Abstract
In this paper, we have considered a single-strain dengue model with saturated incidence rate as well as saturated treatment. Three types of controls, namely vaccination for susceptible humans, treatment for infected humans and mosquitoes killing effort by humans, are considered here. Existence of different equilibrium points and their stability have been investigated in terms of the basic reproduction number \((R_0)\). The system experiences different types of bifurcations such as transcritical bifurcation, backward bifurcation depending on the different model parameters. To verify the validity of the proposed model, we have fitted the model with real reported data of dengue outbreak in Singapore from 18th week, 2014 to 1st week, 2015. Performing sensitivity analysis we have identified most influential model parameters to control the disease. We have discussed estimation of actual and effective reproduction number. Pontryagin’s maximum principle has been used to find out the most effective control strategy for reducing dengue infection. Numerically we have shown the effect of different model parameters on disease spreading. Finally, using efficiency analysis we have identified that treatment for infected humans with mosquitoes killing effort is the most effective among considered control strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The study of vector-borne disease based on mathematical modeling is an important research area in mathematical epidemiology. The vector-borne disease spreads through interaction of susceptible hosts with infected vectors or infected hosts with susceptible vectors. Mosquito-borne dengue disease mainly spreads by the female mosquitoes Aedes aegypti and Aedes albopictus species mosquitoes [1]. The dengue-infected female mosquitoes bite susceptible peoples; then, susceptible peoples become infected by the disease. Symptoms of dengue fever usually appear within three to fifteen days after biting by the infectious mosquitoes [2].
Dengue virus (DENV) is mainly a RNA virus [3]. There are five different strains of dengue virus, namely DENV-1, DENV-2, DENV-3, DENV-4 and DENV-5 [4]. In particular when a person is infected with any strain of dengue, his body develops immunity with respect to that particular strain [5]; therefore, that person may be affected by another types of strains. Thus, infection by any particular strain does not imply person is protected from other types of strains. Dengue epidemic occurred in Asia, Africa, and North America first time simultaneously [6], and it was first reported in 1779–1780, later it was spread more than 127 countries. Different strains of dengue virus were first identified in different times. The DENV-1 was identified in 1977, DENV-2 in 1981, DENV-4 in 1981 and DENV-3 in 1994 [7] but in October 2013, the latest strain DENV-5 has been announced [4]. According to the report of World Health Organization (WHO), approximately 3.97 billion people are living at risk in tropical and subtropical region for this disease [8]. It becomes hyper in many countries like Thailand, Bangladesh, Malaysia, India, Singapore, Sri Lanka, etc. [8]. The peoples are terrified about dengue fever due to its high morbidity and mortality rate. The number of dengue patient has been increasing day by day. According to the report of World Health Organization (WHO), the number of dengue cases was less than one thousand in 1950, but it is highly increasing in last few years, more than 3 million in 2015 [9] and 4.2 million in 2019 [5]. The record number of dengue cases was reported in 2019 throughout the world. The co-infections of dengue occur with rate ranging from 5 to 30% as high as 40–50% for the co-circulation of different strains of dengue in the same area [10, 11]. Due to the presence of three strains, Thailand becomes in danger, total reported dengue case was 128964 from 77 provinces in 2019 [12]. First time in Philippines and Thailand, complex form of dengue, dengue shock syndrome or DHF found during epidemic of dengue in 1950 [12]. First licensed dengue vaccine Dengvaxia (CYD-TDV) [13] become commercially available in highly dengue burden countries, but it has side effects like pain and headache. In 2016, it was approved in 11 countries [14, 15], later in European Union (in 2018) and United States (in 2019) also.
Many researchers from different fields like biology, mathematics etc., are trying to investigate about the dynamics of disease spreading and its control through mathematical modeling. It is one of the most powerful tools to analyze disease transmission dynamics and its control. In 1975, Bailey [16] first proposed a basic SIR dengue model. Esteva and Vargas [17] modified the Bailey’s model considering constant population of human and constant birth rate of vectors. In their work, they established that if the basic reproduction number is less than unity, then the disease free equilibrium point is stable. The environment plays an important role in spreading of dengue disease. The spreading of this disease depends on density of mosquitoes. On the other hand, the growth of mosquitoes is highly dependent on availability of dirty water, which is gathered mainly through rainfall. To understand the effect of rainfall on the dengue transmission, Chanprasopchai [18] proposed an SEIR model in 2017. Derouich and Boutayeb [19] have developed an SIR model to characterize dengue transmission between vectors and humans. The dengue model of Derouich and Boutayeb was extended by Erikson [20] in 2010 adding exposed human class.
In recent decades, some researchers have proposed the multi-strain dengue model to investigate the effect of different strains in the same model [21, 22]. Sriprom [21] introduced a multi-strain dengue model in the presence of two strains and described the dynamics of sequential transmission of dengue virus. Mishra and Gakkar [22] formulated a two-strain dengue model and discussed the effect of vector control, awareness on dengue dynamics. Researchers included some control strategies like the vector control, awareness and treatment control, etc., in their model to identify the most important features to reduce the infection [23, 24]. The vector control policies are: use of adulticide to increase the mortality rate of adult mosquitoes, use of larvicide to kill the eggs of mosquitoes, etc. [23]. The awareness and treatment policies include: protection against mosquitoes bites, vaccination, treatment of the infected human, etc. To study the effect of vaccination, Supriatna [24] proposed a dengue model of single- and two-strain model and discussed the model with vaccination. Zheng and Nie [11] developed a mathematical model of two-strain dengue infection, describing the co-circulation of multiple strains and identified the most important control strategies using optimal control principle. Recently many researchers studied on dengue disease dynamics [25,26,27,28,29].
Optimal control becomes an important key in mathematical modelling to investigate the disease dynamics and control the influence of the infection. Optimal control is used in mathematical modelling to find out optimal value of the most effective strategy when more than one controls are used. Using optimal control, one can identify the most suitable control function which reduces the infected populations as well as minimize the implementation cost [30]. Optimal control policies on dengue disease transmission have been studied by many researchers in recent years [31,32,33,34].
This paper is the extension of the work [35]. In [35], authors considered saturated type incidence rate of the form \(\dfrac{\beta S I}{1 + \alpha I}\) as this type of incidence rate ultimately tends to \(\dfrac{\beta }{\alpha }\) as \(I \rightarrow \infty .\) For crowding effect of infected population, the rate of disease transmission ultimately decreases. For this reason, authors took this type of incidence rate. Also, they considered three types of control parameters, namely protection control against mosquito bites (\(u_1\)), treatment control for dengue-infected individuals (\(u_2\)) and insecticide spray against the mosquito (\(u_3\)). But in this paper, we have considered saturated incidence rate in the form \(\dfrac{\beta S V_I}{1 + \alpha S}\) and saturated treatment in the form \(\dfrac{a u_2 I}{1 +bu_2 I}\). This type of incidence rate ultimately tends to \(\dfrac{\beta }{\alpha }\) as \(S \rightarrow \infty .\) For inhibitory effect and psychological effect of susceptible population, the rate of disease transmission will reduce. For this reason, we have used this type of incidence rate. Here, we have also used three types of controls, namely vaccination for susceptible humans, treatment for infected humans and mosquitoes killing effort by humans. In [35], system does not exhibit backward bifurcation; moreover, authors did not show any types of bifurcation. But in this paper, we show two types of bifurcation, namely transcritical and backward bifurcation. In backward bifurcation, for \(R_0 < 1\) bistability exists (one stable disease-free equilibrium and one stable endemic). Therefore, here eradication of disease depends on model parameter as well as initial population density. In [35], authors used parameter values from some literature to study sensitivity analysis and optimal control. But, here we have estimated model parameters by fitting the model with real reported data of dengue in Singapore to study sensitivity analysis and optimal control problem. Further, to find out best control among applied controls, we have performed efficiency analysis.
