Abstract
Introduction
Some studies have shown an increase in alcohol use disorders (AUD) after Roux-en-Y gastric bypass surgery (RYGB), but its relationship with binge eating disorder (BED) has not been fully explored. The purpose of this study was to determine the prevalence of AUD and BED after RYGB and also to evaluate if BED is predictive of late postoperative occurrence of AUD or BED.
Methods
Patients (n = 46) submitted to RYGB, in a tertiary outpatient weight management service at a Federal University of Sao Paulo, Brazil, were tested for BED and AUD using the Questionnaire on Eating and Weight Patterns-Revised (QEWP-R) and AUDIT, respectively. BED was tested before surgery, while both disorders were evaluated with a follow-up period of 12 ± 1.6 years after RYGB.
Results
No patients reported AUD before RYBP. After a mean period of 12 years from surgery, ten patients (21.7%) were diagnosed with AUD. Before surgery, BED was present in 24 patients (52.2%) and it was detected in seven out of these 24 patients (29.2%) after RYGB. Thirteen new cases of BED (28.2%) were detected after surgery; total of 20 patients (43.5%) with BED. No association was found between pre- and postsurgery BED (p = 0.148). After RYGB, four out of 24 patients (16.6%) with presurgery BED developed AUD, and no association was found between presurgery BED and postsurgery AUD (p = 0.384). Seven out of ten patients (70%) with AUD after RYGB also developed BED, but no statistical significance was found between these two disorders (p = 0.061).
Conclusion
The presence of BED before RYGB did not predict AUD and BED after RYGB. Nevertheless, factors involved in a possible association between BED and AUD after surgery remain to be determined.
Level of evidence
Level III, cohort study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity in many developed countries has reached the status of a public health problem [1]. Bariatric surgery (BS) is now consolidated as an effective treatment against severe obesity, and the Roux-en-Y gastric bypass (RYGB) and Sleeve gastrectomy are the most common techniques of BS in our country. BS are associated with beneficial effects, including a lowered risk for diabetes, cardiovascular problems, sleep apnea, joint pain and quality of life, as well as a sharp reduction in overall mortality [2].
The association between alcohol consumption and disordered eating patterns, such as seen in the proposed drunkorexia, where individuals try to deal with the calorie excess brought by alcohol binges by engaging in unhealthy compensatory behaviors or avoid eating to allow alcoholic indulgencies [3, 4]. Although RYGB causes a marked reduction in weight, an improvement in comorbidities, and an improved quality of life, several studies suggest that RYGB is associated with an increased risk of developing alcohol use disorders (AUD) [5,6,7,8]. Therefore, current alcohol abuse or illicit drug use are the primary exclusion criteria for RYGB in Brazil and the United States [9, 10].
One proposed but not proven mechanism to explain the increased risk of AUD after RYGB is the need for an adjustment in caloric intake [11] such that patients switch from binge eating to excessive alcohol consumption [12,13,14,15,16].
Few studies have examined psychosocial predictors of post-bariatric AUD, and the results of these studies are controversial [17, 18]. Therefore, the objectives of this study were to evaluate the incidence of alcohol abuse during long-term follow-up after RYGB and to determine if binge eating disorder (BED) is a predictive factor for postoperative AUD or BED occurrence.
Patients
A retrospective cohort study was conducted with 46 participants (33 females and 13 males) from a total of 100 patients with severe obesity who underwent both a binge eating disorder investigation at the Obesity Clinic of Hospital of Rim/Paulista School of Medicine and RYGB surgery at São Paulo Hospital/University within the period between 2001 and 2004. After a mean period of 12 ± 1.6 years, and ranging from 9 to 14 years, an effort was made to contact and invite all 100 eligible patients for revaluation of their medical and psychological conditions. Six patients had died, and 48 could not be reached or refused to participate. Thus, 46 patients were revaluated between 2013 and 2014. All the participants provided informed consent and the study was approved by the ethics committee of Universidad Federal de São Paulo.
Methods
Clinical assessment included anthropometric measures for body mass index (BMI) determination before surgery and at the time of the revaluation. The minimum body weight achieved during the follow-up period and the amount of weight regained were also determined.
