased vulnerability to illness, physical dependency and frailty. Frailty, the spiral of reduced functional reserve and increased vulnerability to disease [9], represents a key prognostic marker in acute/hospital care settings [9, 10]. A previous study found that medical, functional, and biological characteristics, linked with the frailty phenotype [9, 10], were among the most important predictors of functional decline associated with hospitalization [11]. Frailty is associated with an increased risk of a range of adverse outcomes during hospitalization, including: (1) the onset of geriatric syndromes, such as delirium, dizziness, functional decline, incontinence, pressure ulcers [12]; (2) polypharmacy and adverse drug events [13]; (3) prolonged hospital stays, institutionalization, re-hospitalization and mortality [14, 15]. Diverse prognostic indexes integrating multiple domains have shown to predict 1- and 2-year mortality in hospitalized elders [8, 16]. However, frailty markers of functionality necessary to maintain high independency and adequate quality of life in an acute event and following have not been precisely identified.
There is a continuously growing rise in hospitalization for acute events in older frail persons with chronic comorbidities. Considering that there are little data for hospitalized older patients, an extremely vulnerable group of individuals, the need for functional and prognostic information remains essential for decisions concerning long-term clinical planning, discharge, and follow-up [17]. In addition, the role of innovative biomarkers of frailty and diseases on adverse outcomes needs investigation compared to traditional prognostic factors [18].
The Report-AGE project is currently ongoing with the main objective to create one of the world’s largest data resources regarding demographics, comprehensive geriatric assessments, clinical and diagnostic information, as well as biological and molecular markers in all older patients admitted to the Hospital Network of the Italian National Research Center on Aging (INRCA) throughout Italy.
Methods/design
Study design
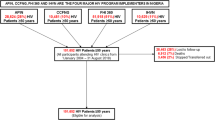
This is an ongoing prospective study of older patients consecutively admitted to participating acute care wards in INRCA acute care research hospitals located in Italian cities of Ancona, Fermo, Cosenza and Casatenovo that started in September 2011 (Fig. 1). After receiving information describing the study, potential participants are screened for eligibility. Eligibility criteria includes: age ≥65 years, admission to one of the INRCA research hospitals, written informed consent. In the case of a participant’s cognitive decline or poor capability of judgment, a proxy (relative or caregiver) is invited to give consent in addition to receiving consent of the participant. Exclusion criteria include: lack of informed consent from the patient or caregiver.
The Ethical Committee of the Italian National Research Center on Aging has approved the study protocol.
Study procedures
The first baseline study data collection occurs at the time of hospital admission. Baseline data collection includes using a minimum data set. The minimum data set comprises demographic information, geriatric multidimensional assessment data, clinical, biological and diagnostic information routinely collected during hospital stay (within the first 24 h following hospital admission). The questionnaires used for data collection are identical in all INRCA participating research hospitals and quality control of data collection is performed at each center. The data regarding ad hoc investigations to identify biological markers are collected using a separate and detailed data sheet. A more detailed description of study procedures has been described [19].
Markers of frailty syndrome include: muscular weakness (handgrip strength) during hospitalization, information (self-reported) regarding other frailty markers before hospitalization will include: unintentional weight loss, poor endurance (exhaustion), slowness and low physical activity [9]. Frailty will be considered present if 3 or more criteria are present. A pre-frail syndrome will be considered if 1 or 2 criteria are present [9].
Collection of biological samples and data storage
Blood samples are taken on the day of study enrolment (within 24 h following admission) and are stored at −80°C until needed to perform analyzes on biological markers and polymorphisms. Trained and dedicated staff in the hospitals properly prepare blood samples before shipment to the centralized laboratory. Biological samples of plasma, serum, buffy coat, and whole blood are coded before being sent to the centralized laboratory in the Scientific and Technological Area of INRCA in Ancona, Italy for biobanking. Laboratory staff of the operating units involved in the project will be allowed access to the samples to carry out the analyses. A database is organized for privacy protection on an INRCA server. Access will be governed through a Hypertext transfer protocol over secure socket layer (HTTPS) secure protocol. Study protocol staff at different sites have access to both the server and the database to insert data. Biological samples will be used only for the purposes of this investigation and destroyed 5 years after the end of the study.