Aim of the manuscript is to formulate a single-strain dengue virus transmission model in the presence of vaccination and treatment. Susceptible humans are affected by bites of infected mosquitoes in saturated form and susceptible mosquitoes are affected by infected humans in bilinear form. To include the effect of limitation of medical facility, we consider the treatment function in saturated form. Three types of controls have been considered, namely vaccination for susceptible humans, treatment for infected humans and mosquitoes killing effort by humans. After discussing qualitative analysis of the proposed model, we have estimated most of the model parameters by fitting the model with real infection data. Sensitivity analysis has been performed to find out the most effective parameters to control the infection and also effect on different model parameters on disease spreading has been discussed here. Estimation of basic reproduction number and effective reproduction number have been studied here. Finally, we have identified the optimal policy of control strategies to minimize the number of infected humans as well as reduce the implementation cost for using different controls.
The novelties of the paper are mentioned here:
-
1.
Here we consider saturated type incidence and treatment.
-
2.
The system (1) exhibits backward bifurcation at \(R_0 = 1\). For backward bifurcation, two endemic equilibria exist when \(R_0^{*}< R_0 < 1\) (where \(R_0^{*}\) is the critical value of \(R_0\)).
-
3.
To validate the model, we have fitted the model with real reported data of dengue in Singapore from 18th week, 2014 to 1st week, 2015.
-
4.
To find out most sensitive parameters and to perform optimal control problem, we go through estimated model parameters by fitting model with real data.
-
5.
Further we find estimation of actual reproduction number and effective reproduction number which are generally uncommon in other literature.
-
6.
To find out optimal value of applied controls to reduce infected human populations and to minimize implemented cost, we have performed optimal control analysis.
-
7.
Finally we have studied efficiency analysis to know which control is best among applied controls.
Organization of this paper is as follows: Formulation of the model is discussed in Sect. 2. Positivity and boundedness of the solutions of the model, expression of basic reproduction number, existence of different types equilibrium points and their stability criteria are presented in Sects. 3, 4, and 5, respectively. Different types of bifurcation are investigated in Sect. 6. Validation of the proposed model and estimation of model parameters are studied in Sect. 7. Sensitivity analysis and effect of model parameters are discussed in Sects. 8 and 9, respectively. In Sects. 10.1 and 10.2, estimation of actual reproduction number and effective reproduction number have been discussed. Optimal control with numerical examples and efficiency analysis are studied in Sects. 11 and 12, respectively. We have summarized the results in Sect. 13.
2 Model formulation
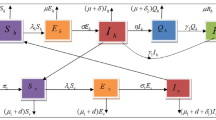
In this paper, we have formulated a single-strain dengue transmission model to study the dengue outbreak in Singapore from 18th week, 2014 to 1st week, 2015. Let total human populations be divided into three disjoint classes, namely susceptible class (S), infected class (I) and recovered class (R), and vector populations be divided into two disjoint class, namely susceptible vectors \((V_{s})\) and infected vectors \((V_{I})\). We have considered disease transmission rate by infected mosquitoes in saturated form \(\dfrac{\beta S V_I}{1 + \alpha S}\), where \(\alpha \) represents inhibitory factor of susceptible population. The above saturated incidence rate is considered here because due to the lack of knowledge about the disease, rate of disease transmission increases with number of infected vectors but for social awareness, inhibitory factor of susceptible human populations and also crowding effect of infected human populations, rate of disease transmission will decrease. To introduce the effect of limited medical resources, we have considered treatment function in saturated form \(\dfrac{a u_2}{1 + b u_2 I}\), where b represents delay in getting treatment. For lower abundance of infected human populations, treatment rate behaves like linear character but for higher abundance of infected human populations it ultimately tends to \(\dfrac{a}{b}\) where a, b both are positive constants. We assume that some of infected human populations are recovered naturally and some other are recovered using treatment. To formulate the model, we assume that the susceptible population has constant growth rate \(\omega \) and \(\omega _1\) for humans and vectors, respectively. The susceptible vectors become infected after biting infected human following mass action law. Here normal death rate of both humans and vectors is taken into consideration. Incorporating the above assumptions dynamics of flow diagram of dengue infection is presented in Fig. 1, and the corresponding model is given in the following,
with the initial conditions \(S(0)> 0, I(0) \ge 0, R(0) \ge 0, V_{S}(0) > 0\) and \(V_{I}(0) \ge 0\). In the next section, we shall investigate the positivity and boundedness of the proposed model to establish the well definiteness of the model. All model parameters in model system (1) are written in Table 1.
3 Basic properties of the proposed model
First we have to check the uniform boundedness criteria of solutions of the proposed model to analyze the model. To check the uniform boundedness first we shall check the positivity of solutions, i.e., we have to establish that all solutions of the proposed model are positive starting from any nonnegative initial conditions. For establishing nonnegativity and boundedness, we shall prove the following two theorems.
Theorem 1
All solutions of the proposed model are nonnegative for any time t satisfying nonnegative initial conditions.
Proof
To prove this, we first show that \(S(t) > 0\) for all time t. From the first equation of (1), we get
Integrating and using the initial conditions, we get \(S(t) \ge S(0)e^{-(\beta V_{I}+\mu + u_{1})t} > 0\). Again from the second equation of (1), we get
Again integrating, we get \(I(t) \ge I(0)e^{-(\mu +d+\gamma +au_{2})t} \ge 0\). Similarly from other three equations of (1), we have \(R(t) \ge R(0)e^{-\mu t}\ge 0\) , \(V_{S}(t) \ge V_{S}(0)e^{-(\mu _{2}+cu_{3} +\sigma I)t} > 0\) and \(V_{I}(t) \ge V_{I}(0)e^{-(cu_{3}+\mu _{2})t} \ge 0\) for all t. \(\square \)
Theorem 2
All solutions of model system (1) are uniformly bounded.
Proof
Let \(H=S+I+R\) and \(V=V_{S}+V_{I}\), taking derivative of H with respect to t we get,
Integrating both sides of above expression and then applying theory of differential inequality [18] , we have
as t goes to infinity, the above inequality changes to
Again derivative of V with respect to t gives
Using the similar arguments as used for H(S, I, R) , we obtain,
Using the results of (2-3), we get
where \(M=\dfrac{\omega }{\mu }+\dfrac{\omega _{1}}{\mu _{2}+cu_{3}}+H(S(0),I(0),R(0))+V(V_{S}(0),V_{I}(0))\).
Hence, all solutions of the system are uniformly bounded. \(\square \)
4 Basic reproduction number \((R_0)\) and existence of different types of equilibrium points
In this section, first we shall determine the basic reproduction number of the proposed model in terms of model parameters, and then, the expression for the existence of different equilibrium points will be discussed.
The basic reproduction number (\(R_{0}\)) plays a significant role to determine whether an epidemic will ensure or not [36]. Since the system (1) has the disease-free equilibrium point (DEF) \(E_{0}(S_{0}, 0,R_{0}^{'}, V_{S_{0}}, 0)\) where \(S_{0}= \dfrac{\omega }{\mu + u_{1}}\), \(R_{0}^{'}=\dfrac{\omega u_{1}}{\mu (\mu + u_{1})}\) and \(V_{S_{0}}=\dfrac{\omega _{1}}{\mu _{1} + c u_{3}}\), hence \(R_0\) of the model exists. Here to find the expression of \(R_{0}\) by the next-generation matrix approach as proposed by Driessche and Watmough [37], we use the notation \( F_1 = (F_{11} \ F_{12})^T\) and \( F_2 = (F_{21} \ F_{22})^T\) where \( F_{11} = \dfrac{\beta S V_{I}}{1+\alpha S}\), \(F_{12} = \sigma V_{S}I \) and \(F_{21} = (\mu + d + \gamma )I + \dfrac{a u_{2}I}{1+bu_{2}I}\) , \(F_{22} = cu_{3}V_{I}+\mu _{1} V_{I}\).
