Abstract
Machining of nickel alloys, particularly Monel 400 alloys, plays an important role in numerous applications of aircraft and marine industries. Machining of these alloys, with complicated shapes, through conventional machine tools is a cumbersome task. Therefore, an experimental research was made to investigate the travelling-wire electrochemical machining (TWECM) characteristics of Monel 400 alloys. TWECM has the benefit of negligible tool wear and stress-free machining. The improper removal of contaminants, mostly oxides on the specimen surface, reduces the performance of TWECM. In this direction, ozonated aqueous NaCl was used as an electrolyte in order to enhance the machining performance of TWECM. The predominant TWECM process parameters i.e. applied voltage (V), electrolyte concentration (EC) and electrolyte flow rate (U) were considered to investigate the performance measures of material removal rate (MRR) and surface roughness (R a). The microstructure of surface of the Monel 400 alloys specimen machined with TWECM was studied to understand the effect of electrolyte during the machining. Maximum of 90% increase in MRR was observed because of this new electrolyte. The surface roughness was also decreased considerably up to 32%. The contour plots were drawn to study the individual and interactive effect of process parameters on performance measures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
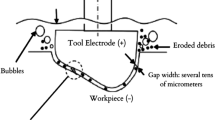
Travelling-wire electrochemical machining (TWECM) is a non-conventional machining technique capable of machining hard materials and complicated shapes. In TWECM, the work piece is the anode and the wire-tool is the cathode and the electrolyte is pumped through the gap between the tool and the work piece. The high-current and low-voltage DC supply is passed through the electrochemical cell for the anodic dissolution of the work piece. The machining is done according to Faraday’s law. The high-velocity jet of electrolyte pumps out the sludge (metal hydroxides) and some by-products (oxides and chlorides) generated during the machining. The TWECM process doesn’t make thermally influenced machining zones. It produces high surface quality products with the forceless machining. Its tool (cathode) is not affected by wear. Low fatigue value and stress-free components are produced in this machining. The experimental investigation on electrochemical machining characteristics of nickel alloys, particularly Monel 400 alloys is vital as these alloys are heavily used in the aircraft and marine applications. These alloys steels are also used as good corrosion resistant materials. Machining of these alloys using conventional machining techniques provides a high pressure to the tool, which shows the way to the use of TWECM for pressure-less machining. The rate of dissolution of the specimen in TWECM is highly dependent on the electrolytes. Many salts solutions i.e. NaCl, LiCl, KCl, MgCl2, CaCl2, and NaNO3 are used as an electrolyte in traditional ECM in the direction to improve the machining performance. The dissolution rates of mild steels in NaCl and NaClO3 salts were exhaustively studied [1, 2]. An experimental investigation was made on 100Cr6 steel specimen with an aqueous NaCl and NaNO3 electrolytes in electrochemical machining (ECM) [3]. The aqueous NaCl is preferred since NaNO3 is a passivating electrolyte. The anodic dissolution rate of hafnium was increased with water-isopropanol-glycerin chloride electrolyte [4]. The catalytic effect of hydroxyl ions accelerates the anodic dissolution rate [5]. Electro-thermochemically treated materials undergo high anodic dissolution rate [6]. Aqueous chloride with ethylene glycol was experimented as an electrolyte to study the anodic dissolution behavior of titanium [7]. The essential characteristics of aqueous solutions in electrochemical machining are conductivity, alkalinity, oxidizing power, the degree of ionization and solubility in solution. Any chance for an increase in conductivity of solution supports the growth of material removal rate (MRR). The oxidizing power of a solution must be greater to have high MRR. The tendency to form an insoluble electrochemical products film on the workpiece surface is a hindrance to efficient machining. This passive film behaves as an insulating layer that doesn’t allow ions to react with the anode. Therefore, MRR reduced significantly [8]. If the aqueous solution posses some oxidizing power, it can break the passive film on the surface. In this direction, the catalytic agents like potassium dichromate, hydrogen peroxide, and ferric nitrate mixed with aqueous NaCl electrolyte are also used as electrolytes for machining 20 MnCr5 alloy steels in ECM. As these chemicals enhanced conductance and oxidizing power, the sensible increase in MRR was observed [9–11]. The pressurized air in the electrolyte improves the accuracy and surface finish while sparking diminishes in inter-electrode gap [12]. Pressurized oxygen in the electrolyte can be used as an electrolyte in electrochemical machining [13]. The dissolved oxygen increases the oxidizing capability of the electrolyte solution. The more corrosive behavior of electrolyte in addition to