Abstract
Purpose of Review
Evidence suggests prenatal polybrominated diphenyl ethers (PBDE) exposure effects on human neurodevelopment, but this is controversial due to conflicting research results. We conducted a systematic review and meta-analysis to summarize available peer-reviewed data of prenatal PBDE exposure effects on cognitive function, motor function, and behavior problems in children.
Recent Findings
Eligible birth cohort studies (January 1996–February 2017) were located through PubMed®, Web of Science®, or Google Scholar® and reported PBDE concentration in cord blood, maternal blood, or colostrum, as well as neurodevelopment assessment scores in children. Comprehensive meta-analysis (v.3.3.070, November 20, 2014) was used to calculate summary effect. Covariates are child age category (≤ 2, 3–5 and 6–7 years), location, and time period.
Summary
Six studies were included in meta-analysis. We found that prenatal PBDE exposure significantly correlated with decreased cognitive function (npooled = 804; k = 6; r = − 0.237; 95% CI − 0.441, − 0.010; p = 0.041), decreased motor function (npooled = 794; k = 5; r = − 0.350; 95% CI − 0.610, − 0.022; p = 0.037), and increased behavior problems (npooled = 307; k = 3; r = 0.393; 95% CI 0.133, 0.602; p = 0.004). Child age category was a significant covariate. The largest summary effect by child age category was ≤ 2 years for cognitive function and 6–7 years for behavior problems. Biomarker type was also a significant covariate. PBDEs measured in colostrum had a similar neurodevelopment effect size to cord blood, but PBDEs measured in maternal blood had a smaller effect size, relative to cord blood. The effect of prenatal PBDE exposure on behavior may be underestimated because only maternal blood was used as the exposure biomarker in eligible behavior assessments. Our study suggests that prenatal PBDE exposure adversely affects neurodevelopment. This study was underpowered due to the low number of available studies meeting eligibility criteria, although the use of pooled data analysis helped to offset the underpowered meta-analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background
Polybrominated diphenyl ether (PBDE) flame retardants are persistent organic pollutants commonly found in the environment [1,2,3,4]. Predominantly used in polyurethane foam and plastics for consumer products, PBDEs entered the commercial market in the 1970s, driven by demand created by California Technical Bulletin 117 (1975) that required certain consumer products sold in California be able to withstand direct contact with an open flame for 12 s without sustaining combustion [2, 5,6,7]. The size of the California consumer market made the flame-resistant standard a de facto requirement for rest of the USA [7].
Theoretically, there are 209 PBDE congeners with one to ten bromine atoms attached to the diphenyl ether substrate; however, only three congeners were commercially produced: pentabromodiphenyl ether (pentaBDE), octabromodiphenyl ether (octaBDE), and decabromodiphenyl ether (decaBDE) [2, 3]. Billions of pounds of PBDEs were incorporated into consumer goods, such as furniture, electronics, textiles, carpet padding, plastics, vehicle seats and car seats, and placed on the market, primarily in the USA [8, 9]. PBDEs are additive flame retardants—they are not covalently bonded to foam or a plastic polymer matrix—so they volatilize or leach into the environment and potentially bioaccumulate [3, 10, 11].
Regulatory Response
Concern regarding persistence, bioaccumulation, and toxicity of some PBDE congeners led to regulatory restrictions and phaseout of most PBDEs by 2014. Regulatory remedies restricting environmental release of PBDEs to reduce human exposure were initiated by the European Union (EU) Commission in 2001 over concerns of bioaccumulation after research detected pentaBDE derivatives in human breast milk [12, 13]. The European Union (EU) subsequently banned pentaBDE and OctaBDE in consumer products in 2004 [14]. The US Environmental Protection Agency (US EPA) implemented a voluntary production phaseout of pentaBDE and octaBDE [8, 15, 16]. By 2008, the EU restricted all commercially produced PBDEs to less than 0.1% by weight in most electronic or electrical devices [17, 18]. In 2009, tetra-, penta-, hexa-, hepta-, and octaBDE were added to the Stockholm Convention on Persistent Organic Pollutants (POPs) [1, 19]. In 2012, the US EPA proposed a Significant New Use Rule (SNUR) for six common PBDE congeners, effectively limiting their continued use in US commerce [20]. This initiated a voluntary production phaseout of decaBDE by the last two manufacturers of the chemical in the USA by December 31, 2013 [20]. California Technical Bulletin 117 was changed to allow furniture manufactures an option of not using flame retardants if their products would not combust when in contact with a smoldering ignition source, rather than an open flame ignition source specified under the 1975 version [7].
Regulatory action has reduced PBDEs detected in the environment and in humans [21, 22•, 23, 24]. However, PBDEs are still detected in house dust, food, animal tissue, and humans, particularly in adipose tissue, breast milk, and blood lipids [5, 8, 25,26,27]. This is due in part to continued use of furniture containing PBDEs and from the environmental persistence and bioaccumulation of several PBDE congeners [3, 5]. In 2002, US adult PBDE sera levels were ten times the levels found in their European or Asian counterparts, but until recently, there were few research studies on human health effects of PBDE exposure [8, 27, 28].