Psychological evaluation was made through the Questionnaire on Eating and Weight Patterns-Revised (QEWP-R), which is a structured instrument based on the criteria proposed by the DSM-IV for BED diagnosis and subclinical binge eating [19]. For some people, eating large quantities of food may not be physically possible after surgery, but when eating episodes are accompanied by a subjective feeling of loss of control (LOC), BED diagnosis was presumed using other DSM-IV criteria [20].
The AUDIT questionnaire was applied to assess the occurrence of alcohol abuse. This is a ten-item instrument developed by the World Health Organization, with its validity well established in Brazil, for the investigation of alcohol abuse and its behavioral consequences during the previous 12 months. The patient’s score on the AUDIT questionnaire defines a classification of substance use as follows: zone I (low risk)—0 to 7 points; zone II (hazardous risk)—8 to 15 points; zone III (harmful use)—16 to 19 points; zone IV (probable dependence)—20 to 40 points. By identifying the risk zone, it becomes possible for the professional to offer customized treatment that is focused on the standards of individual consumption [21]. A semistructured interview was conducted by the mental health professional at the baseline to evaluate the consumption of alcohol. One of the criteria to qualify a patient for bariatric surgery was the absence of alcohol abuse. The question used to identify the consumption of alcohol using the semi-structure questionnaire was Do you drink alcohol? How often do you drink alcohol? How many doses do you usually drink? The AUDIT questionnaire was applied by the main researcher at the end of the follow-up period.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS), version 20 for Windows (SPSS Inc., Chicago, Illinois), was used for data analysis. The quantitative variables were tested assuming the existence of a normal data distribution. The descriptive analysis of the quantitative variables included mean values and standard deviation. The qualitative variables were described in relative and absolute frequencies. The frequency of patients diagnosed with BED before and during the follow-up after surgery and the incidence of AUD during the postsurgery follow-up were calculated. For the comparison between groups with and without alcohol abuse (AUDIT > 20), we used a Student t test for quantitative variables. A Chi-square distribution test was used to determine the association between the frequencies of the qualitative variables. The predictive value of the presence of BED before surgery for the occurrence of AUD and BED during the follow-up after surgery were also determined.
A level of significance of 5% was considered.
Results
The 54 patients who could not be contacted for this study did not differ from the 46 that were reassessed in the long-term follow-up period: female sex (57% vs. 42%, p = 0.125), age (39.89 years vs. 41.35 years, p = 0.476), BMI preoperative (50.57 vs. 51.52, p = 0.978) and binge eating disorder (56.4% vs. 43.6%, p = 0.373).
A total of 46 patients completed the Questionnaire on Eating and Weight Patterns-Revised (QEWP-R) before surgery and during the follow-up period. The participants were predominantly women (n = 33; 71.7%), and the average age before surgery was 41.4 ± 10.5 years. The mean BMI before surgery was 51.1 ± 7.5 kg/m2 and was 36.8 ± 10.4 kg/m2 by the time of revaluation during the follow-up period. Binge eating disorder (BED) was present in 24 (52%) of the 46 patients before surgery and in 20 patients (43%) during the follow-up after RYGB. However, among these 20 patients with BED in the postoperative follow-up period, only seven patients also had BED before surgery. Thus, BED was present in seven patients (24%) in the long-term follow-up group that had previously been diagnosed with BED (n = 24), while 13 patients out of the 22 patients who were without BED before surgery (59%) were diagnosed as new cases of BED during the postoperative follow-up period. No significant association was found between pre- and postsurgical BED (p = 0.125) (Table 1).
No patient referred to consume alcohol in large amounts prior to the RYGB. At the postoperative evaluation with AUDIT, ten patients (21.7%) developed AUD characterized by alcohol addiction symptoms with an AUDIT score ≥ 20, and 4 (6.5%) showed a risk for alcohol abuse (AUDIT score ≥ 6 for women and ≥ 8 for men). Among the ten patients diagnosed with AUD six patients reported receiving treatment for alcohol abuse during the postoperative follow-up period. Four patients reported receiving therapy for alcohol abuse disorder, and two reported having been previously treated in a hospital for more than 30 days.