electrochemical dissolution provides more scope for improvement in MRR. A fresh surface of workpiece forms a thin layer that contains a considerable amount of cations. The presence of activators and the enhanced supply of air oxygen support the formation of additional oxidant Fe3+ incase of Fe specimen, which can cause active corrosion [14]. The oxidizing agent, particularly ozone prevents the premature passivating layer formation. Lindner et al. patented the apparatus of ECM with ozone generator [15]. It made the authors to investigate the ozonated environment in TWECM for machining Monel 400 alloys. Kalaimathi et al. made some experimental analysis on electrochemical machining features of Monel 400 alloys in aqueous NaCl and NaNO3 electrolytes [16, 17]. This article elucidates the effect of ozonated aqueous NaCl solution on Monel 400 alloys in the TWECM in the direction of improving machining performance. Ozone is an outstanding oxidative chemical and finds its application in disinfection processes. It maintains high oxygen content in the aqueous systems and removes cyanides, iron and manganese residues, phenols and detergents. Ozonation leaves fewer potentially harmful residues, unlike chlorination. It is also used for sterilization and odor control in sewage treatment and manufacturing [18]. The ozonated aqueous NaCl solution as an electrolyte in TWECM is not reported in the literature. Therefore, the present work is aimed to study the TWECM characteristics of Monel 400 alloys on MRR and surface roughness (R a) with ozonated aqueous NaCl electrolyte. The response surface methodology (RSM) was used to model the TWECM process variables. Surface roughness (R a) is the measure of irregularities on the surface. The surface roughness of the machined components with TWECM is mainly affected by irregular oxides and chlorides deposited on the surface. The ozonated electrolyte is aimed to remove the accumulation on the surface by which better surface is obtained. Therefore, the measure of surface roughness (R a) is used in addition to MRR in this work. The RSM technique requires few runs to get an equivalent response which needs hours of run-time otherwise. Many researchers [9–11, 13, 17, 19] applied RSM in their research work as an effective design of experiments (DOE) technique. Central composite design (CCD) of RSM is preferred to Box-Behnken design as it does the high-quality design and analysis with less number of runs. Therefore, the present investigation was based on CCD of RSM.
2 Materials and methods
Schematic diagram of TWECM set-up attached with ozone generator is shown in Fig. 1. It comprises of a power supply system, ozone generator system, electrolyte supply, and filtering system, tool and tool feed mechanism, work holding position system and control panel.
Schematic diagram of travelling-wire electrochemical machining setup with ozone generator. 1 Ozone generator 2 Ozone and electrolyte mixing tank 3 Magnetic stirrer 4 Pump 5 Flow control valve 6 Pressure gauge 7 Flow meter 8 Pump 9 Ozonated electrolyte 10 Work holding vice 11 Work piece 12 Flow control valve 13 Wire holding panel 14 Wire guide roller 15 Wire tool 16 Low voltage high current DC supply 17 Ozonated electrolyte tank 18 Filter 19 Pump
Figure 2 shows the photograph of in-house fabricated TWECM setup. This structure consists of DC rectifier (20 V, 50 A), electrolytic tank, cutting panel, work holding vice, DC motors (12 V, 40 W), power circuit to control the movement of cutting panel and control knobs. The workpiece was fixed on vice using the fixture. The wire-tool and work piece are connected with positive and negative charges of DC rectifier. The wire is rolled along the pulleys to position above the workpiece and its travel speed is controlled by DC motor. The Z-axis movement is facilitated to provide the adequate wire feed rate and Y-axis movement is enabled to control the length of cut. The electrolyte mixture in the tank is pumped through the small gap between wire-tool and work piece. Ozone, used in various chemical applications, is known to be a strong oxidant and has a very short half-life. Ozone can easily decompose into secondary oxidants such as highly reactive hydroxyl and peroxyl radicals. These radicals are among the most reactive oxidizing elements and undergo fast, non-selective, free radical reactions with dissolved compounds. These hydroxyl radicals act on organic contaminants resulting in some peroxides, aldehydes, and hydrogen peroxide. Hydrogen peroxide presence also increases the MRR significantly [10]. Since ozone has a very short life when dissolved in water, it is generated in close proximity to the machining cell where it is consumed. For the production of ozone, corona discharge is used commonly because of the advantages of cost-efficient ozone production and the greater durability of the system. The ambient air is passed through an intense, high frequency alternating current electric field. The ozone generator used in this work is shown in Fig. 3 and its details are given in Table 1. This ozone generator is connected to the TWECM setup externally. Ozone is allowed to dissolve in an aqueous electrolyte by the additional equipment of magnetic stirrer.
