Abstract
Parasitic flowering plants are characterized by the development of an organ known as haustorium, which has evolved in multiple independent angiosperms clades. The haustorium has also been deemed “the most plastic of organs” due to its ability to accommodate physiological and anatomical differences between the parasite itself and its host plants. This is achieved through the development of vascular connections, which involve the differentiation of various specialized cell types by the parasite. The development, structure, and evolution of the haustorium and the connections it fosters are reviewed here considering all 12 parasitic plant lineages. A multi-level comparison between “model” parasitic plants, such as Orobanchaceae and Cuscuta species, with members of often neglected groups, such as Lennoaceae, Mitrastemonaceae, and Santalales yields the idea of a shared general body plan of the mature haustorium. This proposed haustorium bauplan is composed of an upper part, including structures associated with mechanical attachment to the host body, and a lower part, including all parasitic tissues and cell types within the host body. The analysis of multi-level convergence is also applied here to the comparison between haustoria and other plant organs. Considering the structure, molecular development, and functionality of this organ under the framework of continuum and process plant morphology, I propose the interpretation of haustoria as morphological misfits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
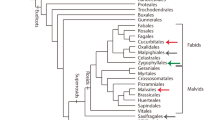
Parasitism is a widespread ecological interaction, observed in all domains of life (Combes 2001). In the Archaeplastida, the clade that harbors red algae, glaycophytes, multiple green algae lineages, and land plants (Baldauf 2008), parasitic interactions are often established by both green and red algae, as well as by land plants (Oborník 2019). Within the later, parasitic lifestyle is manifested in two different nutritional modes. On the one hand, mycoheterotrophism is observed in nearly all main lineages, including liverworts, ferns, conifers, and angiosperms, which exploit fungal hosts to fulfill their nutritional needs (Feild and Brodribb 2005; Merckx et al. 2009; Merckx 2013). On the other hand, plant parasitism is currently observed in 12 independent clades, exclusively among angiosperms (Fig. 1a; Nickrent 2020). These species are unique in their capacity of obtaining water and nutrients directly from other plants, without the aid of a fungal partner or host.

modified from Nickrent 2020) indicating 12 independent origins of the parasitic lifestyle (in red). b Pyrularia pubera Michx. (Santalaceae, Santalales), a root hemiparasitic tree. c Lennoa madreporoides Lex. (Lennoaceae), a root holoparasite. d Castilleja mexicana (Hemsl.) A. Gray (Orobanchaceae), a root hemiparasitic herb. e Cuscuta campestris Yunck. (Convolvulaceae), a parasitic vine. f Phoradendron juniperinum Engelm. ex A. Gray (Santalaceae), a mistletoe. g Mitrastemon matudae Yamam. (Mitrastemonaceae), an endoparasite. p parasite, h host
Diversity of parasitic flowering plants. a Angiosperm phylogeny (
In terms of their diversity, parasitic flowering plants add up to ca. 1% of extant angiosperm species (Westwood et al. 2010). Such taxonomic diversity is matched by a broad variation of plant habits and functional attributes (Těšitel 2016). Most parasitic species, ranging from trees and shrubs, to small herbs and tuberous plants, germinate on the ground and attack the root system of their hosts (Fig. 1b–d; Bell and Adams 2011). Twining vines, i.e., Cassytha (Lauraceae) and Cuscuta (Convolvulaceae), germinate on the ground but develop no functional root system, attaching instead to the stems and branches of a wide variety of host plants (Fig. 1e; Kuijt 1969). Aerial shrubs, i.e., mistletoes, also parasitize host branches (Fig. 1f; Aukema 2003). However, their seeds, which are mostly transported by animal dispersers, germinate directly upon the aerial organs of their hosts (Lamont 1983). A final habit, known as endoparasitism, is observed in a few species that exhibit extreme vegetative body reduction and grow exclusively within the host roots/stems during most of their life cycle (Fig. 1g; Mauseth and Rezaei 2013; Nikolov et al. 2014).