INRCA Scientific Committee has coordinated the study and a contact has been identified at each hospital.
Study measurements of geriatric multidimensional assessment and clinical data (to be performed within 24 h from admission and within 24 h prior to discharge).
-
(1)
Demographics and clinical data collection (identification information, personal data at admission, assessment date, cognitive function, communication and vision, mood and behavior, physical function, incontinence, diagnosis of the disease, health conditions, oral and nutrition status, skin conditions, medications, treatment and procedures, advanced directives, discharge potential, discharge, assessment information, anamnestic–clinical data, standardized clinical assessment, physical performance tests).
-
(2)
The interRAI Acute Care Instrument [20].
-
(3)
Assessment of the functional capacities will be performed using specific performance tests of physical performance, including short physical performance battery (SPPB) [21] and handgrip strength [22].
-
(4)
Information regarding drug consumption: prior to admission, during hospital stay and at discharge [23].
-
(5)
Diagnostic and treatment procedures carried out during hospitalization (including information from X-rays, computerized tomography, ultrasounds, electrocardiograms, magnetic resonance imaging, etc).
-
(6)
Biobanking and study measurements of biological markers (to be performed within 24 h of admission).
Study measurements of biomarkers and analyses
Analysis of biological markers of endothelial function, immunological markers of inflammation and stress, prostrate disease, miRNAs, hematopoietic stem cell precursors, nutritionally important trace metals, alleles of metallothionein and zinc transporters and polymorphisms of disease-associated genes and mitochondrial DNA. Blood samples are collected within 24 h of admission and stored at −80 °C to analyze biological markers and polymorphisms using the following approaches:
-
The quantity of ADMA, SDMA and l-arginine will be determined with plasma isolated by HPLC.
-
Detection of plasminogen activator inhibitor-1 (PAI-1) will be determined with a commercially available enzyme immunoassay kit.
-
Evaluation of plasma trace metals (Zn, Fe, Se, Cu). The elements will be directly measured in serum or plasma collected with heparin by inductively coupled plasma mass spectrometry (ICP-MS). This determination will be performed on all patients and associated with the primary study endpoints.
-
The markers of oxidative stress will be analyzed in plasma samples by ELISA.
-
Analysis of circulating microRNA (miRNA) by miRNA expression profiling through miRNA microarray and real-time PCR analysis
-
Determination of telomere length in circulating leukocytes. The analysis of telomere length will be performed by reverse transcription polymerase chain reaction (RT-PCR), using approximately 50 ng of DNA per sample extracted from peripheral blood.
-
Determination of epigenetic modifications of DNA in circulating leukocytes in order to identify altered gene regions and if these have a prognostic role. (analysis of DNA methylation by methylight method and/or whole-genome tiling arrays)
-
Measurement of cytokines and anti-inflammatory molecules, in addition to neuregulin-1 (NRG-1), which has recently been identified as a prognostic marker for chronic heart disease.
-
Genotyping as follows: (i) a genetic polymorphism that can influence zinc homeostasis (MT1A); (ii) polymorphisms present in the genes coding for both the principal pro- and anti-inflammatory cytokines (IL-6, IL-1, TNF-alpha, IL-10, IL-2, IL-17, IL-8), as well as pro- and anti-inflammatory chemokines (MCP-1 and RANTES); (iii) molecules involved in pathogenetic mechanisms and individual responses to stress from physical activity (e.g., E23 K polymorphism of the KIR 6.2 gene); (iv) polymorphisms of the metalloproteinases, MMP1 MMP2 and MMP9, of tissue inhibitors of metalloproteases (TIMPs) and alpha-2 macroglobulin, that are involved in the maintenance of the extracellular matrix, of particular relevance to cardiovascular disease, including heart failure; (v) polymorphisms associated with the current disease of a patient (e.g., APOE for neurodegenerative diseases and IGF-1 for metabolic diseases such as type 2 diabetes).