Let \( {\mathbf {F}}=\frac{\partial (F_{11},F_{12})}{\partial (I,V_1)}|_{E_0}= \begin{pmatrix} 0&{}\dfrac{\beta \omega }{\mu + u_{1}+\alpha \omega }\\ \dfrac{\sigma \omega _{1}}{\mu _{1}+cu_{3}}&{}0 \end{pmatrix} \textit{and} \ {\mathbf {V}}=\frac{\partial (F_{21},F_{22})}{\partial (I,V_1)}|_{E_0}= \begin{pmatrix} \mu +d+\gamma +au_{2}&{}0\\ 0&{}\mu _{1}+cu_{3} \end{pmatrix}.\) Therefore, next-generation matrix \((F V^{- 1})\) is given by
The spectral radius of the matrix \(FV^{-1}\) is the basic reproduction number, which is given by
Other than the DEF, the system contains the endemic equilibrium point is \( E_{*}(S_{*}, I_{*},R_{*}, V_{S_{*}}, V_{I_{*}}) \) where \(I_{*}\) satisfies the biquadratic equation
with \(S^* = \dfrac{\omega }{\mu + u_1} - \dfrac{(\mu + d + \gamma )I^*}{\mu + u_1} - \dfrac{a u_2 I^*}{(1 + b u_2 I^*)(\mu + u_1)}>0\) if \(\omega (1 + b u_2 I^*) > au_2 I^* + (\mu + d + \gamma )(1 +bu_2 I^*)\), \(V_{I^*} = \dfrac{\sigma \omega _1 I^*}{(c u_3 + \mu _1)(c u_3 + \mu _1 + \sigma I^*)}, V_{S^*} = \dfrac{\omega _1}{\mu _1 + c u_3 + \sigma I^*},\) \(R^* = \dfrac{a u_1 I^*}{\mu (1 + b u_2 I^*)} + \dfrac{\gamma I^*}{\mu } + \dfrac{u_1 S^*}{\mu }\) and the co-efficients \(C_i, i= 1,2,3,4\) are given in “Appendix I”.
It is clear from the expression of \(C_i\)’s that \(C_4\) is always positive, and depending on sign of \(C_1\), \(C_2\), \(C_3\) the number of feasible endemic equilibrium points will be calculated. The existence of endemic equilibrium point is discussed in the following theorem.
Theorem 3
An unique endemic equilibrium point \(E_{*}(S_{*}, I_{*}, R_{*}, V_{S_{*}}, V_{I_{*}})\) exists for \(R_{0} > 1\) if \(C_{1}\), \(C_{2}\) and \(C_{3}\) maintain the same sign.
Proof
To obtain the endemic equilibrium point, we have an expression of I in the form as in Eq. (4) where always \(C_{4} > 0\) and \(C_{0}\) can be expressed as \(C_{0}=(\mu _{1}+cu_{3})^{2}(\mu +d+\gamma +au_{2})(\mu + u_{1} + \alpha \omega )(1-R_{0}^{2})< 0\) if \(R_0 >1\). Therefore, by Descartes Rule of signs, Eq. (4) has at least one positive root if \(C_{1}\), \(C_{2}\) and \(C_{3}\) are of same sign for \(R_{0} > 1\). Thus, the endemic equilibrium point exists if \(C_{1}\), \(C_{2}\) and \(C_{3}\) maintain the same sign for \(R_{0} > 1\). \(\square \)
5 Stability analysis of equilibrium points
We shall now discuss the stability of the system about different equilibrium points. The eradication or persistence of the disease depends on stability of the disease-free equilibrium point and the endemic equilibrium points, respectively.
Theorem 4
The system is locally asymptotically stable around its disease-free equilibrium point if \(R_{0} < 1\).
Proof
The characteristic equation of the system at disease-free equilibrium points is given by:
where \(\varTheta _{1}=\mu +d+\gamma +\mu _{1}+au_{2}+cu_{3} > 0\) and \(\varTheta _{2}=(\mu _{1}+c u_{3})(\mu +d+\gamma +au_{2}) (1 - R_0^2)\).
It is clear from the above equation that among the five roots, three roots are negative which are \(-(\mu + u_{1})\), \(-\mu \), \(-(\mu _{1}+cu_{3})\) and other two roots satisfy the equation \(\lambda ^{2}+\varTheta _{1}\lambda +\varTheta _{2}= 0\). Since if \(R_0 < 1\) then \(\varTheta _{2} > 0\), so by Routh–Hurwitz criteria the last equation will have roots with negative real part if \(R_{0} < 1\) [38]. Thus, if \(R_{0} < 1\), then the system is locally asymptotically stable about disease-free equilibrium point. \(\square \)
Since at \( R_0=1 \) one root of the characteristic equation vanishes and other four becomes negative and the usual eigen analysis method fails. Suppose \( R_0=1 \) occurs at \(u_2=u_2^{[TC]}\) where
Theorem 5
The system is locally asymptotically stable around its endemic equilibrium point if \(F_{2} > 0\), \(F_{3} > 0\) and \(F_{1}F_{2} > F_{3}\), where the symbolic parameters are determined in subsequent steps.
Proof
The characteristic equation of system (1) corresponding to the endemic equilibrium point is given by
where \(F_1,\) \(F_2,\) and \(F_3\) are given in “Appendix II”.
Clearly it has two negative real roots \(-\mu \), \(-(\mu _{1}+cu_{3})\), and remaining three roots satisfy the equation
It is clear from the expressions of the coefficients that \( F_{1} \) is positive. Thus, by Routh–Hurwitz criteria all roots of the above characteristic equation will have negative real part if \(F_{2}> 0, F_{3} > 0\) and \(F_{1}F_{2} > F_{3}\) [38]. Thus, system is locally asymptotically stable around its endemic equilibrium points. \(\square \)
In biological point of view, the stability of disease-free equilibrium point implies disease eradicates from the system and stability of endemic equilibrium point implies the persistence of the disease in the system.
6 Bifurcation analysis
In this section, we investigate different types of bifurcations of the proposed model system (1). First we examine the Transcritical bifurcation at the DFE \(E_0\) in Theorem 6, and Theorem 7 is dedicated for backward bifurcation. The transcritical bifurcation will be discussed with respect to \(u_2\), and the backward bifurcation will be established considering \( \alpha \) as the bifurcation parameter.
Theorem 6
The model system (1) experiences transcritical bifurcation when the model parameter \(u_2\) passes through the critical value \( u_2=u_2^{[TC]} \) if \(\dfrac{u_2^2(\mu _1 + c u_3)^4 }{\sigma ^2 \omega _1^2} \ne \dfrac{(\mu _1 + c u_3)}{\omega _1(\mu + \mu _1 + \alpha \omega )} + \dfrac{\beta (\mu + u_1)}{(\mu + \mu _1 + \alpha \omega )^3}\).
