Abstract
Background
The increased incidence of alcohol use disorders (AUD) after bariatric surgery has been proposed despite limited empirical support. We sought to determine the prevalence of current and lifetime AUD and other Axis I diagnoses in patients who have undergone bariatric surgery, and to test the hypothesis that greater weight loss is associated with a higher incidence of AUD following surgery.
Methods
Individuals who underwent bariatric surgery between 2004 and 2007 were recruited for inclusion in the study. The diagnosis of current and lifetime AUD and other Axis I disorders was assessed using the Structured Clinical Interview for DSM-IV.
Results
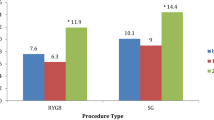
A total of 51 individuals were included. The prevalence of lifetime and current AUD was 35.3% and 11.8%, respectively. No associations were found between weight loss following surgery and the development of an AUD or other Axis I diagnoses. Significantly more current AUD was reported in (1) individuals with a lifetime history of AUD compared to those without a lifetime AUD (p < 0.05), and (2) individuals undergoing Roux-en-Y gastric bypass (RYGB) compared to those undergoing the laparoscopic adjustable gastric banding (LAGB) surgery (p < 0.05).
Conclusions
Individuals undergoing bariatric surgery were found to have a lifetime prevalence of AUD comparable to the general population. Although weight loss was not associated with the development of an AUD following surgery, individuals with a lifetime history of AUD may be at increased risk for relapsing to alcohol use after surgery. All instances of current AUD were identified in individuals undergoing RYGB as opposed to LAGB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As many as two thirds of the adults in the USA are now considered to be overweight or obese [1]. Bariatric surgery has now become a common surgical procedure for morbidly obese individuals, due in large part to the significant difficulty of achieving long-term weight loss with diet and exercise alone [2]. In the USA, approximately 110,000 bariatric procedures are being performed every year [3].
In recent years, reports have emerged that raise concerns that patients may be at increased risk for developing an alcohol use disorder (AUD) following bariatric surgery [4, 5]. Morbidly obese individuals being evaluated for bariatric surgery have been reported to have an elevated lifetime prevalence of AUD in some, but not all, studies to date, with only a small fraction of individuals meeting criteria for a current (i.e., past 12 months) AUD at the time of the surgery [6–12].
Some studies have examined the relationship between weight loss following bariatric surgery and subsequent alcohol intake. In a 1-year follow-up study of 440 laparoscopic adjustable gastric banding (LAGB) surgery patients, Dixon and colleagues found that patients with significantly better rate of weight loss consumed alcohol regularly [9]. Black and colleagues demonstrated a trend toward increased weight loss in association with alcohol use in 44 LAGB patients at 6-month follow-up [10]. On the other hand, alcohol use was not a predictor of weight loss in a long-term follow-up of 60 LAGB patients [11]. In addition, no significant association was noted with alcohol use and weight loss at 1-year follow-up in a study of 119 Roux-en-Y gastric bypass (RYGB) surgery patients [13].
However, little empirical evidence exists to support the notion that undergoing bariatric surgery is associated with a subsequent increase in alcohol use and development of an AUD. In a survey of 70 individuals 6–10 years after undergoing RYGB, Ertelt and colleagues found that 8.6% of those surveyed appeared to meet the criteria for AUD following surgery, using a questionnaire developed by the investigators [5]. In another survey study of 318 bariatric patients conducted on-line, 28.4% reported difficulty controlling alcohol intake after bariatric surgery compared to 4.5% before the surgery [4]. Finally, Welch and colleagues assessed 75 individuals 2 years after undergoing laparoscopic RYGB, and found a prevalence of 1.3% for alcohol abuse, as determined by endorsing three or more questions to the CAGE questionnaire [14]. None of these studies, however, used criteria-based diagnosis of AUD [15].
The purpose of this present study, therefore, was to determine the prevalence of current and lifetime AUD and other Axis I diagnoses in patients who underwent bariatric surgery, using a structured clinical interview validated for diagnosing substance use disorders and other psychiatric illnesses. We hypothesized that weight loss following bariatric surgery would be associated with the development of an AUD.