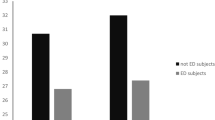
Of the 24 patients diagnosed with BED before surgery, four developed AUD during the follow-up period after surgery (16.7%). However, of the 22 patients without BED before surgery, six patients developed AUD during the follow-up period after surgery (27.3%), and no significant difference was found between these two frequencies (p = 0.484) (Fig. 1). The frequency of AUD in men (38.5%) showed a tendency to be higher than that in women (15.2%), although not statistically significant (p = 0.095).
Of the 20 patients with postsurgical BED, seven (35%) also developed alcohol abuse, while three patients of the 26 without BED (11.5%) were also diagnosed with alcohol abuse. Despite the high prevalence of this association, no statistical significance was found between BED and AUD after surgery (p < 0.061) (Fig. 1). Among those seven patients diagnosed with both postsurgical AUD and BED, only two had BED before surgery. The predictive positive value of the presence of BED before surgery for the occurrence of AUD during the follow-up after surgery was 35%.
Discussion
One of the more important things this study showed was that obese individuals who did not report abusive consumption of alcohol in the preoperative period presented AUD at a frequency of 21.7% in a mean follow-up period of 12 years after RYGB.
Although the present study does not have a control group, this frequency is higher than that reported for the Brazilian adult general population, that is, 13% [22].
This finding is in accordance to other studies findings the hypothesis that point to BS as a risk factor for AUD. excessive alcohol consumption. It is also in accordance with the results of other studies with control group such as The SOS study evaluated alcohol intake for over 20 years after different modalities of bariatric surgery and showed that, compared to the presurgical condition, patients who have undergone RYGB increased their risk of AUD to 5.9 times higher than that of patients in the control group who did not submit to surgery. Over time, those undergoing surgery also showed a gradual increase in alcohol consumption. The risk of AUD in patients undergoing RYGB also proved to be higher than that observed in patients who were submitted to restrictive-only procedures [23]. In another study, patients submitted to laparoscopic sleeve gastrectomy were evaluated after 1 year and a decrease in alcohol consumption was found [24].
Another prospective study evaluated 2.348 patients who submitted to RYGB and laparoscopic adjustable gastric banding (LAGB) in ten hospitals in the United States. It found an incidence of AUD symptoms of 20.8% in the 7 years after surgery, which is very similar to the incidence found in our study. When the two surgical groups were compared, the risk of AUD was two times higher in patients who submitted to RYGB than in those who submitted to LAGB [25]. A study by Mitchell et al. [26] also used AUDIT questionnaire to assess 78 patients who submitted to RYGB after a follow-up period of 13–15 years. The findings differ from ours and showed a 12.8% frequency of alcohol abuse before surgery that decreased to 7.7% after surgery.
A study by Ertelt et al. [27] used a survey by mail to evaluate symptoms of AUD in 70 patients within a period of 6 to 10 years after bariatric surgery and noted that the frequency of patients with symptoms of AUD did not differ in the pre- and postoperative periods (8.6% and 10.0%). In addition, most individuals with AUD after surgery also mentioned AUD symptoms in the preoperative state. Using a group of 1945 participants, King et al. [17] evaluated prospectively the prevalence of AUD both before and 1 or 2 years after bariatric surgery (69.6% RYGB). There was no difference between the prevalence found in the preoperative prevalence and the prevalence 1 year after surgery (7.6% vs. 7.3%). However, when only the 1339 patients who were submitted to RYGB were considered, the prevalence rose from 7.6% after 1 year of surgery to 9.6% after 2 years of surgery (p < 0.001) which did not occur with the other restrictive-only procedures (gastric band and sleeve gastroplasty). As in our study, these results indicate increased chances of abusive alcohol consumption after RYGB.