Proof
To establish the above theorem, we have to verify transversality condition of Sotomayor’s theorem [39] for transcritical bifurcation at disease-free equilibrium point \(E_{0}\). We investigate the bifurcation with respect to the model parameter \(u_{2}\). Let \(f(S, I, R, V_S, V_I)=(f_1, f_2, f_3, f_4, f_5)^T\) where
One of the characteristic roots of \(J(E_0)\) is zero for \(u_2 = u_2^{[TC]}\). Let \(V = (v_1 \ v_2 \ v_3 \ v_4 \ v_5)^T\) and \(W = (w_1 \ w_2 \ w_3 \ w_4 \ w_5)^T\) be eigenvectors corresponding to the zero eigenvalue of the matrices \(J(E_0)_{u_2^{[TC]}}\) and \(J^T(E_0)_{u_2^{[TC]}}\), respectively, where \(v_1 = - \dfrac{\beta S_0}{(1+\alpha S_0)(\mu + u_1)},\) \(v_2 = \dfrac{c u_3 + \mu _1}{\sigma V_{S_0}},\) \(v_3 = \dfrac{\gamma (c u_3 + \mu _1)}{\mu \sigma V_{S_0}} - \dfrac{\beta S_0 u_1}{\mu (1+\alpha S_0)(\mu + u_1)},\) \(v_4 = -1,\) \(v_5 = 1\) and \(w_1 = 0,\) \(w_2 = \dfrac{\sigma }{(\mu + d + \gamma )},\) \(w_3 = 0,\) \(w_4 = 0,\) \(w_5 = 1\).
Since for this system
if the condition stated in the theorem is satisfied.
Therefore, all conditions of Sotomayor’s theorem for transcritical bifurcation are satisfied, and hence, Sotomayor’s theorem ensures the proposed system experiences transcritical bifurcation at the disease-free equilibrium point \(E_0\). \(\square \)
Transcritical bifurcation diagram with respect to \(u_2\) with \(\omega =1.95\), \(\beta =0.8\), \(\alpha =2.01\), \(\mu =0.1\), \(\mu _1=0.01\), \(d=0.19\), \(\gamma =0.15\), \(b=0.001\), \(a=1.9\), \(\omega _1=5.2\), \(u_3=0.2\), \(c=0.9\), \(\sigma =0.2\), \( \mu _2=0.2\); the blue and red lines correspond the stable equilibrium and the unstable equilibrium, respectively. (Color figure online)
To interpret the transcritical bifurcation numerically, we have drawn the stable–unstable branches of the solution curve with respect to \(u_2\) (see Fig. 2). It is clear from Fig. 2 that for \( u_2 > u_2^{[TC]}=1.2 \) the disease-free equilibrium point is stable and for \(u_2 < u_2^{[TC]}\) the disease-free equilibrium point becomes unstable through creation of stable endemic equilibrium. Therefore, the system exchanges the stability when \( u_2 \) crosses the critical value \( u_2 = u_2^{[TC]} \). Biologically the above result has high importance, because there is a critical value of the treatment control parameter above which the disease will eradicate from the system.
Next we study backward bifurcation of the proposed model (1) at \( R_0 = 1 \) with respect to bifurcation parameter \(\alpha \). Suppose \( R_0 = 1 \) occurs at \(\alpha =\alpha ^* \) where \(\alpha ^* = \dfrac{1}{\omega }\left\{ \dfrac{\sigma \beta \omega \omega _1}{(c u_3 + \mu _1)^2 (\mu + d + \gamma + a u_2)} - (\mu + u_1) \right\} >0 \) when other parameters are fixed.
Theorem 7
The model system (1) undergoes through backward bifurcation at \( R_0 = 1 \) ( equivalently for the critical value of the bifurcation parameter \(\alpha = \alpha ^*\)) if \(\dfrac{a b u2^2 (c u_3^2 + \mu _1)^2}{\sigma ^2 S_0 (\mu + d + \gamma )} > \dfrac{\beta ^2 \sigma S_0^2 V_{S_0}}{(1+\alpha S_0)^3 (\mu + \mu _1) (\mu + d + \gamma )} + \dfrac{c u_3 + \mu _1}{V_{S_0}}. \)
Proof
Here Castillo–Chavez and Song’s theorem [40] is used to find the condition for backward bifurcation of model system (1). Again consider the function \(f(S, I, R, V_S, V_I)\) which is already defined explicitly in the previous theorem. For critical value of the bifurcation parameter \(\alpha = \alpha ^*\) ( which is equivalent to \(R_0 = 1\)) \( J(E_0) \) has one zero eigenvalue. Let \( W = (w_1 \ w_2 \ w_3 \ w_4 \ w_5)^T \) be the right eigen vector of the Jacobian matrix \( J(E_0) \) corresponding to zero eigenvalue where \( w_1 = - \dfrac{\beta S_0}{(1+\alpha S_0)(\mu + u_1)},\) \(w_2 = \dfrac{c u_3 + \mu _1}{\sigma V_{S_0}},\) \(w_3 = \dfrac{\gamma (c u_3 + \mu _1)}{\mu \sigma V_{S_0}} - \dfrac{\beta S_0 u_1}{\mu (1+\alpha S_0)(\mu + u_1)},\) \(w_4 = -1,\) \(w_5 = 1\). Also, let \(V = (v_1 \ v_2 \ v_3 \ v_4 \ v_5)\) be the left eigenvector of the Jacobian matrix \(J(E_0)\) corresponding to zero eigen value where \(v_1 = 0,\) \(v_2 = \dfrac{\sigma }{(\mu + d + \gamma )},\) \(v_3 = 0,\) \(v_4 = 0,\) \(v_5 = 1\). To use Castillo–Chavez and Song’s theorem [40], we need to find bifurcation coefficients \(\psi \) and \(\phi \) which are given by \(\psi = \varSigma _{k,i,j = 1}^5 v_k w_i w_j \dfrac{\partial ^2 f_k}{\partial x_i \ \partial x_j}=\dfrac{2 a b u2^2 (c u_3^2 + \mu _1)^2}{\sigma ^2 S_0 (\mu + d + \gamma )} - \dfrac{2 \beta ^2 \sigma S_0^2 V_{S_0}}{(1+\alpha S_0)^3 (\mu + \mu _1) (\mu + d + \gamma )} + \dfrac{2 c u_3 + \mu _1}{V_{S_0}} \) and \(\phi = \varSigma _{k,i = 1}^5 v_k w_i \dfrac{\partial ^2 f_k}{\partial x_i \ \partial \alpha }=\dfrac{\beta \sigma S_0 V_{S_0}}{(\mu + d + \gamma ) (1 + \alpha S_0)^2} > 0.\)
Now model system (1) undergoes through backward bifurcation at \(R_0 = 1 \) with respect to bifurcation parameter \(\alpha = \alpha ^*\) if \(\psi > 0\), i.e., \(\dfrac{a b u_2^2 (c u_3^2 + \mu _1)^2}{\sigma ^2 S_0 (\mu + d + \gamma )} > \dfrac{\beta ^2 \sigma S_0^2 V_{S_0}}{(1+\alpha S_0)^3 (\mu + \mu _1) (\mu + d + \gamma )} + \dfrac{c u_3 + \mu _1}{V_{S_0}}.\)
Hence the theorem is proved. \(\square \)
Backward bifurcation diagram with respect to \(R_0 (\alpha ) \) with \(\omega = 2.9\), \(\beta = 0.9\), \(\mu = 0.13\), \(\mu _1 = 0.03\), \(d = 0.0109\), \(\gamma = 0.15\), \(a = 60\), \(\omega _1 = 5.2\), \(u_3 = 0.2\), \(c = 0.9\), \(\sigma = 0.23\), \(\mu _2 = 0.2\), \(\alpha = 1.6\), \(u_2 = 0.75\), \(b = 30\)
In Fig. 3, we have presented the one-parameter bifurcation diagram with respect to \( R_0\). In figure, the blue line corresponds to the stable branch of the solution curve and the red line corresponds to the unstable branch. It is clear from Fig. 3 that for \( R_0 < 1 \) disease-free equilibrium point is stable and for \(R_0 > 1\) disease-free equilibrium point is unstable. For \( R_0{^*}< R_0 < 1\), two endemic equilibrium points exist, endemic equilibrium point with lower density infected is unstable and endemic equilibrium point with higher density infected is stable where \(R_0{^*}\) is a critical value of \(R_0\). Biologically this bifurcation is important because for \( R_0{^*}< R_0<1 \) disease may persists in the system depending on initial population density. On the other hand, there is a critical value of the inhibitory factor above which disease will eradicate from the system and below which eradication of disease depends on initial population size.