PBDE Fate and Transport, Routes of Human Exposure
PBDEs are lipophilic and have a moderate to high octanol-water partition coefficient, depending on amount of bromination [8]. Partitioning coefficient estimates the environmental fate of a chemical regarding how likely the chemical will dissolve in water or bioaccumulate in lipids of plants and animals. The higher the octanol-water coefficient, the more likely a chemical is to bioaccumulate. All PBDE congeners have varying levels of environmental persistence [4, 9, 29, 30]. Gaseous PBDEs or airborne PBDE-contaminated dust are the main forms of transport in the environment [8, 31,32,33]. The primary fate of PBDEs is deposition in soil and sediment, which is a route of PBDE uptake into the food chain [8, 31, 34, 35].
Environmental exposure studies measuring PBDE concentration in various human biomarkers showed that PBDE exposure increased rapidly after commercialization [2, 36,37,38,39,40,41,42,43]. Common congeners found in human tissue include tetraBDE, hexaBDE, and decaBDE [28, 44]. Humans are exposed to PBDEs through their environment, primarily via air, food, and house dust and fetal exposure via maternal exposure that crosses the placenta [8, 26, 28, 43, 45•, 46,47,48].
The half-life of PBDE congeners varies by bromination level and by the environmental compartment or tissue [30]. The relatively consistent level of decaBDE measured in human blood sera by surveillance programs in several countries, combined with a relatively short half-life of decaBDE in human sera (11 to 18 days), suggests that humans are continuously exposed to this PBDE congener [26, 30, 49, 50•].
PBDE Exposure and Risk to Neurodevelopment
Prenatal exposure to neurotoxic agents, such as PBDEs, can interrupt neurodevelopment processes and have lasting adverse effects. Fetal neurodevelopment occurs in early gestation and is especially susceptible to environmental toxins [51]. Previous research indicates a positive association between prenatal PBDE exposure and adverse neurodevelopment outcomes, but conflicting results in the research exist regarding the significance, magnitude and direction of association [2, 5, 8, 46, 47, 52,53,54,55,56]. Data from animal studies suggest exposure to certain PBDEs disrupts normal endocrine function associated with neurodevelopment, although the results are not consistent [5]. Toxicokinetic studies show reduced ability of young animals to excrete PBDEs, resulting in a higher body burden compared to adults [5].
With few epidemiological available studies available, the US Environmental Protection Agency (US EPA) established PBDE exposure thresholds for neurobehavioral effects based on animal studies [37,38,39,40]. The US EPA reference dose for oral exposure (RfD-oral) for neurobehavioral effects is 0.1 μg/kg/day for BDE-47 (tetraBDE) and BDE-99 (pentaBDE), 0.2 μg/kg/day for BDE-153 (hexaBDE), and 7 μg/kg/day for BDE-209 (decaBDE) [37,38,39,40]. PBDE concentrations in cord blood and colostrum from recent birth cohort studies are close to and, in some cases, exceed the US EPA RfD-oral threshold for neurobehavioral effects [52, 53, 55].
Study Objective
The aim of this research is to determine the summary effect of prenatal PBDE exposure on neurodevelopment outcomes (cognitive function, motor function, and behavior problems) in children. To accomplish this aim, we conducted a systematic review and meta-analysis of eligible birth cohort studies reporting a measure of association between prenatal PBDE exposure measured in cord blood, prenatal maternal blood or colostrum, and neurodevelopment test scores assessed in children.
Rationale
There are two motivations for conducting this research. First, while regulatory actions have removed PBDEs in new foam-containing furniture and electronics, there is a large stock of existing consumer products in use that contain PBDEs. Consumers using older furniture are likely to be of a lower socio-economic status (SES), such as college students and young families. Prenatal exposure to PBDEs might be modifying the effect of SES on neurodevelopment outcomes. Second, the rigor employed in conducting a systematic review and meta-analysis provides value in summarizing the effect of an exposure on a health outcome when controversy in existing research results exist, especially when the exposure occurs in utero.
Methods
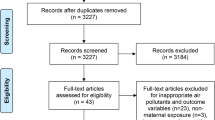
The systematic review a priori protocol began with developing a literature search strategy and eligibility criteria for the selection of birth cohort studies. Case-control and cross-sectional studies were not eligible because temporality of prenatal PBDE exposure prior to or shortly after birth was a necessary condition. Search engines used were (1) PubMed®, (2) Google Scholar®, and (3) Web of Science®. Study selection followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart [57], shown in Fig. 1. Study eligibility criteria included type of study (birth cohort), study participants (consenting pregnant women and their infants/children), exposure (PBDEs detected prenatally or shortly after birth), outcome (neurodevelopment assess using a validated instrument by trained and competent personnel), measure of association, and effect size. Any study meeting eligibility criteria was screened for relevance without consideration to geography.