Methods
Sample and Recruitment
The Partners Human Research Committee approved this study. Prospective participants were selected from those who completed the required psychiatric evaluation with one of the study authors (FH) prior to the bariatric surgery at Brigham and Women's Hospital; this group represented at least 95% of all patients undergoing bariatric surgery at the institution. Inclusion criteria for this study were age ≥18, at least 2 years post-surgery, underwent the surgery at Brigham and Women's Hospital, and sufficient English language skills to complete the questionnaires. Prospective participants were mailed a letter explaining the nature of the study and inviting them for participation. The letter also included a 12-question survey about their current health status (SF-12 [16]) immediately followed by a 3-question screening of alcohol use patterns with the Alcohol Use Disorder Identification Test Consumption Questions (AUDIT-C [17]). The AUDIT-C was initially selected for inclusion in order to oversample the subjects with possible AUD. The letter explained that we were interested in “studying how patients are doing after bariatric surgery,” but did not explicitly state that the goal of the study was to determine the incidence of AUD following surgery. Those individuals returning the survey were contacted by telephone to further explain the nature of the study, and a copy of the consent form was mailed or faxed to the prospective subject. The consent form explained the purpose of the study as follows: “This research is being done to evaluate how patients are doing after bariatric surgery.” The consent form also described the interview as follows: “You will be asked questions about your weight, type of surgery performed, any complications since the surgery, as well as about your mood, anxiety, and use of alcohol, drugs, and food.” After written informed consent was obtained, subjects were then recontacted over the telephone for the interview. Each participant received $25.00 in the mail after completion of the interview.
Procedures
After enrollment, the presurgery psychiatric evaluations were retrospectively examined. The evaluations were completed by a single clinician (FH) with substantial experience conducting prebariatric surgery evaluations. This evaluation was a requirement for all patients seeking bariatric surgery at our institution, and was not conducted for research purposes. The diagnosis of an AUD was made based on this clinical interview, and the information was extracted for inclusion in this study. During the study interview, further demographic and clinical information was obtained. The Structured Clinical Interview for the DSM-IV Non-Patient Edition (SCID-I/NP [18]) for the following modules was also administered in the following order: mood disorder, anxiety disorder, substance use disorder, and eating disorder. The SCID was administered to all subjects by one author (JS). Each subject's lifetime and current alcohol use history were carefully reviewed, including the pattern of alcohol use at the time of surgery.
Statistical Analysis
All analyses were conducted with SAS (SAS Institute, Cary, NC). Where appropriate, descriptive statistics, chi-square, Fisher's exact test, and t test for significance were performed. p values of <0.05 were considered statistically significant. The sensitivity and specificity of the AUDIT-C instrument in identifying current AUD were calculated using the SCID as the gold standard.
Results
A total of 530 individuals were contacted by mail inviting them to participate in the study. Seventy surveys (13.2%) were returned undeliverable because of address changes, and no further contact was attempted. Of the remaining 460, 103 (22.4%) individuals responded to the initial survey, and 51 (11.1%) individuals agreed to participate in the interview and completed all portions of the assessment.
All 51 subjects were included in the analysis of the study. As summarized in Table 1, participants were predominantly female (N = 44, 86.3%), and the mean age was 51.3 (SD = 8.7) years. The mean weight prior to surgery was 296.1 (SD = 56.1) lbs. The mean weight loss was 100.1 (SD = 47.6) lbs. The mean time since surgery until the time of the interview was 43.4 (SD = 6.8) months.
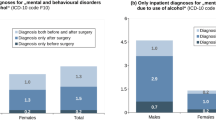
The overall prevalence of lifetime and current Axis I diagnoses is summarized in Table 2. Lifetime and current AUD were diagnosed in 18 (35.3%) and 6 (11.8%) individuals, respectively. None of the individuals met the criteria for AUD at the time of the surgery based on the clinical assessment conducted prior to surgery, and then confirmed during the SCID interview. Those individuals with a current AUD were significantly more likely to be diagnosed with a lifetime history of AUD (Fisher's exact test, p = 0.017). Weight loss as a continuous variable following surgery was not associated with the diagnosis of current AUD or any lifetime or current Axis I diagnoses. The lifetime prevalence for drug use disorders in this study sample was 13.7%, primarily due to either cocaine use (11.8%) or stimulant use (7.8%) disorders. Nevertheless, no individual met the criteria for any drug use disorder at the time of the surgery or after the surgery.