7 Model validation and parameter estimation
In this section, we shall validate the proposed model with real reported data. To fit the model, we consider the real data of dengue outbreak in Singapore from 18th week of 2014 to 1st week of 2015 [41]. To fit the proposed model with real reported data, we have used MATLAB minimization software package fmincon [38] and estimated best-fitted model parameters. To obtain best-fitted model parameters, we have minimized the sum of squares error (SSE), which is defined as \( SSE = \sum _{j = 1}^{n}\left( Y_j - I(t_j) \right) ^2\) where \(Y_j\) is the cumulative number of real reported data of jth week and \(I(t_j)\) is model predicted cumulative infected density of the same week. By finding residuals of the data fit, we can verify about the fitness of the model. If the residuals \(\left\{ Y_j - I(t_j) | j= 1,2,...,n \right\} \) are randomly distributed, then we can say that model is well fitted.
To perform the data fit process, we consider initial number of susceptible, infected and recovered human population are 5525628, 251, 0, respectively, whereas initial number of susceptible and infected mosquitoes is 200000 and 1500, respectively. Best-fitted curve of cumulative infected data, residuals and bar diagram of new infection per week are shown in Fig. 4. From the figures, it is clear that our model fitting with real reported data is reasonably good. Among fifteen model parameters, we have estimated twelve parameters and remaining model parameters are taken from [42]. We have summarized estimated values of all model parameters in Table 2.
8 Sensitivity analysis
Since the proposed model contains many parameters among them some of the model parameters are highly sensitive on \(R_0.\) Sensitivity analysis is performed to find out the model parameters, which have most significant affect on \(R_0.\) To perform sensitivity analysis, we apply normalized forward sensitivity index method [43, 44]. The normalized forward sensitivity index method of \( R_0 \) with respect to model parameter \( \phi \) is defined by \( \varLambda _{\phi }^{R_0} = \dfrac{\partial R_0}{\partial \phi } \dfrac{\phi }{R_0} \). Using this method, one can identify the model parameters, which have positive or negative impact on basic reproduction number. The basic reproduction number shows same behavior with the model parameters for positive sensitivity index parameter and opposite behavior for negative sensitivity index parameter. In Table 3, we have enlisted the sensitivity indexes of the model parameters. It is clear from Table 3, \(\omega \), \(\omega _1\), \(\beta \), \(\sigma \) have positive-sensitive indexes and c, \(u_3\), \(u_1\), \(\gamma \) have negative indexes.
9 Effect of different model parameters on disease spreading
In this section, we have studied the effect of highly sensitive model parameters on the disease dynamics. For this purpose, we have found abundance of infected humans changes with increasing or decreasing values of model parameters considering base value of the parameters as given in Table 2. Here we have studied the effect of four state parameters, namely \(\beta ,\) \(\sigma ,\) \(\gamma ,\) c and two control parameters \(u_2\), \(u_3.\) In the following subsections first we discuss the effect of \(\beta \) and \(\sigma \) on disease dynamics.
9.1 Effect of \(\beta \) and \(\sigma \) on disease spreading
In Fig. 5, we have presented the density of infected humans for different values of \(\beta \) and \(\sigma \). We have observed from Fig. 5a that number of infected humans increases when disease transmission rate from infected mosquitoes to susceptible humans increases and vice versa. Similarly from Fig. 5b we have seen that the number of infected humans also increases when disease transmission rate from infected humans to susceptible mosquitoes increases. Thus, the number of infected humans increases with the increase of \(\beta \) and \(\sigma \) both and vice versa. We can conclude that to control the density of infected humans, i.e., spreading of disease, we have to take policy such that \(\beta \) and \(\sigma \) both decrease. The value of \(\beta \) and \(\sigma \) will decrease if we can minimize the interaction among the humans and mosquitoes. The said interaction can be minimized if we isolate the humans from mosquitoes. The isolation can be done using mosquito nets, wearing long-covered clothes, etc.
9.2 Effect of auto immune rate \((\gamma )\) and mosquito killing efficiency (c)
In Fig. 6, we have presented the time series of infected humans for different values of \(\gamma \) and c. We have seen from Fig. 6a that the number of infected humans decreases with increase of \(\gamma \). Also from Fig. 6b we have seen that the number of infected humans decreases with the increase of c. Therefore, number of infected humans decreases with the increase of both \(\gamma \), c and vice versa. This result is biologically important because the increase of \(\gamma \) and c will decrease the density of infected humans but increase of \(\gamma \) can be done taking healthy foods, proper physical exercise and increase of c can be performed by destroying mosquito larvae, killing adult mosquitoes, cleaning dirty water, increasing mortality of mosquito’s eggs, etc.
9.3 Effect of treatment control parameter \((u_2)\) and mosquitoes killing effort control parameter \((u_3)\)
In Fig. 7, we have shown density of infected humans for different values of \(u_2\) and \(u_3\). It is clear from Fig. 7a that the number of infected humans decreases with increase of \(u_2\) and vice versa. Similarly, from Fig. 7b it is clear that the number of infected humans decreases or increases with the increase or decrease of \(u_3\), respectively. Therefore, with increase in both the control parameters \(u_2\) and \(u_3\), the number of infected humans decreases and vice versa. Biologically disease transmission can be reduced taking proper treatment as well as using adulticide for killing adult mosquito, larvicide for increasing mortality of eggs, destroying mosquitoes larvae, etc.
10 Estimation of actual and effective reproduction number
In this section, we shall estimate \( R_0 \) from actual data and estimate the values of effective reproduction number from actual data of Dengue outbreak in Singapore 2014.
10.1 Estimation of actual \( R_0 \) for dengue outbreak
There are several analytical as well as statistical methods for estimating \(R_0\) from the actual data for infectious disease. To estimate \( R_0 \) from the initial growth phase of the disease [45], we assume that at early stage of the disease, the number of cumulative cases (Q(t)) varies as exponentially with force of infection \((\varLambda )\) which can be expressed mathematically as \(Q(t) \propto \mathrm{exp}(\varLambda t).\) Similarly number of infected humans, infected mosquitoes vary as \(\mathrm{exp}(\varLambda t)\). So we have
where \(I_0\) and \(V_{I_0}\) are constants. Here we assume that both susceptible populations remain constant and they are given by \(S_0 = \dfrac{\omega }{\mu + u_1}\) and \(V_{S_0} = \dfrac{\omega _1}{\mu _1 + c u_3}\). Substituting (7) in second and fifth equation of equation (1) and putting \(b = 0\), we get
and
where \(\zeta _1 = \mu + d + \gamma + a u_2\) and \(\zeta _2 = \mu _1 + c u_3.\) From Eqs. (8) and (9), we get
Putting the value of \(\beta \sigma \) from the above relation in expression of \( R_0 \), we obtain
Now first we estimate the force of infection (\(\varLambda \)) and then we estimate basic reproduction number \(R_0\). The relation between new number of cases per day (q(t)) with cumulative cases per day (Q(t)) as follows \(q(t) \approx \varLambda Q(t)\).
To obtain the estimation of the force of the infection \((\varLambda )\), we maintain the following steps one by one. First we plot new number of cases along \( y- \) axis and cumulative number of cases along x-axis. Then, from scatter diagram we obtain threshold cumulative values up to which it shows exponential growth. Next using least-square method, we fit a linear regression curve based on the collected exponential growth data [46]. The slope of the regression line is force of infection \((\varLambda )\).