The cutting was achieved by moving the tool wire against the workpiece in the aqueous electrolyte. All the experiments were carried on the Monel 400 alloys of size 20 × 20 × 15 mm. The chemical composition (weight %) of commercially obtained Monel 400 alloys is given in Table 2.
High wire diameter increases the contact surface area of the workpiece, which results in an increased MRR. However, if the wire diameter is increased to some specific value, it constrains the electrolyte flow in the inter-electrode gap (IEG) to result in insufficient electrolytic ions. Therefore, the wire diameter was optimized as 1.3 mm which produces better machining performance [20]. Hence, in this work, 1.3 mm diameter wire is used. MRR is directly proportionate to wire feed rate. Higher wire feed rates not only increase MRR but also surface roughness [20]. Hence, the wire feed rate is set to the possible minimum as 0.1 mm/min in this work. The higher applied voltage causes greater machining current in the machining gap, which increases MRR. Low voltage leads to poor machining performance and high voltage leads to burning out of wire. The voltage range of 11–16 V was considered in TWECM in previous works [21, 22]. Therefore, the voltage range (10–14 V) available in the setup is used. High concentration decreases the mobility of ions which results in poor anodic dissolution. It was reported that concentration levels from 100 g/L to 220 g/L influences MRR and surface roughness [16]. The travelling-wire speeds of 60–140 mm/min influences significantly [19]. Therefore, the travelling-wire speed of 80 mm/min is considered. The inter-electrode gap between wire-tool and workpiece is kept as 0.1 mm. The current of 10 A is set in DC rectifier. The machining was done for 10 min. Since the ozone is mixed with aqueous NaCl electrolyte, various flow rates of this ozonated aqueous NaCl are considered. The operating conditions of TWECM are summarized in Table 3. By considering the results of literature and pilot experiments, the TWECM process parameters i.e. voltage (V), electrolyte concentration (EC) and flow rate (U) were selected as significant control factors. MRR and Ra were taken as the performance measure.
Table 4 shows the actual and coded values of process parameters selected for the experiments. The Eq. (1) helps to compute the interval of parameters (ΔX i ) [19].
where, X i, max upper bound of parameters; X i, min lower bound of parameters; n number of levels.
In this work, MRR was measured based on the rate of weight loss during machining.
Weights of the specimen before machining and after machining were measured with the weighing machine with the least count of 1 mg. The surface roughness (R a) is measured with the MarTalk measuring device and its sample plot is shown in Fig. 4. The results are the average of the three measurements.
The central composite design and experimental results are shown in Table 5.
The electrolyte and water undergo ionic dissociation [23, 24] as the potential difference is applied between the work piece (anode) and the tool (cathode). The positive ions are attracted towards the tool and the negative ions are progressed towards the work piece. Metals in the surface would react with hydroxyl ions and form metal hydroxides. These metal hydroxides (sludge) get precipitated in the electrolyte solution.
3 Results and discussions
To characterize the current experiments of TWECM performances, regression models for the outputs of MRR and R a are developed based on Eq. (3).