Lifetime and current binge eating disorders (BED) were diagnosed in 58.8% and 25.5%, respectively. Lifetime or current BED was not associated with either lifetime or current AUD. Individuals with current BED tended to lose less weight compared to those without BED, although the difference was not statistically significant (90.5 lbs vs. 103.4 lbs, p = 0.26).
Demographic and clinical variables were compared between those participants undergoing RYGB and LAGB (Table 3). Those individuals undergoing RYGB were significantly more likely to develop a current AUD than those undergoing LAGB (p < 0.05). However, no difference in current BED was noted between the two surgery groups. There were no significant differences in demographic and clinical variables or other Axis I diagnoses between the two surgery groups, including total weight loss.
The AUDIT-C was positive (score ≥4 for men and ≥3 for women [17]) in 12 individuals (23.5%). The instrument correctly identified 83.3% (5 out of 6) of those with current AUD, and correctly identified 84.8% (39 out of 46) of those without an AUD.
Discussion
This study found that individuals with morbid obesity seeking bariatric surgery have rates of lifetime AUD (35.3%) comparable to the general population. The National Epidemiological Survey on Alcohol and Related Conditions found that the lifetime prevalence of any alcohol use disorder was 30.3% in 2001–2002, although the prevalence was significantly higher in men (42.0%) than in women (19.5%) [19]. Consistent with earlier reports, none of the individuals was found to have a current AUD at the time of the presurgery evaluation [12]. Nevertheless, about 10% of the individuals met the criteria for a current AUD 2–5 years after surgery, which is also comparable to the prevalence found in the general population (8.5% overall, 12.4% in men, 4.9% in women) [19]. The majority (83.3%) of those meeting criteria for a current AUD also had a lifetime history of AUD that was in full sustained remission at the time of the surgery. Hence, these cases represent relapses after a period of remission, rather than de novo development of an AUD. However, this study did not find any association between weight loss following surgery and the development of an AUD.
Interestingly, every individual who met the criteria for a current AUD had undergone RYGB rather than LAGB. This is a novel finding, although not entirely unanticipated. Previous studies on the pharmacokinetics of ethanol after RYGB have revealed significant alterations—specifically, serum ethanol levels reach their peak much sooner and take longer to reach zero than compared to those who have not had the surgery [20, 21]. In addition, individuals have noted the more rapid onset of the intoxicating effects of ethanol, and that these effects are experienced after consuming fewer drinks than before surgery [5].
Ethanol is metabolized partially in the stomach by the gastric ADH enzyme, which reduces ethanol bioavailability [22]. Gastrectomy has been shown to limit this first-pass metabolism, and higher than expected serum ethanol levels are achieved [23]. By limiting the amount of ethanol being metabolized by gastric ADH, RYGB similarly leads to a reduction in the first-pass metabolism so that higher serum ethanol levels are obtained. Similar alterations in the ethanol pharmacokinetics are demonstrated with vertical sleeve gastrectomy [24]. To date, no studies have been conducted to demonstrate changes in ethanol pharmacokinetics following LAGB. Furthermore, no studies have been conducted to determine the pharmacokinetic changes for other substances of abuse following bariatric surgery.
Consistent with prior reports, the results of this study suggest that individuals who have undergone bariatric surgery have a high lifetime prevalence of BED [25]. The very low rates of current AUD but a higher lifetime AUD and BED found in individuals seeking bariatric surgery raise the question of whether some morbidly obese individuals reduce the consumption of alcohol because food (or binging) provides sufficient rewards that was previously provided by alcohol [26]. Evidence has been accumulating that palatable foods produce effects in the brain that are similar to that produced by substances of abuse [27]. In addition to increasing endogenous opioids in the mesolimbic reward circuits, foods that are high in sugar or fat cause an increase in dopamine, although not as dramatically as alcohol and drugs [28]. Furthermore, Wang and colleagues have demonstrated reduced dopamine activity in the striatum of severely obese individuals that is similar in magnitude to that reported in alcohol and drug users, and that D2 receptor density is inversely related to body mass index [29]. The low D2 density may lead such individuals to consume compulsively more alcohol, drugs, or food to experience the rewarding effects [30]. Hence, it has been speculated that patients who are no longer able to continue their previous eating habits following bariatric surgery (such as consuming highly palatable foods or engaging in binging) may seek out similar rewards from alcohol or other substances of abuse [28, 31]. Nevertheless, the results of this study did not find an association between the resolution of BED after surgery and the re-emergence of AUD.