We obtain from Fig. 8b \(\varLambda = 0.1506101534251 \pm 0.0189325690942 \) \(\mathrm{day}^{-1}\). Putting the values of \( \varLambda \) in (10) and using other parameters from Table 2, we obtain the estimated value of basic reproduction number as \(R_0 = 2.218450740\) with lower and upper limit as 2.082235564 and 2.352455166, respectively.
10.2 Effective reproduction number R(t)
In mathematical epidemiology, basic reproduction number plays a crucial role to control or eradicate the infection from the community. \(R_0\) is defined as the average number of secondary infection produced by a single infective in its entire life span as an infected host, i.e., it is a constant quantity. But in reality initially disease spreads rapidly in higher rate among population and after reaching its maximum limit position, starts to decrease that means basic reproduction number \(R_0\) is not always constant. In this section, we study time-varying reproduction number that means reproduction number per week. This type of time-varying reproduction number is known as effective reproduction number and is denoted by R(t) [47,48,49]. Based on the values of effective reproduction number R(t), researchers can predict about influence of the disease among the population and can give idea about the useful control or preventive measures to decrease the invade of the infection. For estimating effective reproduction number R(t) from per week wise infection curve of dengue outbreak data, we use the formula as follows
where b(t) denotes new number of cases at t th week and \(g(\lambda )\) represents generation interval distribution of the disease. Let the rate of leaving infected humans, infected mosquitoes be represented by \(c_1 = \mu + d + \gamma + a u_2,\) \(c_2 = c u_3 + \mu _1\), respectively, and generation interval distribution is the combination of \(c_1 e^{- c_1 t}\), \(c_2 e^{- c_2 t}\) then explicit formula is given by
The above expression is valid when it satisfies the condition \(\varLambda > \mathrm{min}\left\{ - c_1, - c_2\right\} \), and mean of the above distribution is given by \(T = \dfrac{1}{c_1} + \dfrac{1}{c_2}\). Using daily new cases of dengue data and formula (11), we can estimate effective reproduction number R(t) using Eq. (12).
We have calculated effective reproduction number estimating model parameters, and the corresponding figure of effective reproduction number is presented in Fig. 9. It is clear from Fig. 9 that values of effective reproduction number oscillate and it lies near about unity except few weeks. It is also clear from the figures that value of effective reproduction number is dropped from 4.291 to 0.6942.
11 Application of optimal control technique to the proposed model
In this section, we shall apply Pontryagin’s maximum principle varying the control parameter to obtain the optimal path of the control strategies. The main objective to formulate an optimal control problem is to minimize the infected human populations as well as infected mosquitoes and also to minimize cost applied for controls [30]. There are three types of control strategies, those are: control \(u_1\) represents vaccination applied for susceptible humans, control \(u_2\) represents treatment for infected human populations and control \(u_3\) represents mosquitoes killing effort by humans. In the previous sections, we have studied the proposed model considering \(u_1, u_2, u_3\) as constants but here we are considering each of these parameters as time dependent.
11.1 Formulation of optimal control problem
To formulate optimal control problem, we consider control strategies as a function of time t. Since if applied controls are constant then implementation cost may be very high. So we have to change the control policies as time changes such that the implementation cost becomes minimum with minimum number of infected humans. Now we reformulate model system (1) in the following form:
where
and integral of cost functional is given by L(x, u) = \(A_1 S + A_2 I + A_3 V_S + A_4 V_I + \dfrac{1}{2} B_1 u_1^2 + \dfrac{1}{2} B_2 u_2^2 + \dfrac{1}{2} B_3 u_3^2\), which is also known as the Lagrangian of the optimal control problem (13). In the expression of L(t, x(t), u(t)) , the constants \( A_1(> 0),\) \(A_2(> 0) \) represent per capita loss for presence of susceptible humans and infected humans, respectively, whereas \( A_3(> 0),\) \(A_4(> 0) \) represent per capita loss for presence of susceptible mosquitoes and infected mosquitoes, respectively. \(B_1, B_2, B_3\) represent weighted cost for applying controls \(u_1(t),\) \(u_2(t),\) \(u_3(t)\), respectively. The control variables are Lebesgue-measurable function and are given as below,
11.2 Existence and Uniqueness of optimal control problem
Theorem 8
The optimal control problem (13) subject to condition Eq. (1) admits optimal control variables \(u_1^*(t),\) \(u_2^*(t)\) and \(u_3^*(t)\) such that \(M(u_1^{*}(t), u_2^{*}(t), u_3^{*}(t)) = min \left\{ M(u_1(t),\right. \left. u_2(t), u_3(t)): (u_1, u_2, u_3)\in U \right\} \) where U is defined in (14).
Proof
The control variables and state variables both are non-empty and nonnegative. The control constraint set U is convex set.
Adding all equation of model (1), we get

Therefore, \(N(t) \le \dfrac{k}{\mu ^{'}} + \left( N_0 - \dfrac{k}{\mu ^{'}} \right) e^{- \mu ^{'}t}\), i.e., \(N(t) \longrightarrow \dfrac{k}{\mu ^{'}} \ as \ t \longrightarrow \infty \), which implies all state variables \(S, I, R, V_S, V_I\) are bounded.
At the same time, integrand of the objective functional \(M(u_1(t), u_2(t), u_3(t))\) is convex set with respect to \(u_1, u_2 \,\) and \( \, u_3 \).
Again system (1) can be written in the following form:
Therefore,
i.e. \(|| G(\phi _1) - G(\phi _2) || < B || \phi _1 - \phi _2 || \ where \ B= max \left\{ \frac{2 k\beta }{\mu '}, \frac{2 k\sigma }{\mu '} \right\} \).
If we denote \(F(\phi )=E \phi + G(\phi )\), then \(||F(\phi _1) - F(\phi _2) || \le || E|| || \phi _1 - \phi _2 || + B || \phi _1 - \phi _2 || \le C || \phi _1 - \phi _2 || \ where\ \left( || E || + B \right) \le C < \infty \). So all state variables satisfy Lipschitz condition. Therefore, there exist optimal control variables \(u_1^*(t),\) \(u_2^*(t)\) and \(u_3^*(t)\) such that
Hence the theorem is proved. \(\square \)
11.3 Characterization of optimal control problem
Theorem 9
For optimal control variables \(u_1^*(t),\) \(u_2^*(t)\), \(u_3^*(t)\) and state variables of Eq. (1) which minimizes \(M(u_1(t), u_2(t), u_3(t))\) over U, adjoint variables \(\lambda _1,\) \(\lambda _2,\) \(\lambda _3,\lambda _4\) and \(\lambda _5\) satisfying
with \(\lambda _i(T) = 0, i = 1,2,3,4,5\) and corresponding control variables \(u^*(t) = (u_1{^*}(t), u_2{^*}(t), u_3{^*}(t))\) is as follows
where \({\overline{u}}_{2}\) is nonnegative root of \(B_2 u_2 ( 1 + b u_2 I)^2 = a I(\lambda _2 - \lambda _3).\)
Proof
Pontryagin’s maximum principle is used to characterize the optimal control problem of the proposed model [50,51,52,53]. The Hamiltonian of the optimal control problem is given by:
The adjoint equations can be found using Pontryagin’s maximum principle which satisfies the following relations:
\(\dfrac{\mathrm{d} \lambda _1(t)}{\mathrm{d}t} = - \dfrac{\partial H}{\partial S},\) \(\dfrac{\mathrm{d} \lambda _2(t)}{\mathrm{d}t} = - \dfrac{\partial H}{\partial I},\) \(\dfrac{\mathrm{d} \lambda _3(t)}{\mathrm{d}t} = - \dfrac{\partial H}{\partial R},\) \(\dfrac{\mathrm{d} \lambda _4(t)}{\mathrm{d}t} = - \dfrac{\partial H}{\partial V_S},\) \(\dfrac{\mathrm{d} \lambda _5(t)}{\mathrm{d}t} = - \dfrac{\partial H}{\partial V_I}\) with \(\lambda _i(T) = 0, i = 1, 2, 3, 4, 5.\)
Using above relations, we get
with
Using optimality conditions \(\dfrac{\partial H}{\partial u_1} = 0,\) \(\dfrac{\partial H}{\partial u_2} = 0 \) and \(\dfrac{\partial H}{\partial u_3} = 0\) we get \(u_1 = u_1^*\), \(u_2 = u_2^*\) and \(u_3 = u_3^*\) where \(u^*\) is defined in the statement of the theorem.