-
Evaluation of polymorphisms of mitochondrial DNA, since these play a primary role in several age-associated diseases, using the Affymetrix platform; (vi) Determination of the “whole genome” using Affymetrix Chip Array 6.0. and polymorphism analysis using real-time PCR and specific primers. Frequency allele will be evaluated in the various subjects. The presence of specific allelic variants will be associated with plasma levels of trace elements and endpoints of the study.
Outcome measurements
The principal goals of the Report-AGE project are:
-
1.
To assess the association of baseline frailty parameters with:
-
incident functional limitation (loss in at least 1 point in ADL or SPPB, or walking speed ≤1 m/s)
-
incidence and change in severity of frailty-related health conditions (pre-frail condition to frailty)
-
recovery of physical function after an acute event (improvements in ADLs, SPPB scores, walking speed)
-
baseline measures of strength and physical performance (handgrip strength, SPPB, walking speed)
-
-
2.
To access the contribution of episodes of severe acute illness to changes in body weight and composition [anthropometric measures of body mass and bioelectrical impedance analysis (BIA)] and the relationship of these episodes to risk of functional limitation, re-hospitalization and mortality.
-
3.
To assess the ways how changes in physical functionality parameters can influence disease-related states such as cardiovascular disease risk factors such as lipids, blood pressure and glucose tolerance.
-
4.
To assess the interdependency of nutrition and physical functional, co-morbid health conditions, and their association with change in frailty parameters in older hospitalized adults
-
5.
To provide a solid scientific database which can be used to test for critical outcomes to develop effective strategies for maintaining health in older persons following an acute event.
The collection completion date to begin analyses on primary outcomes is estimated for September 2016.
Statistics
Required sample size calculations (G-Power Version 3.1.5) will be used for all potential investigations on clinical outcomes. All statistical analyses will be conducted using the SPSS software version 18 (Chicago, IL, USA). Continuous variables will be expressed using as means with standard deviations (SD) and medians with ranges. For variables with a normal distribution, statistical comparisons between groups will be made using an analysis variance test. If the data are not normally distributed, statistical comparisons will be made using the Kruskal–Wallis test. Measurements with discrete distribution will be expressed as percentages (%) and analyzed by the Chi squared or Fisher’s exact test when appropriate.
Risk estimates on clinical outcomes will be carried out by applying logistic regression analyses and Cox regression models to calculate the odds ratio (OR) with a confidence interval of 95 %.
Results
Preliminary results of the study indicate that there are a total of 3479 older persons enrolled. As expected, there are more women (n = 1936) compared to men (n = 1543) enrolled. The population is largely represented by older persons living in the community (approximately 75 %) (see Table 1). The variations regarding the changes in activities of daily living, cognitive performance before hospital admission, during hospital admission and at discharge are described in Table 2. Variations in pain and balance are reported in Table 2. At the moment, data regarding routine biochemical assays are available.
Discussion
This is the first ongoing epidemiological study of an existing integrated healthcare system aimed at providing high-quality data to identify predictable biological and clinical markers of health outcomes in older acute care patients. Aging is associated with adaptive changes of organs and systems, predisposing them to reduced functional reserve, resulting in increased vulnerability to illness and physical dependency. This state constitutes the condition of “frailty”, which is associated with increased risk of multiple adverse events such as falls, fractures, disability, institutionalization and death [10, 24]. To reduce the burden of disease and disability, which significantly reduce the quality of life of the elderly, it is necessary to reach a high level of management efficiency to implement needed prevention measures of disability in the frail elderly. This type of study will be able to identify necessary markers for future randomized trials in older persons.
Age-related physical and cognitive decline are not only the result of the aging process itself, but also highly prevalent in chronic diseases, such as cardiovascular disease, chronic obstructive pulmonary disease (COPD), diabetes, cancer, all of which often coexist [25–27]. An increased number of comorbidities is also a risk factor for malnutrition, which in turn leads to disability. Protein and calorie malnutrition, together with sarcopenia, contributes to creating a vicious cycle leading from frailty to disability. Sarcopenia is known to be associated with worsening of cognitive capacity [28, 29]. Indeed, studies have shown that physical activity is associated with a slowing of cognitive and functional decline [30, 31] and a lower incidence of comorbidities [32]. Maintaining cognitive function has shown to improve morbidity and mortality rates [33]. Recent data also indicate that certain disorders of cognitive function may hold important prognostic significance in elderly patients with COPD and heart failure. In particular, in older patients with COPD, deterioration in executive functioning has been associated with increased mortality [34]. However, specific biomarkers associated with physical and cognitive decline, especially following an acute event are unknown. This study will allow to identify not only new biomarkers, but also the role of existing biomarkers on physical and cognitive outcomes in older frail persons.