The mechanisms involved in the increased risk of AUD after bariatric surgery are not clear. It has been proposed that changes in the digestive system caused by RYGB might be involved in the development of a higher consumption of alcohol. With the surgical reduction of the gastric chamber and resulting faster draining, part of the ingested alcohol remains to be metabolized and, with a greater and faster absorption, a high concentration of alcohol can be found circulating in the blood. In addition, when a certain amount of alcohol is rapidly absorbed, the ability of the liver to metabolize the alcohol may be exceeded, which would slow down its elimination from systemic circulation. This explains why patients undergoing bariatric surgery feel the effects of alcohol earlier, more intensely and for a longer period of time, thus becoming more sensitive to alcohol [28,29,30]. It has been suggested that this increased sensitivity to alcohol following surgery could expose, earlier and more frequently, the individuals to the rewarding properties of alcohol, which might be a contributing factor to the increased risk of AUD post-RYGB [10, 31]. However, experimental studies using intravenous administration of alcohol suggest that RYGB in obese rats increases the effects of alcohol independent of its direct gastric effects or a change in alcohol absorption after the surgery [32]. Therefore, the biological mechanism proposed could be an enabling factor that contribute with AUD post-BS, but do not explain the primary behavior alteration found in these patients.
Another mechanism to explain increased AUD after surgery would be a shift in reward processing in the central nervous system, in which the intake of large quantities of solid foods would be replaced by excessive alcohol consumption [12, 33]. “Filling the void” theory also has been discussed in a recently paper as a possible mechanism which participants drink alcohol after BS instead of eating to manage unsolved psychological issues [16]. Idea of an “addiction change” is not supported by the results of our study, however, since of the ten patients with AUD in the postoperative stage, only four belonged to the group of 24 patients with BED in the preoperative stage. Therefore, the occurrence of BED before BS did not show a predictive value for the occurrence of postoperative AUD. It should be emphasized that only two patients with BED and AUD in the postoperative period presented with BED in the preoperative condition, leading us to believe that the development of AUD in the follow-up period after RYGB was not a transfer of compulsive behavior from excessive food intake to abusive alcohol consumption.
It is interesting to note that of the 20 patients who presented with BED in the follow-up period after RYGB, 13 were new cases of BED. The new cases were detected 2 years after the procedure in patients not presenting compulsive behavior before surgery. In addition, five of these 13 patients also presented with AUD. The reasons for this association are unclear (“addiction change”; cause/effect relationship; different phenotypes of an underlying disorder or different disorders with common pathophysiological pathways) as it is unclear if there is in fact any kind of true association other than pure chance, as none of these possibilities account for the differences in AUD rates among the various BS techniques. Yet, another possible unproved reason may be speculated: in some patients, AUD could represent a late and unwanted result from a defense mechanism against a reduction in caloric intake or energy stores following surgery. Particularly excessive alcohol intake could be a compensatory mechanism since alcohol provides a large amount of energy in a small volume, which would be an appropriate explanation for those with a reduced stomach chamber. In other words, by increasing alcohol consumption, RYGB patients would be compensating for their inability to consume calories from solid food. It must be pointed out that this hypothesis also does not shed a light upon the aforementioned rate differences.
Considering that AUD is not uncommon following RYGB, it is important to diagnose abusive alcohol intake before surgery and advise all patients about the risks of AUD in the postoperative period, particularly those used to alcohol intake or with a history of alcohol abuse in the family.
One limitation of this study is that alcohol abuse is a contraindication for BS and that patients may decrease the amount they use prior to surgery or may omit information on the amount of their use when interviewed by a mental health professional before surgery. Also, the small sample size is small especially after 12-year follow-up and it was a convenience sample, not representative which may limit the applicability of our results. The strengths of our study are a retrospective cohort with incidence data, long-term follow-up (mean 12-year follow-up) and long-term outcomes.
The clinical implications of this study is the need for long-term patient psychological evaluation post-operatively due to the high risk of developing AUD, among other psychological and psychiatric issues. Future research should evaluate the association between caloric restriction, weight loss, BED and AUD disorders after BS.
In conclusion, our study showed that the occurrence of AUD is frequent in long-term follow-up of BS. The presence of BED before BS is not predictive of BED or AUD in the postoperative period, but the association of these two conditions is not uncommon in the years following surgery.