This study highlights the potential importance of inquiring about both current and lifetime histories of AUD in those seeking bariatric surgery. Even if no alcohol is being consumed at the time of surgery, those with a prior history of AUD may be at risk of relapsing following surgery. Regardless of the prior history of AUD, patients should be informed that the intoxicating effects of alcohol may be experienced sooner than compared to before surgery, and that fewer drinks are needed to become intoxicated. Moreover, the intoxication will be longer lasting than before the surgery.
A preliminary finding of this study was that AUDIT-C may effectively identify individuals with an AUD. The AUDIT-C tool was included as a screening tool to oversample subjects with a current AUD. However, all survey respondents who agreed to participate were enrolled in the study due to the lower than anticipated response rate. The instrument correctly identified 83.3% (5 out of 6) of those with current AUD, and correctly identified 84.8% (39 out of 46) of those without an AUD. While the AUDIT-C has been found to be an effective screening tool in other populations, further research is required before the AUDIT-C can be recommended as a routine screening tool given the small sample size of this study [32].
There are numerous limitations to this study. The sample size of this study was small, and the results may not be generalizable to the larger bariatric surgery population. The low response rate to the initial survey may have led to a selection bias. For instance, those individuals who failed to lose weight following surgery may have been more reluctant to participate in the study. Another possibility for the low response rate is that individuals who were drinking alcohol may have been less likely to respond to the initial survey due to the inclusion of the AUDIT-C, leading to an underestimation of the prevalence for AUDs following surgery. Another limitation of this study is the reliability of the AUD diagnosis at the time of surgery—some patients may have underreported their alcohol consumption in order to appear as a more suitable candidate for surgery. Even though each subject's lifetime alcohol use was re-examined during the SCID administration, it is possible that those who underreported their alcohol use continued to underreport their use several years later. Therefore, an alternative interpretation of the results is that those subjects meeting criteria for current AUD following surgery may not have relapsed at all because they have been actively drinking from the time prior to surgery. Nevertheless, the very low prevalence for current AUD has been reported previously in those seeking bariatric surgery [12]. The higher risk of developing AUD following RYGB may be due to sampling subjects from only those undergoing surgery between 2004 and 2007—those undergoing LAGB may in fact develop AUD but do so at a later time than compared to RYGB. Finally, the participants were not assigned randomly to the types of surgery, leading to differences in characteristics that may have influenced the different rates of current AUD.
The implications for further research include the need for a trial that employs a prospective design to evaluate the incidence of AUD and other Axis I diagnoses following surgery. Studies with a larger sample size to better characterize the relationship between alcohol use and bariatric surgery are also needed. Pharmacokinetic studies of ethanol in individuals who have undergone bariatric surgeries may help to shed light on the possible mechanisms for the difference in the prevalence of current AUD observed between RYGB and LAGB. Finally, further studies are needed to explore the use of AUDIT-C as a screening tool for AUDs in the bariatric surgery population.
Conclusion
The results of this study suggest that individuals undergoing bariatric surgery have a lifetime prevalence of AUD comparable to the general population. Those with a lifetime history of AUD in particular may be at increased risk for relapsing to alcohol use following bariatric surgery, particularly if undergoing RYGB as opposed to LAGB. At the time of the presurgery evaluation, potential candidates may need to be evaluated not only for current alcohol use but also for lifetime histories of alcohol use.
References
Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2002;286:1195–200.
Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg. 2002;184:9S–16.
Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200:378–385.
Buffington CK. Alcohol use and health risks: survey results. Bariatric Times. 2007;4(2):21–3.