Now we have \(\dfrac{\partial ^2 H}{\partial u_1^2} = B_1 >0,\) \( \begin{vmatrix} \dfrac{\partial ^2 H}{\partial u_1^2}&\dfrac{\partial ^2 H}{\partial u_1 \partial u_2} \\ \dfrac{\partial ^2 H}{\partial u_2 \partial u_1}&\dfrac{\partial ^2 H}{\partial u_2^2} \end{vmatrix} = B_1 B_2 > 0 \) and \( \begin{vmatrix} \dfrac{\partial ^2 H}{\partial u_1^2}&\dfrac{\partial ^2 H}{\partial u_1 \partial u_2}&\dfrac{\partial ^2 H}{\partial u_1 \partial u_3} \\ \dfrac{\partial ^2 H}{\partial u_2 \partial u_1}&\dfrac{\partial ^2 H}{\partial u_2^2}&\dfrac{\partial ^2 H}{\partial u_2 \partial u_3} \\ \dfrac{\partial ^2 H}{\partial u_3 \partial u_1}&\dfrac{\partial ^2 H}{\partial u_3 \partial u_2}&\dfrac{\partial ^2 H}{\partial u_3^2} \end{vmatrix} = B_1 B_2 B_3 > 0. \) Thus the minimality condition for H is satisfied at \(u^* = (u_1{^*}, u_2{^*},u_3{^*})\). \(\square \)
To solve optimal control problem numerically, we use forward–backward sweep method. In this method, forward application of fourth-order Runge–Kutta method of system (1) is combined with backward application of fourth-order Runge–Kutta method of system (15) with transversality condition (16). To draw the problem numerically, we consider time interval [0, 36], i.e., after 36 weeks all the controls are terminated automatically. To solve the control effect, we consider values of model parameters as in Table 2 and the cost coefficients \(A_1 = 0.00015\), \(A_2 = 0.2\), \(A_3 = 0.000015\), \(A_4 = 0.000131\), \(B_1 = 0.01\), \(B_2 = 0.3\), \(B_3 = 0.1\) satisfying \(S(0) = 5525628\), \(I(0) = 251,\) \(R(0)= 0,\) \(V_S = 200000\), \(V_I = 1500.\) In Fig. 10 , the blue line and the red line represent time series of infected components with control and without control, respectively. From Fig. 10a, b, it is clear that number of infected decreases when controls are used. In Fig. 10c–e, time series of all controls are represented.
12 Efficiency analysis
In this section, our target is to find out best effective control strategy using efficiency analysis. In the proposed model, three types of control strategies are used. Among three controls always \(u_2\) control is used since treatment is more essential to get recovery from the dengue disease. Therefore, two cases may arise (i) Strategy 1: \(u_1 \ne 0,\) \(u_2 \ne 0,\) \(u_3 = 0\) (ii) Strategy 2: \(u_1 = 0,\) \(u_2 \ne 0,\) \(u_3 \ne 0\). Among these two cases which strategy is better to control dengue outbreak, to know this we need to apply efficiency analysis. The efficiency index (E. I.) is defined by E. I. = \(\left( 1 - \dfrac{A^c}{A^o}\right) \times 100\), where \(A^c\) and \(A^o\) are number of cumulative infected humans with and without control, respectively. We find the value of \(A^o\) and \(A^c\) using Simpson’s \(\dfrac{1}{3}\)rd rule where \(A^o = \int _0^{36} I(t) \mathrm{d}t = 101.8026\). The values of \(A^c\) and efficiency index (E.I.) are given in Table 4 for different strategies. The strategy with highest number efficiency index (E.I.) is the best strategy [50, 52, 54]. In our problem, the best efficient policy is the second strategy. In epidemiological point of view, mosquitoes killing effort by humans with taking treatment for infected humans is the best efficient policy among the two strategies.
13 Conclusion
In this study, we have proposed a single-strain dengue model with saturated type incidence rate. To get recovery from mosquitoes bites, two types of recovery functions have been introduced for human populations, namely vaccination for susceptible populations and saturated treatment for infected populations. Three types of control functions are considered, namely vaccination for susceptible populations, treatment for infected populations and mosquitoes killing effort to recover from dengue infection. We have proved that disease-free equilibrium point is stable for \(R_0 < 1\) and unstable for \(R_0 > 1\). Similarly we have shown that endemic equilibrium point is stable under some conditions. Using Sotomayor’s theorem, we have proved that model system undergoes through transcritical bifurcation about disease-free equilibrium point when model parameter \(u_2\) passes through its critical value \(u_2^{[TC]}\). This result is biologically significant because below the critical value of the treatment parameter (i.e., \(u_2 <u_2^{[TC]}\)) disease will persist in the system. Also we have shown using Castillo–Chavez and Song’s theorem that model system has backward bifurcation at \(R_0 = 1\). We have observed that for \(R_0^{*}< R_0 < 1\) two endemic equilibrium points exist, one with smaller infected is unstable and other one with higher infected is stable where \(R_0^{*}\) is critical value of \(R_0\). Biologically we can conclude that eradication of disease depends not only on model parameter but also on initial population density.
We have fitted model with real reported data of dengue outbreak in Singapore from 18th week, 2014 to 1st week, 2015 to check the validity of the proposed model and estimated model parameters. To identify the highly effective model parameter, sensitivity analysis has been performed; these parameters need to control to reduce the disease spreading.
To find the suitable path for control parameters, we have used optimal control policy which will minimize the implementation cost with minimum number of infected populations. Numerically we have shown the positive impact of the different controls for eradicating dengue transmission. Using efficiency analysis, we have found that the best effective control policy is use of treatment for infected humans and mosquitoes killing effort simultaneously. Thus from this work we can conclude that spreading of dengue can be controlled using proper preventive actions.
Code Availability
Not applicable.
Data Availability
Data of this study will be made available from the corresponding author on reasonable request.