References
Bruschi Kelles SM, Diniz MFHS, Machado CJ, Barreto SM (2014) Mortality rate after open Roux-in-Y gastric bypass: a 10-year follow-up. Braz J Med Biol Res 47(7):617–625. https://doi.org/10.1590/1414-431X20143578
Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Bengtsson C (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357(8):741–752 https://doi.org/10.1056/NEJMoa066254
Gorrell S et al (2018) Gender differences in relations between alcohol-related compensatory behavior and eating pathology. Eat Weight Disord. https://doi.org/10.1007/s40519-018-0545-7
Thompson-Memmer C, Glassman T, Diehr A (2018) Drunkorexia: a new term and diagnostic criteria. J Am Coll Health 4:1–7. https://doi.org/10.1080/07448481.2018.1500470
Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A (2013) Substance use following bariatric weight loss surgery. JAMA Surg 148(2):145–150. https://doi.org/10.1001/2013.jamasurg.265
Östlund MP, Backman O, Marsk R, Stockeld D, Lagergren J, Rasmussen F, Näslund E (2013) Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg 148(4):374–377. https://doi.org/10.1001/jamasurg.2013.700
Suzuki J, Haimovici F, Chang G (2012) Alcohol use disorders after bariatric surgery. Obes Surg 22(2):201–207. https://doi.org/10.1007/s11695-010-0346-1
White GE et al (2018) Alcohol use thresholds for identifying alcohol-related problems before and following Roux-en-Y gastric bypass. Ann Surg. https://doi.org/10.1097/SLA.0000000000003078
Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Guven S et al (2008) American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic and bariatric surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis 4(5):S109–S184. https://doi.org/10.1038/oby.2009.28
Segal A, Fandiñob J (2002) Indications and contraindications for performing bariatric operations Bariatric surgery indications and contraindications. Rev Bras Psiquiatr 24(Supl III): 68–72. https://doi.org/10.1590/S1516-44462002000700015
Ivezaj V, Stoeckel LE, Avena NM, Benoit SC, Conason A, Davis JF et al (2017) Obesity and addiction: can a complication of surgery help us understand the connection? Obes Rev 18(7):765–775. https://doi.org/10.1111/obr.12542
Blum K, Bailey J, Gonzalez AM, Oscar-Berman M, Liu Y, Giordano J et al (2012) Neuro-genetics of reward deficiency syndrome (RDS) as the root cause of “addiction transfer”: a new phenomenon common after bariatric surgery. J Genet Syndr Gene Ther. https://doi.org/10.4172/2157-7412.S2-001
Schulte EM, Yokum S, Potenza MN, Gearhardt AN (2016) Neural systems implicated in obesity as an addictive disorder: from biological to behavioral mechanisms. Prog Brain Res 223: 329–346. https://doi.org/10.1016/bs.pbr.2015.07.011
James GA, Gold MS, Liu Y (2004) Interaction of satiety and reward response to food stimulation. J Addict Dis 23(3):23–37. https://doi.org/10.1300/J069v23n03_03
Steffen KJ, Engel SG, Wonderlich JA, Pollert GA, Sondag C (2015) Alcohol and other addictive disorders following bariatric surgery: prevalence, risk factors and possible etiologies. Eur Eat Disord Rev 23(6):442–450. https://doi.org/10.1002/erv.2399
Yoder R, MacNeela P, Conway R, Heary C (2018) How do individuals develop alcohol use disorder after bariatric surgery? A grounded theory exploration. Obes Surg 28(3):717–724
King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG et al. (2012) Prevalence of alcohol use disorders before and after bariatric surgery. JAMA 307(23): 2516–2525. https://doi.org/10.1001/jama.2012.6147
Mitchell JE, Steffen K, Engel S, King WC, Chen JY, Winters K et al (2015) Addictive disorders after Roux-en-Y gastric bypass. Surg Obes Relat Dis 11(4):897–905. https://doi.org/10.1016/j.soard.2014.10.026