In addition, polypharmacy and inappropriate drug prescribing are associated with comorbidity. An increased number in inappropriate medications can increase the risk for adverse drug reactions (ADR) [35–37], and on the other hand, non-adherence to therapy can also further increase disability and mortality [38] [39]. There is an urgent need to identify specific agents associated with ADRs, especially during hospital stay and following discharge.
Comprehensive geriatric assessment has been shown to predict health outcomes in older persons [40, 41].
At the moment, hospital-acquired disability issues have only been addressed in a few studies [8, 42, 43]. The Report-AGE project is designed to implement a standardized geriatric assessment aimed at identifying the functional components and their potential interactions with acute illness in older persons. This project will encompass biological, clinical and social perspective in acute settings on disability measures. Existing data from epidemiological and clinical Italian studies testing biomarkers on age-related outcomes have been conducted in community-dwelling elders [44, 45]. Lastly, strategies involving targeted intervention to frail patients and/or a systematic review of hospital care practices will stimulate quality improvement, which in turn could yield a significant reduction in negative outcomes. In conclusion, the Report-AGE study will provide evidence on specific clinical, biological, functional and social characteristics of older patients during hospitalization in the context of a multidimensional approach. Results from this study are expected to improve health outcomes in this increasing population.
Trial status: Recruiting was underway at the time of manuscript submission.
References
US census bureau international data (2009). https://www.census.gov/prod/2009pubs/p95-09-1.pdf
Merrill CT, Elixhauser A (2005) Hospitalization in the United States, 2002. AHRQ Publication 05-056, Washington (DC)
Landefeld CS (2003) Improving health care for older persons. Ann Intern Med 139(5 Pt 2):421–424
Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, Guralnik JM, Ferrucci L (2009) Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 64(3):377–384
Maggi S, Noale M, Gallina P, Marzari C, Bianchi D, Limongi F, Crepaldi G (2004) Physical disability among older Italians with diabetes. The ILSA study. Diabetologia 47(11):1957–1962
Khan H, Kalogeropoulos AP, Georgiopoulou VV, Newman AB, Harris TB, Rodondi N, Bauer DC, Kritchevsky SB, Butler J (2013) Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J 166(5):887–894
Volpato S, Bazzano S, Fontana A, Ferrucci L, Pilotto A (2015) Multidimensional prognostic index predicts mortality and length of stay during hospitalization in the older patients: a multicenter prospective study. J Gerontol A Biol Sci Med Sci 70(3):323–329
Abbatecola AM, Spazzafumo L, Corsonello A, Sirolla C, Bustacchini S, Guffanti E (2011) Development and validation of the HOPE prognostic index on 24-month posthospital mortality and rehospitalization: Italian National Research Center on Aging (INRCA). Rejuvenation Res 14(6):605–613
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146–M156
Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, Lattanzio F (2010) Frailty and muscle metabolism dysregulation in the elderly. Biogerontology 11(5):527–536
Chiarantini D, Volpato S, Sioulis F, Bartalucci F, Del Bianco L, Mangani I, Pepe G, Tarantini F, Berni A, Marchionni N et al (2010) Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail 16(5):390–395
Inouye SK, Studenski S, Tinetti ME, Kuchel GA (2007) Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 55(5):780–791
Corsonello A, Pedone C, Lattanzio F, Lucchetti M, Garasto S, Di Muzio M, Giunta S, Onder G, Di Iorio A, Volpato S et al (2009) Potentially inappropriate medications and functional decline in elderly hospitalized patients. J Am Geriatr Soc 57(6):1007–1014