The adequacy of regression models is tested by the method of analysis of variance (ANOVA) and the Fisher’s test (F-ratio). The probability values less than 0.05 suggest the significance of model and terms for the confidence interval of 95%. The insignificant terms are identified by the probability values greater than 0.1. ANOVA results of both MRR and Ra models for ozonated aqueous NaCl electrolyte are shown in Table 6.
The MRR model F-value of 4.71 implies that the model is significant. In this case V, V2, EC2 are significant model terms. The coefficient of determination (R 2) for the model is 0.81. Therefore, the developed model for MRR as in Eq. (4) proves to be adequate and can be used to predict the performances for further analysis.
The R a model F-value of 6.97 implies that the model is significant. In this model, V, U, V*EC, V*U are significant model terms. The coefficient of determination (R 2) for the model is 0.86. Thus, the developed model as in Eq. (5) is sufficient.
In Fig. 5, it is observed that MRR is higher with the ozonated aqueous NaCl solution for all experiments compared with aqueous NaCl. This is due to the efficient removal of sludge and oxides on the surface by O3 mixed electrolyte. Since the deposits are continuously removed, the specimen surface becomes fresh and more conductive. The metal sludge which is deposited on the specimen surface is removed completely by the process of ozonation [15]. The ozone (O3) often break into oxygen form which supports the removal of oxides on the surface of the specimen [10].
The ozone (O3) is decomposed into secondary oxidants of hydroxyl and peroxyl radicals. These radicals are among the most reactive oxidizing elements and undergo fast, non-selective, free radical reactions with dissolved compounds such as oxides and metal hydroxides. By this way, any form of sludge is further oxidized and is pumped out of the machining gap. This process helps to expose the fresh surface for efficient machining, which results in improved MRR. All the readings show the considerable improvement in MRR. Figure 6 shows that the surface roughness (R a) is reduced due to the ozonated aqueous NaCl electrolyte. Sludge and oxides deposition create the uneven surface finish. But the ozonated aqueous NaCl removes the contaminants on the surface resulted in better R a. Uniform reduction in R a is observed in all readings. Figure 7 portrays the influence of process parameters on MRR for ozonated aqueous NaCl electrolyte environment. The first contour plot shows the influence of voltage (V) and the electrolyte concentration (EC) on the MRR. The increase in voltage enhances the machining current in the inter-electrode gap, which increases the MRR. The second plot in Fig. 7 explains the effect of flow rate (U) and the voltage (V) on MRR. It is observed that the flow rate around 4–8 L/min produces good MRR. The given electrolyte concentration range is sufficient for producing good MRR. Third contour plot in Fig. 7 explicates the effect of EC and U on MRR at the voltage of 12 V. Since the voltage is not sufficient at this condition, the figure doesn’t show the promise in MRR. The MRR changes more with the change in voltage than a change in the cumulative effect of EC and U.
Figure 8 shows the scanning electron microscopy (SEM) surface structure of the specimen machined at the conditions of V = 12 V, EC = 150 g/L, U = 5.3 L/min using plain aqueous NaCl electrolyte. A lot of irregularities can be noted in the surface structure as oxides and hydroxides deposits available on the surface due to improper removal. These contaminants (oxides and hydroxides) must be properly removed to get the proper surface. The interactive effect of parameters on R a during the machining using ozonated aqueous NaCl is shown in Fig. 9. The first contour plot in Fig. 9 explains the effect of voltage and electrolyte concentration on surface roughness of machined components in ozonated aqueous NaCl environment while flow rate is 5.3 L/min. High voltage increases the metal dissolution rapidly which in turn increases the surface roughness. Higher flow rates support the removal of sludge on the surface to some extent which results in better R a. But these flow rates do not promise the efficient removal of oxides on the surface. The electrolyte concentration range of 130–180 g/L is just sufficient to make active dissolution but the variation in voltage and electrolyte flow rate make significant effect on R a, which could be seen from second and third contour plots of Fig. 9. The irregularities on the surface are taken away by the effect of ozonation to afford better finishes.