Borges MBF, Morgan CM, Claudino AM, Silveira DXD (2005) Validation of the Portuguese version of the Questionnaire on Eating and Weight Patterns: revised (QEWP-R) for the screening of binge eating disorder. Revista Brasileira de Psiquiatria 27(4):319–322. https://doi.org/10.1590/S1516-44462005000400012
White MA, Kalarchian MA, Masheb RM, Marcus MD, Grilo CM (2010) Loss of control over eating predicts outcomes in bariatric surgery: a prospective 24-month follow-up study. J Clin Psychiatry 71(2):175. https://doi.org/10.4088/JCP.08m04328blu
Méndez EB (1999) Uma versão brasileira do AUDIT (Alcohol Use Disorders Identification Test) [tese], Universidade Federal de Pelotas, Pelotas. https://doi.org/10.1590/S0101-60832012000300005
Macinko J, Mullachery P, Silver D, Jimenez G, Neto OLM (2015) Patterns of alcohol consumption and related behaviors in Brazil: evidence from the 2013 National Health Survey (PNS 2013). PLoS One 10(7):e0134153. https://doi.org/10.1371/journal.pone.0134153
Svensson PA, Anveden Å, Romeo S, Peltonen M, Ahlin S, Burza MA et al (2013) Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity 21(12):2444–2451. https://doi.org/10.1002/oby.20397
Coluzzi I et al (2018) Alcohol consumption after laparoscopic sleeve gastrectomy: 1-year results. Eat Weight Disord. https://doi.org/10.1007/s40519-018-0486-1
King WC, Chen JY, Courcoulas AP, Dakin GF, Engel SG, Flum DR et al (2017) Alcohol and other substance use after bariatric surgery: prospective evidence from a US multicenter cohort study. Surg Obes Relat Dis 13(8):1392–1402. https://doi.org/10.1016/j.soard.2017.03.021
Mitchell JE, Lancaster KL, Burgard MA, Howell LM, Krahn DD, Crosby RD et al (2001) Long-term follow-up of patients’ status after gastric bypass. Obes Surg 11(4):464–468. https://doi.org/10.1016/j.soard.2017.03.021
Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ, Marino JM (2008) Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surg Obes Relat Dis 4(5):647–650. https://doi.org/10.1016/j.soard.2008.01.004
Cederbaum AI (2012) Alcohol metabolism. Clin Liver Dis 16(4):667–685. https://doi.org/10.1016/j.cld.2012.08.002
Pepino MY, Okunade AL, Eagon JC, Bartholow BD, Bucholz K, Klein S (2015) Effect of Roux-en-Y gastric bypass surgery: converting 2 alcoholic drinks to 4. JAMA surgery 150(11):1096–1098. https://doi.org/10.1001/jamasurg.2015.1884
Walther L, Brodén CM, Isaksson A, Hedenbro JL (2018) Alcohol consumption in obese patients before and after gastric bypass as assessed with the alcohol marker phosphatidylethanol (PEth). Obes Surg. https://doi.org/10.1007/s11695-018-3165-4
Spadola CE et al (2018) A qualitative examination of increased alcohol use after bariatric surgery among racially/ethnically diverse young adults. Obes Surg 28(6):1492–1497. https://doi.org/10.1007/s11695-017-3022-x
Polston JE, Pritchett CE, Tomasko JM, Rogers AM, Leggio L, Thanos PK, Hajnal A (2013) Roux-en-Y gastric bypass increases intravenous ethanol self-administration in obese rats. PLoS One 8(12):e83741. https://doi.org/10.1371/journal.pone.0083741
Blumenthal DM, Gold MS (2010) Neurobiology of food addiction. Curr Opin Clin Nutr Metab Care 13(4):359–365. https://doi.org/10.1097/MCO.0b013e32833ad4d4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of Universidad Federal de São Paulo and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Freire, C.C., Zanella, M.T., Arasaki, C.H. et al. Binge eating disorder is not predictive of alcohol abuse disorders in long-term follow-up period after Roux-en-Y gastric bypass surgery. Eat Weight Disord 25, 637–642 (2020). https://doi.org/10.1007/s40519-019-00663-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-019-00663-2