PRISMA flow diagram on record selection and exclusion criteria [2]
Study Search Strategy
Study inclusion criteria were limited to studies in English language conducted between January 1, 1996 and February 28, 2017. The search strategy was confined to peer-reviewed scientific articles, books, government documents, conference proceedings, technical reports, reviews, theses, and dissertations; the last source was reviewed for the citation list only. Exclusion criteria restricted patents, audio/visual sources, blogs, microfilm, newspaper articles, and studies on populations already covered under another study. Table S-1 in the Supplemental Material lists all search terms used. Study search terms included the use of a Boolean multi-character wildcard. Search terms were: “PBDE*” OR “polybrominated diphenyl ether” OR “brominated flame*” OR “BFR” AND/OR “neuro*” AND/OR “develop” AND/OR “*natal” AND/OR “infant” AND/OR “child*” AND/OR “in utero” AND/OR “review” AND/OR “meta-analysis.”
Study Screening Strategy and Data Extraction
Titles of identified records were first screened for relevance and duplicates were removed. Studies were then screened for relevance using keyword searches, such as a “mouse,” “rat,” or “animal.” Citation lists of non-relevant studies were reviewed and relevant studies from the citation list were then screened. Abstracts were screened by reading each abstract twice by one reviewer, yielding 17 eligible studies for full-text review, which were also read twice by one reviewer. The reference list of each eligible study was examined to identify additional studies meeting eligibility criteria. No new study was identified from this examination. Data extraction took place after full-text review for eligibility. Data from each eligible study was entered onto a systematic review coding sheet, yielding seven studies that met eligibility criteria for meta-analysis.
Study Characteristics
The characteristics of participants in each study are listed in Table 1. The first birth cohort study to measure prenatal PBDE exposure and neurodevelopment outcomes was Roze [56], followed by Herbstman [55]; Shy [54]; Gascon, 2011 [53]; Gascon, 2012 [52]; Eskenazi [58]; and Chen [46]. Reporting choice of measures of association and dispersion were not consistent across studies. Shy et al. [54] met eligibility criteria for the systematic review but was not included in the meta-analysis because a measure of dispersion was not reported. Risk of bias at individual study level was assessed and estimated as low, given the narrow eligibility criteria of recent birth cohort studies published in peer-reviewed journals and that each study sample size was greater than 50 participants.
Covariates
Common study characteristics reported across all studies were location, type of biomarker collected as a substitute for prenatal PBDE exposure, timing of biomarker collection, and age of the infant/child when neurodevelopment was assessed. Highest age of neurodevelopment testing in eligible studies was 7 years old. Maternal age was reported across studies, but as a categorical variable in some studies and a continuous variable in others. An estimate of mean maternal age is 27–30 years old. An infant/child age category variable was created, with categories delineating development phases of infant/toddler (0–2 years) preschool (3–5 years) and early school years (6–7 years) [59]. Other covariates included region (the USA, EU, or Asia), latitude (absolute), time period of study, and neurodevelopment category.
The neurodevelopment assessment instruments utilized in individual studies included in this meta-analysis (k = 6) are described in detail in Table 2. Several neurodevelopment assessment instruments have overlapping primary measures. Based on a review and categorization of each assessment instrument’s scales and subscales, we created a neurodevelopment outcome category with three variables, cognitive function, motor function, and behavior problems, for use in this meta-analysis. Not all instruments in each study were included in the meta-analysis. Only Gascon et al. [53] utilized a social competence instrument (California Preschool Social Competence Scale, CP-SCS) so it was not included in the meta-analysis. The Development Coordination Disorder Questionnaire (DCD-Q), utilized by Roze et al. [56], was included in the meta-analysis using inverse value as the effect size direction of the DCD-Q is opposite of other motor function assessments.
Data Analysis
Comprehensive Meta-Analysis (CMA, v. 3.3.070, November 20, 2014) was used for descriptive statistics and to calculate summary effect and meta-regression [60]. A random effects model was used as the comparison model for meta-analysis since there was a high level of heterogeneity in both the exposure and the outcome between studies. Due to non-normal distributions of effect sizes across studies, Fisher’s Z transformation with 95% confidence interval (95% CI) was chosen as the meta-analysis effect size. Fisher’s Z was calculated directly from Pearson’s correlation coefficient or from odds ratio or risk ratio using CMA software. Effect size was estimated from beta coefficients using a multi-step process. First, CMA software converted beta coefficients and 95% CI to point estimates and standard errors. Next, Peterson’s imputation formula was used to convert point estimates to correlation coefficient, r, and converted to the effect size used in the meta-analysis with Fisher’s Z transformation equation [60,61,62,63].
Power analysis for a random effects meta-analysis followed the recommended approach from Borenstein et al. and is described in detail in the Supplemental Material [60]. Power analysis for each neurodevelopment category, as well as estimates for the number of studies needed to achieve a power of 0.80, is shown in Table 3.
The potential influence of publication bias was examined using techniques also recommended by Borenstein et al., including Rosenthal’s fail-safe N, Orwin’s fail-safe N, Duval and Tweedie’s trim and fill with funnel plot, and restricting analysis to larger studies [60]. Detailed descriptions of the publication bias tests are in the Supplemental Material.
This systematic review protocol was registered with PROSPERO® on January 20, 2017 as CRD42017055622 and followed the guidance provided PRISMA statement checklist [57], provided in Table S-2 of the Supplemental Material. IRB review determined this study did not meet the definition of research using human subject as set forth by the Department of Health and Human Services, 45 CFR 46.