The energy dispersive X-ray spectrum (EDS) analysis in Fig. 10 shows the elements on the specimen in the particular focus area (300 µm). In EDS, the x-axis represents X-ray energy (keV) and the y-axis represents the number of counts per channel. It is shown in Table 7 that the chlorides and oxides are present significant level on the surface. This is the main reason for the poor MRR and Ra.
Figure 11 shows the SEM structure of machined specimen at the machining conditions of V = 12 V, EC = 150 g/L and U = 5.3 L/min for ozonated aqueous NaCl electrolyte. The presence of O3 influences more on the surface of the specimen. The increase in electrolyte conductivity enhances the anodic dissolution. The sludge and oxides on the metal surface are further oxidized by ozonation, which makes the work piece surface clean. Therefore, the efficient removal of sludge and oxides due to ozonation makes the surface clean, results in better R a. High voltage increases the machining current which facilitates the high MRR and also high R a. High flow rates remove the some deposited contaminants and make the surface uniform, which results in better R a (Fig. 12).
The addition of O3 removes the sludge and contaminants completely thereby reducing R a further. More contaminants on the machined surfaces can be noted with plain aqueous NaCl as shown in Fig. 8. But the surface in Fig. 11 is uniform and no deposits found on the surface. Considerable reduction in elements of oxides and chlorides can be noted in Fig. 12 and Table 8.
4 Conclusions
In this work, the ozonated aqueous NaCl electrolyte is used as an efficient electrolyte to increase the performance of travelling-wire electrochemical machining of Monel 400 alloys. This investigational analysis justified that the ozonated aqueous NaCl electrolyte along with TWECM process parameters influence the machining performances i.e. material removal rate (MRR) and surface roughness (R a) for Monel 400 alloys. Response surface methodology (RSM) was employed to analyze the ECM process. The MRR increase of 3 to 90% is noted in ozonated aqueous NaCl electrolyte due to the removal of oxides and sludge deposits on the specimen surface. The reason for the variation in MRR increase is due to the influence of other parameters i.e. flow rate of electrolyte and voltage in addition to the effect of ozonation. The surface roughness (R a) is decreased to the maximum of 32%. Ozone is decomposed into secondary oxidants such as highly reactive hydroxyl and peroxyl radicals. These radicals are among the most reactive oxidizing elements and undergo fast, non-selective, free radical reactions with dissolved compounds such as oxides and metal hydroxides. These reactions improve the MRR and reduce R a as the fresh surface of the specimen is exposed for better machining. The scanning electron microscopy and energy dispersive X-ray spectrum images confirm the better surface obtained due to the ozonated aqueous NaCl electrolyte. The developed regression equations are tested through the analysis of variance (ANOVA) and are proved adequate.
References
Mao KW (1973) Anodic polarization study of mild steel in NaCl solution during electrochemical machining. J Electrochem Soc 120(8):1056–1060
Mao KW, Chin DT (1974) Anodic behavior of mild steel in NaClO3 at high current densities. J Electrochem Soc 121(2):191–194
Haisch T, Mittemeijer EJ, Schultze JW (2001) Electrochemical machining of the steel 100Cr6 in aqueous NaCl and NaNO3 solutions: microstructure of surface films formed by carbides. Electrochim Acta 47(1–2):235–241
Lilin SA, Balmasov AV, Shmukler MV, Rumyantsev EM (2000) Anodic behavior of Hafnium in water–alcohol solutions of sodium chloride. Prot Met 36(3):228–231
Byk MV, Tkalenko DA, Tkalenko MD (2004) On participation of hydroxide ions in the anodic dissolution of metals in aqueous electrolyte solutions. Prot Met 40(3):294–296