Lattanzio F, Laino I, Pedone C, Corica F, Maltese G, Salerno G, Garasto S, Corsonello A, Incalzi RA (2012) Geriatric conditions and adverse drug reactions in elderly hospitalized patients. J Am Med Dir Assoc 13(2):96–99
Fusco S, Corsonello A, Chiatti C, Fabbietti P, Salerno G, De Bonis E, Corica F, Lattanzio F (2015) Migrant care workers and rehospitalization among older patients discharged from acute care hospitals. Geriatr Gerontol Int 15(2):196–203
Pilotto A, Ferrucci L, Franceschi M, D’Ambrosio LP, Scarcelli C, Cascavilla L, Paris F, Placentino G, Seripa D, Dallapiccola B et al (2008) Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res 11(1):151–161
Graf CE, Zekry D, Giannelli S, Michel JP, Chevalley T (2011) Efficiency and applicability of comprehensive geriatric assessment in the emergency department: a systematic review. Aging Clin Exp Res 23(4):244–254
FUTURAGE Roadmap (2011) www.futurage.group.shef.ac.uk
https://clinicaltrials.gov/ct2/show/study/NCT01397682?term=INRCA&rank=1
Wellens NI, Verbeke G, Flamaing J, Moons P, Boonen S, Tournoy J, Milisen K (2013) Clinical changes in older adults during hospitalization: responsiveness of the interRAI acute care instrument. J Am Geriatr Soc 61(5):799–804
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49(2):M85–M94
Bassey EJ, Harries UJ (1993) Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clin Sci (Lond) 84(3):331–337
Carosella L, Pahor M, Pedone C, Zuccala G, Manto A, Carbonin P (1999) Pharmacosurveillance in hospitalized patients in Italy. Study design of the ‘Gruppo Italiano di Farmacovigilanza nell’Anziano’ (GIFA). Pharmacol Res 40(3):287–295
Drey M, Wehr H, Wehr G, Uter W, Lang F, Rupprecht R, Sieber CC, Bauer JM (2011) The frailty syndrome in general practitioner care: a pilot study. Z Gerontol Geriatr 44(1):48–54
Okonkwo OC, Cohen RA, Gunstad J, Tremont G, Alosco ML, Poppas A (2010) Longitudinal trajectories of cognitive decline among older adults with cardiovascular disease. Cerebrovasc Dis 30(4):362–373
Dodd JW, Getov SV, Jones PW (2010) Cognitive function in COPD. Eur Respir J 35(4):913–922
Maggi S, Limongi F, Noale M, Romanato G, Tonin P, Rozzini R, Scafato E, Crepaldi G (2009) Diabetes as a risk factor for cognitive decline in older patients. Dement Geriatr Cogn Disord 27(1):24–33
Landi F, Abbatecola AM, Provinciali M, Corsonello A, Bustacchini S, Manigrasso L, Cherubini A, Bernabei R, Lattanzio F (2010) Moving against frailty: does physical activity matter? Biogerontology 11(5):537–545
Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J (2008) Functional decline in cognitive impairment—the relationship between physical and cognitive function. Neuroepidemiology 31(3):167–173
Miller ME, Rejeski WJ, Reboussin BA, Ten Have TR, Ettinger WH (2000) Physical activity, functional limitations, and disability in older adults. J Am Geriatr Soc 48(10):1264–1272
Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K (2001) A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med 161(14):1703–1708
Meisner BA, Dogra S, Logan AJ, Baker J, Weir PL (2010) Do or decline? Comparing the effects of physical inactivity on biopsychosocial components of successful aging. J Health Psychol 15(5):688–696
Yaffe K, Lindquist K, Vittinghoff E, Barnes D, Simonsick EM, Newman A, Satterfield S, Rosano C, Rubin SM, Ayonayon HN et al (2010) The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc 58(5):889–894
Antonelli-Incalzi R, Corsonello A, Pedone C, Trojano L, Acanfora D, Spada A, Izzo O, Rengo F (2006) Drawing impairment predicts mortality in severe COPD. Chest 130(6):1687–1694
Hanlon JT, Fillenbaum GG, Kuchibhatla M, Artz MB, Boult C, Gross CR, Garrard J, Schmader KE (2002) Impact of inappropriate drug use on mortality and functional status in representative community dwelling elders. Med Care 40(2):166–176
Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, Cadoret C, Fish LS, Garber L, Kelleher M et al (2003) Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 289(9):1107–1116
Tosato M, Landi F, Martone AM, Cherubini A, Corsonello A, Volpato S, Bernabei R, Onder G (2014) Potentially inappropriate drug use among hospitalised older adults: results from the CRIME study. Age Ageing 43(6):767–773
Corsonello A, Pedone C, Lattanzio F, Lucchetti M, Garasto S, Carbone C, Greco C, Fabbietti P, Incalzi RA (2009) Regimen complexity and medication nonadherence in elderly patients. Ther Clin Risk Manag 5(1):209–216
Onder G, Corsonello A, Bustacchini S, Lattanzio F (2009) L’uso dei farmaci nell’anziano. Rapporto Nazionale 2009 sulle Condizioni e pensieri degli anziani, INRCA, Ageing & Society, Federsanità ANCI, ottobre
Costa AP, Hirdes JP, Heckman GA, Dey AB, Jonsson PV, Lakhan P, Ljunggren G, Singler K, Sjostrand F, Swoboda W et al (2014) Geriatric syndromes predict postdischarge outcomes among older emergency department patients: findings from the interRAI Multinational Emergency Department Study. Acad Emerg Med 21(4):422–433
Brand CA, Martin-Khan M, Wright O, Jones RN, Morris JN, Travers CM, Tropea J, Gray LC (2011) Development of quality indicators for monitoring outcomes of frail elderly hospitalised in acute care health settings: study protocol. BMC Health Serv Res 11:281
Volpato S, Onder G, Cavalieri M, Guerra G, Sioulis F, Maraldi C, Zuliani G, Fellin R (2007) Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J Gen Intern Med 22(5):668–674
Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R, Guralnik JM (2011) Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci 66(1):89–96
Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L (2004) Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 59(3):242–248
Mocchegiani E, Malavolta M, Lattanzio F, Piacenza F, Basso A, Abbatecola AM, Russo A, Giovannini S, Capoluongo E, Bustacchini S et al (2012) Cu to Zn ratio, physical function, disability, and mortality risk in older elderly (ilSIRENTE study). Age (Dordr) 34(3):539–552
Acknowledgments
This study is exclusively funded by INRCA. The trial registration number is: NCT01397682.
Conflict of interest
All authors declare no conflicts of interest.
Human and Animal Rights
The Ethical Committee of the Italian National Research Center on Aging has approved the study protocol which was in accordance with 1964 Helsinksi declaration. This protocol does not contain any studies with animals performed by any of the authors.
Informed consent
Signed informed consent was obtained from all participants in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of Report-Age study group. The members of this group are listed in “Appendix”.
Appendix
Appendix
This paper was prepared on behalf of the Report-Age Study Group at IRCCS INRCA Research Hospitals of Ancona, Fermo, Casatenovo and Cosenza (Italy). Scientific Coordinator: F. Lattanzio. Coordinating Working Group: S. Bustacchini, A. Corsonello, R. Antonicelli, A. Cherubini, E. E. Guffanti, D. Postacchini, L. Spazzafumo, P. Fabbietti, M. Di Rosa, M. Provinciali, F. Busco, R. Galeazzi, A. R. Bonfigli, G. Di Stefano, R. Lisa, C. Maffei, S. David, L. Ferrara, C. Chiatti. Technical Support: R. Arzeni, L. Rossi, R. Firmani, M. Nacciariti, F. Marchegiani, R. Rossini, M. G. Palermi. Clinical Centers: P. Gasparrini, A. Scrimieri, G. Dell’Aquila, P. L. Dessì Fulgheri, E. Espinosa, O. Scarpino, S. Castellani, M. Dellabella, L. Gasparri, C. Giuli, M. Migale, A. Bianchi, C. Misuraca, B. Mazzei, S. Garasto.
Rights and permissions
About this article
Cite this article
Bustacchini, S., Abbatecola, A.M., Bonfigli, A.R. et al. The Report-AGE project: a permanent epidemiological observatory to identify clinical and biological markers of health outcomes in elderly hospitalized patients in Italy. Aging Clin Exp Res 27, 893–901 (2015). https://doi.org/10.1007/s40520-015-0350-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-015-0350-3