Results
The pooled sample size of each neurodevelopment category for the six studies included in the meta-analysis (npooled), and a number of studies in each neurodevelopment category (k) are as follows: n = 804, k = 6 for cognitive function, n = 794, k = 5 for motor function, and n = 307, k = 3 for behavior problems. Results indicate that prenatal PBDE exposure is significantly correlated with decreased cognitive function (β = − 0.237; 95% CI − 0.441, − 0.010; p = 0.041), decreased motor function (β = − 0.350; 95% CI − 0.610, − 0.022; p = 0.037), and increased behavior problems (β = 0.393; 95% CI 0.133, 0.602; p = 0.004). Figure 2a–c provides the forest plots for each neurodevelopment category.
a Summary effect (random model) of prenatal PBDE exposure on cognitive function, npooled = 804, k = 6. b Summary effect (random model) of prenatal PBDE exposure on motor function, npooled = 794, k = 5. c Summary effect (random model) of prenatal PBDE exposure on behavior problems, npooled = 307, 0 = 3
Multivariate analysis from meta-regression indicates biomarker type and infant/child age category are significant moderator variables. Meta-regression analysis on other covariates was not significant. Colostrum had a similar effect size to cord blood as a biomarker estimate for prenatal PBDE exposure, but the effect size was smaller when prenatal PBDE exposure was estimated with maternal blood. Studies that assessed cognitive function and motor function used cord blood, colostrum, and prenatal maternal blood to estimate prenatal PBDE exposure. Studies that measured behavior problems only used maternal blood as the biomarker.
Meta-regression analysis results on cognitive and motor function for biomarker type are summarized in Table 4. Meta-regression was not conducted on behavior problems for biomarker type since all studies in this neurodevelopment category only used maternal blood as the prenatal PBDE exposure biomarker, and hence, there were no other biomarkers available for comparison. The summary in Table 4 indicates that using maternal blood as an estimate of prenatal PBDE exposure may reduce the effect size on cognitive function and motor function.
Power calculations indicate that this meta-analysis is underpowered. The power for each neurodevelopment category is 0.304, 0.313, and 0.354 for cognitive function, motor function, and behavior problems, respectively. The summary of power calculations in Table 3 lists the number of studies needed to achieve 0.50 and 0.80 power for each neurodevelopment category. For cognitive function, motor function, and behavior problems, respectively, 12, 9, and 5 studies are needed to achieve a power of 0.50; 23, 19, and 10 studies are needed to achieve a power of 0.80, respectively.
The influence of publication bias on the summary effect is summarized in Table 5. Based on the analysis of the four tests recommended by Borenstein et al. [60], the likelihood there are unpublished studies that could influence the significance or change the substantive importance of the meta-analysis summary effect is low.
Discussion
The results of this study indicate that prenatal PBDE exposure is associated with a significant decrease in cognitive and motor function and a significant increase in behavior problems among children 7 years of age or younger.
There is a difference between the types of PBDE congener manufactured and types of congeners detected in human biomarkers, suggesting that PBDE metabolism includes debromination. Smaller PBDE congeners are more persistent, bioaccumulative and toxic, and have structural similarities to thyroid hormones [64•, 65••, 66]. From conception through the 10th week of gestation—a critical window of human neurodevelopment—maternal thyroid hormones signal differentiation and relocation to neuronal cells in the embryo, forming main structures in the brain, such as the limbic system, as well as the spinal cord and major peripheral nerves [67,68,69]. The nervous system continues to develop in the second trimester when the fetal thyroid gland is able to synthesize thyroid hormones [67]. The last trimester is a time of rapid brain growth and the ramping up of synaptogenesis and myelination processes, which extend into early childhood [67]. The limbic system, formed during the first trimester of fetal development, directs sensory stimuli to the cerebral cortex, where cognition, motor coordination, and executive function take place [67]. Thus, damage to the developing limbic system can impact cognition, motor function, and behavior. Prenatal PBDE exposure may contribute to limbic system damage and subsequent adverse neurodevelopment assessment test scores [64, 65••, 70•, 71].
Although, regulation restricts PBDEs in new consumer products, upholstered furniture, mattresses, vehicles, and car seats manufactured before 2014 are still likely to contain PBDEs. Consumers using older durable goods are likely to be in a lower socio-economic strata [72•]. Infants born to families living in a lower SES are more vulnerable to factors associated with cognitive and behavior problems [72•, 73]. In 2008, Zota et al. discovered people living in a lower SES are more likely to have a higher body burden of PBDEs [74•]. Thus, it is important to consider prenatal PBDE exposure as a potential effect modifier when investigating the effect of SES attributes on neurodevelopment outcomes. Some factors to consider in prenatal PBDE exposure include the age of the home, bed mattress, upholstered furniture, and car, as well as the amount of time spent inside the home, cleaning frequency and method, and time spent in the car.