References
World Health Organization (2014) Dengue and severe dengue. Technical report, Regional Office for the Eastern Mediterranean
Kularatne S (2015) Dengue fever. Bmj 351:h4661
Gebhard L, Filomatori CV, Gamarnik AV (2011) Functional rna elements in the dengue virus genome. Viruses 3(9):1739–1756
Mustafa MS, Rasotgi V, Jain Gupta S (2015) Discovery of fifth serotype ofdengue virus (denv-5): a new public health dilemma in dengue control. Med J Armed Forces India 71(1):67–70
World Health Organization (2016) Dengue and severe dengue fact sheet, Geneva, Switzerland, Available at https://www.who.int/mediacentre/factsheets/fs117/en
Kuno G (2015) A re-examination of the history of etiologic confusion between dengue and chikungunya. PLoS Negl Trop Dis 9(11):e0004101
Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11(3):480–496
Brady OJ, Gething PW, Bhatt S, Messina JP, Brown-stein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay S (2012) Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6(8)
Reyes AA, Escaner JML (2018) Dengue in the philippines: model and analysis of parameters affecting transmission. J Biol Dyn 12(1):894–912
Dhanoa A, Hassan SS, Ngim CF, Lau CF, Chan TS, Adnan NAA, Han Eng WW, MingGan H, Rajasekaram G (2016) Impact of dengue virus (denv) co-infection on clinical manifestations, disease severity and laboratory parameters. BMC Infect Dis 16(1):406
Zheng T, Nie L (2018) Modelling the transmission dynamics of two-strain dengue in the presence awareness and vector control. J Theor Biol 443:82–91
Rigau-Perez JG (2006) Severe dengue: the need for new case definitions. Lancet Infect Dis 6(5):297–302
Organisation mondiale de la Sante, World Health Organization (2018) Dengue vaccine: Who position paper-september 2018-note de synthese de loms sur levaccin contre la dengue-septembre 2018. Week Epidemiol Rec Releve epidemiologique hebdomadaire 93(36):457–476
East S (2016) World’s first dengue fever vaccine launched in the philippines. CNN, Archived from the original on 18
Zachri E, Planasari S (2016) Dengue fever vaccine available in indonesia, WIB, October, 17
Bailey NTJ (1975) The mathematical theory of infectious diseases and its applications, Charles Griffin and Company Ltd, 5a Crendon Street, High Wycombe, Bucks HP13 6LE
Esteva L, Vargas C (1998) Analysis of a dengue disease transmission model. Math Biosci 150(2):131–151
Chanprasopchai P, Pongsumpun P, Tang M (2017) Effect of rain fall for the dynamical transmission model of the dengue disease in thailand, Computational and Mathematical Methods in Medicine
Derouich M, Boutayeb A, Twizell EH (2003) A model of dengue fever. BioMed Eng Online 2(1):4
Erickson RA, Presley SM, Allen LJS, Long KR, Cox SB (2010) A stage-structured, aedes albopictus population model. Eco-logical Model 221(9):1273–1282
Sriprom M, Barbazan P, Tang M (2007) Destabilizing effect of the host immune status on the sequential transmission dynamic of the dengue virus infection. Math Comput Model 45(9–10):1053–1066
Mishra A, Gakkhar S (2014) The effects of awareness and vector control on two strains dengue dynamics. Appl Math Comput 246:159–167
Abboubakar H, Kamgang JC, Nkamba LN (2018) Bifurcation thresholds and optimal control in transmission dynamics of arboviral diseases. J Math Biol 76:379–427
Supriatna AK, Nuraini N, Soewono E (2010) Mathematical models of dengue transmission and control: a survey. Encyclopedia Virol Res 431
Dwivedi A, Keval R (2021) Analysis for transmission of dengue disease with different class of human population. Epidemiol Methods 10(1):20200046
Jan R, Khan M, Gomez-Aguilar J (2020) Asymptomatic carriers in transmission dynamics of dengue with control interventions. Optim Control Appl Methods 41(2):430–447
Ghosh I, Tiwari PK, Chttopadhyay J (2019) Effect of active case finding on Dengue control: implication from a mathematical model. J Theor Biol 464:50–62
Zhu M, Xu Y (2019) A Time-Periodic dengue fever model in a heterogeneous environment. Math Comput Simul 155:115–129
Anggriani N, Tasman H, Ndii M, Supriatna A, Soewono E, Siregar E (2019) The effect of reinfection with the same serotype on dengue transmission dynamics. Appl Math Comput 349:62–80
Pontryagin LS, Boltyanskii VG, Gamkrelidze RV, Mishchenko EF (1962) The mathematical theory of optimal processes. Wiley, New Jersey
Khan Fatmawati M (2021) Dengue infection modeling and its optimal control analysis in East Java, Indonesia. Heliyon 7:e06023
Salcedo L, Vasilieva O, Svinin M (2020) Optimal control of dengue epidemic outbreaks under limited resoorces. Srud Appl Math 144(2):185–212
Bock W, Jayathunga Y (2019) Optimal control of a multi-patch dengue model under the influence of Wolbachia bacterium. Math Biosci 315:108219
Buonomo B, Marca R (2018) Optimal bed net use for a dengue disease model with mosquito seasonal pattern. Math Methods Appl Sci 41(2):573–592
Khatua A, Kar TK (2020) Dynamical behavior and control strategy of a dengue epidemic model. Eur Phys J Plus 135:643
Zhu D, Ren J, Zhu H (2018) Spatial-temporal basic reproduction number and dynamics for a dengue disease diffusion model. Math Methods Appl Sci 41(14):5388–5403
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease trans-mission. Math Biosci 180:29–48
Martcheva M (2015) An introduction to mathematical epidemiology. Springer, New York
Perko L (2000) Differential equations and dynamical systems, vol 7. Springer
Castillo-Chavez C, Song B (2004) Dynamical model of tuberculosis and their applications. Math Biosci Eng 1:361–404
Weekly number of dengue and dengue haemarrhagic fever cases, Ministry of Health, Singapore, data.gov.sg/dataset
www.statista.com \(>\) Economy and Politics \(>\) International \(>\) Singapore
Chitnis N, Hyman JM, Cushing JM (2008) Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull Math Biol 70:1272–1296
Kamrujjaman M, Saha P, Islam MS, Ghosh U (2022) Dynamics of SEIR model: a case study of COVID-19 in Italy. Results Control Optim. https://doi.org/10.1016/j.rico.2022.100119
Massad E, Coutinho FAB, Burattini MN, Amaku M (2010) Estimation of \(R_0\) from the initial phase of an outbreak of a vector-borne infection. Trop Med Int Health 15(1):120–126
Favier C (2006) Early determination of the reproduction number of vector-borne diseases: the case of dengue in Brazil. Trop Med Int Health 11:332–340
Sardar T, Rana S, Bhattacharya S, Khaled K, Chattopadhyay J (2015) A generic model for a single strain mosquito transmitted disease with memory on the host and the vector. Math Biosci 263:18–36
Wallinga J, Lipsitch M (2007) How generation intervals shape the relationship between growth rates and reproduction numbers. Proc R Soc B 274:599–604
Pinho S, Ferreira C, Esteva L, Barreto F, Silva V (2010) Modelling the dynamics of dengue real epidemics. Philos Trans R Soc A 368:5679–5692
Saha P, Ghosh U (2021) Global dynamics and control strategies of an epidemic model having logistic growth, non-monotone incidence with the impact of limited hospital beds. Nonlinear Dyn 105:971–996
Srivastav AK, Kumar A, Srivastava PK, Ghosh M (2021) Modeling and optimal control of dengue disease with screening and information. Eur Phys J Plus 136:1187
Saha P, Ghosh U (2022) Complex dynamics and control analysis of an epidemic model with non-monotone incidence and saturated treatment. Int J Dyn Control. https://doi.org/10.1007/s40435-022-009697
Saha S, Samanta GP (2022) Analysis of a host-vector dynamics of a dengue disease model with optimal vector control strategy. Math Comput Simul 195:31–55
Carvalho SA, Silva SO, Cunha IC (2015) Mathematical modeling of dengue epidemic: control methods and vaccination strategies. arXiv:1508.00961
Acknowledgements
We would like to thank all of the reviewers for their valuable comments and suggestions to improve the manuscript significantly. Pritam Saha would like to thank UGC, Government of India, for the financial assistance under Junior Research Fellowship Scheme(UGC Ref. No.: 1222/(CSIR-UGC NET JUNE 2019)) toward this research work.
Funding
UGC, Govt. of India fellowship through UGCJRF scheme.
Author information
Authors and Affiliations
Contributions
Authors have contributed equally in simulation, preparation of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this paper declare no conflicts of interest
Appendices
Appendices
1.1 Appendix I: Expressions of \(C_{i}\)
1.1.1 Appendix II: Expressions of \(F_{i}\)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saha, P., Sikdar, G.C. & Ghosh, U. Transmission dynamics and control strategy of single-strain dengue disease. Int. J. Dynam. Control 11, 1396–1414 (2023). https://doi.org/10.1007/s40435-022-01027-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40435-022-01027-y