Ertelt TW, Mitchell JE, Lancaster K, et al. Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surg Obes Rel Dis. 2008;4:647–50.
Black DW, Goldstein RB, Mason EE. Prevalence of mental disorder in 88 morbidly obese bariatric clinic patients. Am J Psych. 1992;149:227–34.
Maddi S, Khobasa D, Persico M, et al. Psychosocial correlates of psychopathology in a national sample of the morbidly obese. Obes Surg. 1997;7:397–404.
Wadden T, Sarwer D, Womble LG, et al. Psychosocial aspects of obesity and obesity surgery. Surg Clin North Am. 2001;81:1001–24.
Dixon JB, Dixon ME, O'Brien PE: Pre-operative predictors of weight loss at 1-year after Lap-Band surgery. Obes Surg; 2001;11:200–7.
Black DW, Goldstein RB, Mason EE. Psychiatric diagnosis and weight loss following gastric surgery for obesity. Obes Surg. 2003;13:746–51.
Kopec-Schrader EM, Gertler R, Ramsey-Stewart G, et al. Psychosocial outcome and long-term weight loss after gastric restrictive surgery for morbid obesity. Obes Surg. 1994;4:336–9.
Kalarchian MA, Marcus MD, Levine MD, et al. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry. 2007;164:328–34.
Sears D, Fillmore G, Bui M, et al. Evaluation of gastric bypass patients 1 year after surgery: changes in quality of life and obesity-related conditions. Obes Surg. 2008;18:1522–5.
Welch G, Wesolowski C, Zagarins S, et al. Evaluation of clinical outcomes for gastric bypass surgery: results from a comprehensive follow-up study. Obes Surg. 2010 (in press).
Sogg S. Alcohol misuse after bariatric surgery: epiphenomenon or “Oprah” phenomenon? Surg Obes Rel Dis. 2006;3:366–8.
Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33.
Bush K, Kivlahan DR, McDonnell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789–95.
First, MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition. (SCID-I/NP) New York: biometrics research, New York state psychiatric institute, November 2002.
Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(7):830–42.
Klockhoff H, Naeslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54:587–91.
Hagedorn JC, Encarnacion B, Brat GA, et al. Does gastric bypass alter alcohol metabolism? Surg Obes Relat Dis. 2007;3(5):543–8.
Lieber CS, Gentry RT, Baraona E. First pass metabolism of ethanol. Alcohol Alcohol Suppl. 1994;2:163–9.
Frezza M, Buda A, Terpin MM, et al. Gastrectomy, lack of gastric first pass metabolism of ethanol and alcohol liver disease. Results of a multicenter study. Ital J Gastroenterol Hepatol. 1997;29:243–8.
Maluenda F, Csendes A, De Aretxabala X, et al. Alcohol absorption modification after laparoscopic sleeve gastrectomy due to obesity. Obes Surg. 2010;20:744–8.
Niego SH, Kofman MD, Weiss JJ, et al. Binge eating in the bariatric surgery population: a review of the literature. Int J Eat Dis. 2007;40:349–59.
Barry D, Clarke M, Petry NM. Obesity and its relationship to addictions: is overeating a form of addictive behavior. Amer J Addict. 2009;18:439–51.
Del Parigi A, Chen K, Salbe AD, et al. Are we addicted to food? Obes Res. 2003;11:493–5.
Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–60.
Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7.
Wang GJ, Volkow ND, Thanos PK, et al. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53.
Wendlin A, Wudyka A: Narcotic addiction following gastric bypass surgery—a case study. Obes Surg. 2010 (in press).
Dawson DA, Grant BF, Stinson FS. The AUDIT-C: screening for alcohol use disorders and risk drinking in the presence of other psychiatric disorders. Compr Psychiatry. 2005;46:405–16.
Acknowledgments
This study was supported by the Norman E. Zinberg Fellowship in Addiction Psychiatry Research and the Livingston Fellowship, Department of Psychiatry, Harvard Medical School (JS), and AAK2400289 (GC).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, J., Haimovici, F. & Chang, G. Alcohol Use Disorders After Bariatric Surgery. OBES SURG 22, 201–207 (2012). https://doi.org/10.1007/s11695-010-0346-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-010-0346-1