A goal of conducting epidemiological research is to quantify the magnitude and direction of association between exposure and outcome, but this can be especially difficult for prenatal exposures. Prenatal exposure to lipophilic environmental contaminants, like PBDEs, partition into lipids of blood, adipose tissue and breast milk. Breast milk is a common biomarker surrogate for prenatal exposure because it is considered non-invasive relative to using maternal blood during pregnancy. Umbilical cord blood is also considered non-invasive and, as our results indicate, may be more representative of fetal exposure. The use of colostrum as a biomarker for prenatal PBDE exposure in Gascon et al. [52] was novel and was more likely to approximate third trimester prenatal exposure than samples of breast milk collected after 1-week postnatal. The results of our study indicate PBDE concentrations measured in colostrum had a similar summary effect to that of cord blood for cognitive and motor function assessment scores.
Behavior problem was the neurodevelopment category requiring the least number of additional studies [10] to achieve a study power of 0.80, indicating a larger effect size associated with prenatal PBDE exposure and behavior problems, even with a small number of studies (k = 3). Studies that assessed behavior problems in this meta-analysis only used maternal blood as a biomarker, which attenuated the effect size of cognitive and motor function in the results of our study. This suggests that the summary effect of behavior problems might have been higher if cord blood or colostrum was the biomarker. Hence, the summary effect size of prenatal PBDE exposure on behavior problems may be underestimated in this study.
Infant/child age category relative to the type of neurodevelopment assessment was a significant moderator variable on test scores, suggesting assessing cognitive function and motor function in age category 0–2 years. A larger summary effect was observed for behavior problems in the 6–7 years age category, suggesting that assessing specific neurodevelopment categories in these respective age categories may reveal a more pronounced effect size.
Strengths and Limitations
This research had several strengths. The narrow eligibility criteria of birth cohort studies and exposure biomarkers provide evidence of strength of association, temporality, plausibility and biological gradient. Development of a neurodevelopment assessment category for use in the meta-analysis streamlined the coding and statistical analysis process and helped visualize trends in meta-regression. Studies that reported beta coefficients were not discarded. Instead, they were converted to a point estimate that could be used with the CMA® software to calculate summary effect, which increased the number of eligible studies, pooled sample sizes and overall power of the meta-analysis results.
There are also limitations. First, only one reviewer conducted the eligibility screening and assessment coding for the systematic review. Various guides on systematic reviews recommend screening and coding activities be conducted in parallel by at least two reviewers and results compared and measured with a kappa statistic [75]. Utilizing one reviewer creates potential susceptibility to selection and information bias. To control for this, the reviewer read each abstract twice and each full-text study included in meta-analysis at least twice. Second, the creation and use of a neurodevelopment category (i.e., cognitive function, motor function, and behavior problems) from the primary measures of neurodevelopment assessment instruments somewhat diluted the effect size of subscales that overlapped two or more neurodevelopment categories. This was viewed as an acceptable tradeoff because it made for a more conservative summary effect estimate, reduced processing time and complexity, and increased pooled sample sizes for different ages and neurodevelopment categories. Finally, the project was underpowered due to the low number of studies included in the meta-analysis. Power = 1—type II error (false negative). For meta-analysis, a false negative is failing to detect an effect when the effect is present. Thus, a low-powered study suggests an underestimation of effect size.
Conclusions
The epidemiologic utility of a systematic review and meta-analysis is the rigor employed in study selection and review and the value to translational science in summarizing evidence from existing studies to communicate a clearer picture of overall public health effect to clinicians, policy-makers, and the public. There is increasing evidence that PBDE exposure affects endocrine disruption, especially with regard to thyroid function, which in turn affects neurodevelopment. The effect of prenatal PBDE exposure on behavior needs further investigation, and we recommend exploring this effect using cord blood or colostrum to estimate fetal exposure.
We also recommend conducting additional systematic reviews and meta-analyses using broader eligibility criteria to include birth cohort studies that use breast milk or meconium along with colostrum and maternal/cord blood as biomarkers for prenatal PBDE exposure and conducting a systematic review and meta-analysis on the efficacy of various human biomarkers in approximating postnatal exposures to PBDEs and other halogenated organics that are suspected of endocrine disruption.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stockholm Convention. SC-4-14 listing of hexaBDE and heptaBDE (commercial octaBDE) under the Stockholm convention; 2009. Available from: http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx
US EPA. Polybrominated diphenyl ethers (PBDEs) project plan. 2006. Available from: https://www.epa.gov/sites/production/files/2015-09/documents/proj-plan32906a.pdf
Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112(1):9–17.
WHO I. UNEP—Brominated diphenylethers (EHC 162, 1994). UNEP 1994. Available from: http://www.inchem.org/documents/ehc/ehc/ehc162.htm
Pohl HR, Odin M, McClure P, Zaccaria K, LLados F, Kawa M, et al. Toxicological profile for polybrominated diphenyl ethers (PBDEs). CDC-ATSDR 2017; Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp68.pdf
Carey CM, Joseph W, Leake PH, Poel WH, Rodgman A, Tovey H, et al. California Technical Bulletin 117. 2000
Congleton J. California Policy Linked to higher exposures to harmful flame retardants. Environ Work Group 2016; Available from: https://cdn.ewg.org/sites/default/files/EWG_Biomonitoring.pdf
Lorber M, Cleverly D, Birnbaum LS, Axelrad D, Boethling B, Frithsen J, et al. An exposure assessment of polybrominated diphenyl ethers. U.S. Environmental Protection Agency, National Center for Environmental Assessment; 2010.