Silkin SA, Pasinkovskii EA, Petrenko VI, Dikusar- AI (2008) High rate anodic dissolution in chloride solutions of steel after electrothermochemical treatment. Surf Eng Appl Electrochem 44(5):343–352
Fushimi K, Kondo H, Konno H (2009) Anodic dissolution of titanium in chloride-containing ethylene glycol solution. Electrochim Acta 55(1):258–264
Davis JR (2000) ‘Corrosion-understanding the basics’. ASM International, Russell, p 563
Ayyappan S, Sivakumar K (2015) Investigation of electrochemical machining characteristics of 20MnCr5 alloy steel using potassium dichromate mixed aqueous NaCl electrolyte and optimization of process parameters. Proc Inst Mech Eng B J Eng Manuf 229(11):1984–1996
Ayyappan S, Sivakumar K (2016) Enhancing the performance of electrochemical machining of 20MnCr5 alloy steel and optimization of process parameters by PSO-DF optimizer. Int J Adv Manuf Technol 82(9):2053–2064
Ayyappan S, Sivakumar K, Kalaimathi M (2015) Electrochemical machining of 20MnCr5 alloy steel with ferric nitrate mixed aqueous NaCl. Int J Mach Mach Mater 17(1):79–94
Ghabrial SR, Ebeid SJ (1981) Beneficial effect of air-electrolyte mixtures in stationary electrochemical machining. Precis Eng. 3(4):221–223
Ayyappan S, Sivakumar K (2014) Experimental investigation on the performance improvement of electrochemical machining process using oxygen-enriched electrolyte. Int J Adv Manuf Technol 75(1–4):479–487
Kunieda M, Mizugai K, Watanabe S, Shibuya N, Iwamoto N (2011) Electrochemical micromachining using flat electrolyte jet. Ann CIRP 60:251–254
Linder HJ, Ufer PP, Heck K, Schmoger G. “Method and apparatus for electro chemical machining”. 1985. US Patent 4504370
Kalaimathi M, Venkatachalam G, Sivakumar M (2014) Experimental investigations on the electrochemical machining characteristics of monel 400 alloys and optimization of process parameters. Jordan J Mech Ind Eng. 8(3):143–151
Kalaimathi M, Venkatachalam G, Makhijani N, Agrawal A, Sivakumar M (2014) Investigations on machining of monel 400 alloys using electrochemical machining with sodium nitrate as electrolyte. Appl Mech Mater 592–594:467–472
Andrews CC, Murphy OJ. ‘Generation and delivery device for ozone gas and ozone dissolved in water’. 2006. US Patent 6,984,304 B2
Kalaimathi M, Venkatachalam G, Sivakumar M. “An experimental investigation and modeling for Traveling wire electrochemical machining of Monel 400 alloys”. Int J Manuf Technol Manag. 2017. (In press)
El-Taweel TA, Gouda SA (2011) “Performance analysis of wire electrochemical turning process—RSM approach”. Int J Adv Manuf Technol 53:181–190
Qu N, Fang X, Li W, Zeng Y, Zhu D (2013) Wire electrochemical machining with axial electrolyte flushing for titanium alloy. Chin J Aeronaut 26(1):224–229
Qu NS, Ji HJ, Zeng YB (2014) Wire electrochemical machining using reciprocated traveling wire. Int J Adv Manuf Technol 72(5–8):677–683
Trasatti S (2009) “Oxygen evolution”, electro chemical theory, reference module in chemistry, molecular sciences and chemical engineering, encyclopedia of electrochemical power sources. Elsevier Inc, Amsterdam, pp 49–55
Trasatti S (2009) “Hydrogen evolution”, electro chemical theory, reference module in chemistry, molecular sciences and chemical engineering, encyclopedia of electrochemical power sources. Elsevier Inc, Amsterdam, pp 41–49
Acknowledgement
The authors would like to acknowledge the support rendered by the Department of Science and Technology, Government of India sponsored SEM and EDS testing facility at VIT University, Vellore-632014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Technical Editor: Márcio Bacci da Silva.
Rights and permissions
About this article
Cite this article
Kalaimathi, M., Venkatachalam, G., Sivakumar, M. et al. Experimental investigation on the suitability of ozonated electrolyte in travelling-wire electrochemical machining. J Braz. Soc. Mech. Sci. Eng. 39, 4589–4599 (2017). https://doi.org/10.1007/s40430-017-0748-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40430-017-0748-2