Alaee M. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int. 2003;29(6):683–9. https://doi.org/10.1016/S0160-4120(03)00121-1.
Kierkegaard A, Sellström U, McLachlan MS. Environmental analysis of higher brominated diphenyl ethers and decabromodiphenyl ethane. J Chromatogr A. 2009;1216(3):364–75. https://doi.org/10.1016/j.chroma.2008.05.058.
Talsness CE. Overview of toxicological aspects of polybrominated diphenyl ethers: a flame-retardant additive in several consumer products. Environ Res. 2008;108(2):158–67. https://doi.org/10.1016/j.envres.2008.08.008.
Meironyte D, Noren K, Bergman Å. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972-1997. J Toxicol Environ Health A. 1999;58(6):329–41.
Proposal for a Directive of the European Parliament and of the Council amending for the 24th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether. 2001/C 154 E/09 Dec 31, 2002. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52001PC0012&from=EN
European Parliament and of the Council. EU Marketing & Use Directive, Amendment 2003/11/EC. 76/769/EEC Feb 6, 2003 p. 4. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003L0011&from=en
Cooke M. EPA technical fact sheet—PBDEs and PBBs. US EPA 2014. Available from: https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_perchlorate_january2014_final_0.pdf
Axelrad D. US EPA—BFRs: regulatory actions and EPA activities—slide presentation. An Exposure Assessment of PBDEs 2009. Available from: https://www.nist.gov/sites/default/files/documents/el/fire_research/1-Axelrad.pdf
European Commission. Electrical and electronic waste—environment—European Commission. The RoHS Directive; 2016. Available from: http://ec.europa.eu/environment/waste/rohs_eee/index_en.htm
Merenyi S. REACH: Regulation (EC) No 1907/2006. 2014; Available from: http://content.grin.com/document/v276400.pdf
Stockholm Convention. SC-4-18. Listing of tetraBDE and pentaBDE under the Stockholm Convention. Stockholm Convention; 2009. Available from: http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx
US EPA. Assessing and managing chemicals under TSCA: polybrominated diphenyl ethers (PBDEs). 2016. Available from: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/polybrominated-diphenyl-ethers-pbdes
Dien NT, Hirai Y, Miyazaki T, Sakai S. Factors influencing atmospheric concentrations of polybrominated diphenyl ethers in Japan. Chemosphere. 2016;144:2073–80. https://doi.org/10.1016/j.chemosphere.2015.10.119.
Guo W, Holden A, Smith SC, Gephart R, Petreas M, Park J-S. PBDE levels in breast milk are decreasing in California. Chemosphere. 2016;150:505–13. https://doi.org/10.1016/j.chemosphere.2015.11.032. Provides evidence that chemical policy restricting PBDEs appears to be effective in reducing exposure to PBDEs in California.
Airaksinen R, Hallikainen A, Rantakokko P, Ruokojärvi P, Vuorinen PJ, Parmanne R, et al. Time trends and congener profiles of PCDD/Fs, PCBs, and PBDEs in Baltic herring off the coast of Finland during 1978–2009. Chemosphere. 2014;114:165–71. https://doi.org/10.1016/j.chemosphere.2014.03.097.
Crimmins BS, Pagano JJ, Xia X, Hopke PK, Milligan MS, Holsen TM. Polybrominated diphenyl ethers (PBDEs): turning the corner in Great Lakes trout 1980–2009. Environ Sci Technol. 2012:120906080022005. https://doi.org/10.1021/es302415z.
Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45(12):5323–31. https://doi.org/10.1021/es2007462.
Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs—a review of levels and sources. Int J Hyg Environ Health. 2009;212(2):109–34. https://doi.org/10.1016/j.ijheh.2008.04.005.
Sjodin A. A review on human exposure to brominated flame retardants, particularly polybrominated diphenyl ethers. Environ Int. 2003;29(6):829–39. https://doi.org/10.1016/S0160-4120(03)00108-9.
Hites RA. Polybrominated Diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–56. https://doi.org/10.1021/es035082g.
Abbasi G, Buser AM, Soehl A, Murray MW, Diamond ML. Stocks and flows of PBDEs in products from use to waste in the U.S. and Canada from 1970 to 2020. Environ Sci Technol. 2015;49(3):1521–8.
Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman Å, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114(2):176–81. https://doi.org/10.1289/ehp.8350.
Frederiksen M, Thomsen C, Frøshaug M, Vorkamp K, Thomsen M, Becher G, et al. Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort. Int J Hyg Environ Health. 2010;213(4):233–42. https://doi.org/10.1016/j.ijheh.2010.04.008.
Kang Y, Wang HS, Cheung KC, Wong MH. Polybrominated diphenyl ethers (PBDEs) in indoor dust and human hair. Atmos Environ. 2011;45(14):2386–93. https://doi.org/10.1016/j.atmosenv.2011.02.019.
Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA. Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ Sci Technol. 2005;39(4):925–31. https://doi.org/10.1021/es0486824.
Törnkvist A, Glynn A, Aune M, Darnerud PO, Ankarberg EH. PCDD/F, PCB, PBDE, HBCD and chlorinated pesticides in a Swedish market basket from 2005—levels and dietary intake estimations. Chemosphere. 2011;83(2):193–9. https://doi.org/10.1016/j.chemosphere.2010.12.042.
Darnerud PO, Atuma S, Aune M, Bjerselius R, Glynn A, Grawé KP, et al. Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, e.g. DDT) based on Swedish market basket data. Food Chem Toxicol. 2006;44(9):1597–606. https://doi.org/10.1016/j.fct.2006.03.011.
Alvarez J. Stockholm convention—commercial octabromodiphenyl ether. 2007; Available from: http://www.pops.int/documents/meetings/poprc/drprofile/drp/DraftRiskProfile_OctaBDE.pdf
Morrissey Donahue J, Galal-Gorchev H, Manibusan M, Jones SJ. Toxicological review of 2,2′,4,4′5,5’-hexabromodiphenyl ether (BDE-153). 2008. Available from: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/1009tr.pdf
Morrissey Donahue J, Galal-Gorchev H, Manibusan M, Jones SJ. Toxicological review of 2,2′,4,4’-tetrabromodiphenyl ether (BDE-47). 2008. Available from: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/1010tr.pdf
Morrissey Donahue J, Galal-Gorchev H, Zhao Q, Gadagbui B, Maier A, Jones SJ. Toxicological review of decabromodiphenyl Ether (BDE-209). 2008. Available from: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0035tr.pdf
Morrissey Donahue J, Galal-Gorchev H, Manibusan M, Jones SJ. Toxicological review of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99). 2008. Available from: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/1008tr.pdf
Domingo JL. Human exposure to polybrominated diphenyl ethers through the diet. J Chromatogr A. 2004;1054(1–2):321–6.
Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, et al. High body burdens of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Perspect. 2003;111(9):1175–9. https://doi.org/10.1289/ehp.6220.
Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111(9):1249–52. https://doi.org/10.1289/ehp.6146.
Birnbaum LS, Cohen-Hubal EA. Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect. 2006; https://doi.org/10.1289/ehp.9061.
Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M, et al. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ Res. 2016;147:365–72. https://doi.org/10.1016/j.envres.2016.02.034. Study employed passive sampling - a novel personal environmental exposure measure - in assessing children's organic compound exposure.
Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjödin A, et al. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study. Environ Health Perspect. 2014; Available from: http://ehp.niehs.nih.gov/1307562; https://doi.org/10.1289/ehp.1307562.
Eskenazi B. In Utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121(2):257–62. https://doi.org/10.1289/ehp.1205597.
Besis A, Samara C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments—a review on occurrence and human exposure. Environ Pollut. 2012;169:217–29. https://doi.org/10.1016/j.envpol.2012.04.009.
Fromme H, Körner W, Shahin N, Wanner A, Albrecht M, Boehmer S, et al. Human exposure to polybrominated diphenyl ethers (PBDE), as evidenced by data from a duplicate diet study, indoor air, house dust, and biomonitoring in Germany. Environ Int. 2009;35(8):1125–35. https://doi.org/10.1016/j.envint.2009.07.003.
Darnerud PO, Lignell S, Aune M, Isaksson M, Cantillana T, Redeby J, et al. Time trends of polybrominated diphenylether (PBDE) congeners in serum of Swedish mothers and comparisons to breast milk data. Environ Res. 2015;138:352–60. https://doi.org/10.1016/j.envres.2015.02.031. Provided further evidence that restricting lower brominated PBDEs in the EU has been effective in reducing infant exposure to these compounds in breastmilk in Sweden. The temporal trend of the highest brominated PBDE (BDE-209) concentration in breastmilk did not change, indicating the EU policy delay in restricting BDE-209 might have contributed to wide-spread exposure in breastfed children in Sweden.
Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–78. https://doi.org/10.1016/S0140-6736(06)69665-7.
Gascon M, Fort M, Martinez D, Carsin A-E, Forns J, Grimalt JO, et al. Polybrominated dihpenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect. 2012; https://doi.org/10.1289/ehp.1205266.
Gascon M, Vrijheid M, Martínez D, Forns J, Grimalt JO, Torrent M, et al. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4years of age. Environ Int. 2011;37(3):605–11. https://doi.org/10.1016/j.envint.2010.12.005.
Shy C-G, Huang H-L, Chang-Chien G-P, Chao H-R, Tsou T-C. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol. 2011;87(6):643–8. https://doi.org/10.1007/s00128-011-0422-9.
Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposures to PBDEs and neurodevelopment. Environ Health Perspect. 2010;
Roze E, Meijer L, Bakker A, Van Braeckel KNJA, Sauer PJJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117(12):1953–8. https://doi.org/10.1289/ehp.0901015.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(7716):332–6.
Eskenazi B, Fenster L, Castorina R, Marks AR, Sjödin A, Rosas LG, et al. A comparison of PBDE serum concentrations in Mexican and Mexican-American children living in California. Environ Health Perspect. 2011;119(10):1442–8. https://doi.org/10.1289/ehp.1002874.
MacDonald M. In-person meetings with Dr. Megan MacDonald, Assistant Professor in the Department of Kinesiology, Oregon State University. 2017.
Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Comprehensive meta-analysis, v3. 2009.
Tarlow S. Comprehensive meta-analysis technical support. 2017.
Nieminen P, Lehtiniemi H, Vähäkangas K, Huusko A, Rautio A. Standardised regression coefficient as an effect size index in summarising findings in epidemiological studies. Epidemiol Biostat Public Health 2013;10(4). Available from: http://ebph.it/article/view/8854
Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol. 2005;90(1):175–81. https://doi.org/10.1037/0021-9010.90.1.175.
Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119(10):1454–9. https://doi.org/10.1289/ehp.1003235.
Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. https://doi.org/10.1016/j.neuroscience.2015.09.070. Provides an in-depth overview of the importance of adequate thyroid hormone levels during pregnancy for normal fetal/infant neurodevelopment, including neuronal cell migration and differentiation.
Carson BL, Masten S. Review of tox literature on penta-, hexa-, octa- and deca BDE. NIEHS. 2001. Available from: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/pbdes_508.pdf
Fitzgerald MJT, Gruener G, Mtui E. Clinical neuroanatomy and neuroscience, vol. 1. 6th ed. Edinburgh: Saunders/Elsevier; 2012. 417 p.
Bellinger DC. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ Res. 2004;95(3):394–405. https://doi.org/10.1016/j.envres.2003.07.013.
Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(s3):511–33. https://doi.org/10.1289/ehp.00108s3511.
Vuong AM, Webster GM, Romano ME, Braun JM, Zoeller RT, Hoofnagle AN, et al. Maternal polybrominated diphenyl ether (PBDE) exposure and thyroid hormones in maternal and cord sera: the HOME study, Cincinnati, USA. Environ Health Perspect 2015 ;123(10). Available from: http://ehp.niehs.nih.gov/1408996. Study results indicate thyroglobulin antibodies significantly modified the negative association between BDE-47, BDE-99 and total PBDEs with thyroid-stimulating hormone (TSH) and free T3 levels in cord blood.
Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118(10):1444–9. https://doi.org/10.1289/ehp.1001905.
Ronfani L, Brumatti LV, Mariuz M, Tognin V, Bin M, Ferluga V, et al. The complex interaction between home environment, socioeconomic status, maternal IQ and early child neurocognitive development: a multivariate analysis of data collected in a newborn cohort study. PLoS One. 2015;10(5):e0127052. https://doi.org/10.1371/journal.pone.0127052. In a birth cohort in Italy, authors confirmed the complex relation between home environment, socioeconomic status, maternal IQ and early child neurocognitive development. The home environment had the greatest influence on child neurodevelopment.
Dietrich KN, Eskenazi B, Schantz S, Yolton K, Rauh VA, Johnson CB, et al. Principles and practices of neurodevelopmental assessment in children: lessons learned from the centers for children’s environmental health and disease prevention research. Environ Health Perspect. 2005;113(10):1437–46. https://doi.org/10.1289/ehp.7672.
Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42(21):8158–64. https://doi.org/10.1021/es801792z. First study to examine socioeconomic status and PBDE exposure in California. Results suggest lower household income is positively associated with higher PBDE body burden.
Selvin S. Statistical tools for epidemiologic research, vol. 1. 2nd ed. Oxford: Oxford University Press; 2011. 494 p
Acknowledgements
Dr. Molly Kile, ScD, Associate Professor, Oregon State University.
Dr. Veronica Irvin, MPH, Ph.D., Assistant Professor, Oregon State University.
Dr. Meghan MacDonald, Assistant Professor, Oregon State University.
Dr. David Bellinger, Professor, Harvard University; Boston Children’s Hospital, Department of Neurology.
Dr. Shelley Su, Instructor, Oregon State University.
Dr. Ellen Smit, Professor, Oregon State University.
Author information
Authors and Affiliations
Contributions
Hudson-Hanley wrote the manuscript, which is based on an independent project final report in fulfillment of a Ph.D. program requirements. Kile, Irvin, and Flay reviewed the final report and manuscript and provided recommendations for improvement. MacDonald provided guidance neurodevelopment assessment instruments that aided in the creation of the neurodevelopment category covariate as an outcome measure in the meta-analysis. MacDonald also reviewed the draft final report and provided guidance. Bellinger provided guidance on neurotoxicity and neurodevelopment assessments that informed the creation of the neurodevelopment category used in the meta-analysis. Su provided guidance on toxicology. Smit provided guidance on manuscript format.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Environmental Epidemiology
Rights and permissions
About this article
Cite this article
Hudson-Hanley, B., Irvin, V., Flay, B. et al. Prenatal PBDE Exposure and Neurodevelopment in Children 7 Years Old or Younger: a Systematic Review and Meta-analysis. Curr Epidemiol Rep 5, 46–59 (2018). https://doi.org/10.1007/s40471-018-0137-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-018-0137-0