Among the species exhibiting each of these growth habits, photosynthetic ability also varies widely, ranging from fully and partially photosynthetic plants, to species completely devoid of chlorophyll or chloroplast genome (Bromham et al. 2013; Molina et al. 2014). These non-green species are usually termed holoparasites (Fig. 1c, g), while species that are at least partially photosynthetic are termed hemiparasites (Fig. 1b, d, e, f) (Musselman and Press 1995). Parasitic plants also show great diversity in terms of geographical distribution, having colonized the most different environments, from arctic to tropical regions (Irving and Cameron 2009; Heide-Jørgensen 2013). This widespread occurrence is frequently associated with a wide host range, from ferns to cacti, from trees to crops, and from shrubs to lianas (Heide-Jørgensen 2008).
The unifying feature of this broad diversity of species is their capacity to develop an organ known as a haustorium, which represents the “very essence of plant parasitism” (Kuijt 1969). The haustorium acts in the initial attachment of a parasite to a suitable host, in the penetration of host tissues, and in the establishment of vascular connections between the two plants, enabling the exchange of water, nutrients, and genetic information (Joel 2013; Yoshida et al. 2016). Given the importance of this organ for the parasitic nutritional mode, a wealth of information is available in the literature regarding haustorium morphology, anatomy, and ultrastructure.
However, most of the research attention to date has been dedicated to parasitic plants considered as either forest pathogens, or weeds to horticultural and agricultural crops, including Cuscuta, Orobanchaceae, and a few mistletoe species (Hawksworth 1983; Clarke et al. 2019; Watson et al. 2020). Indeed, much of the available information on haustorium structure, development, functionality, and evolution is based on the study of these parasites. This reflects both the considerable focus of current research on these economically important parasites and the need for more work on other parasitic plant species (Riopel and Timko 1995). The need for increased work dealing with non-pathogenic and non-weedy parasitic plants becomes even more relevant when one considers that the interest in parasitic plants has increased over the past three decades (Nickrent 2020).
In this context, this review discusses the development, structure, and functionality of the parasitic plant haustorium, with a special focus on the vascular connections between parasite and host. The broad diversity of parasitic plant clades is considered here, including “model lineages,” such as Orobanchaceae and Cuscuta (Cesarino et al. 2020), as well as groups that are often neglected in literature reviews, such as Santalales, Lennoaceae, and Mitrastemonaceae. The goal is to provide a comparison across the different angiosperm groups that include parasitic plants. Furthermore, based on insights from evolutionary developmental biology and continuum morphology, an approach that acknowledges gradations between typical plant structures (Sattler 1996), a new perspective and interpretation of haustorium identity and development is discussed here.
2 Haustorium development
The continuum process of haustorium development can be divided into three phases, namely initiation, intrusion, and conduction (Kokla and Melnyk 2018), each of which involves the formation of a different set of structures. Upon initiation, which is often triggered by chemical and/or physical stimuli provided by the host (Thoday 1951; Goyet et al. 2019; Shimizu and Aoki 2019), appendages associated with mechanical anchorage to the host surface are formed. These include the modified root hairs of some Balanophoraceae and most Orobanchaceae species (Holzapfel 2001; Cui et al. 2016), as well as expansions of the haustorium upper part, such as the attaching folds of Santalales and Krameriaceae root parasites (Kusano 1902; Musselman 1977) and the holdfast of mistletoes (Sallé 1983). Adhesion to the host surface is also attained through the release of cementing substances by structures such as the papillae of Orobanche spp. (Joel and Losner-Goshen 1994), the secretory trichomes of Cuscuta (Vaughn 2002), and the adhesive disc, which corresponds to the external, flattened part of the young haustorium of Cassytha and mistletoe species (Sallé 1983; Heide-Jørgensen 1991).
Attachment to the host surface allows the parasite to mechanically penetrate host dermal tissues. Cell-wall-degrading enzymes are also considered to play an important role in loosening the middle lamellae of host cells, thus facilitating penetration (Nagar et al. 1984; Losner-Goshen et al. 1998; Ouyang et al. 2016). Invasion of the host body leads to the development of a penetration peg, also known as intrusive organ, which develops either endogenously, from the pericycle, or exogenously, from the epidermis and outer cortex of the parasitic haustorium (Lee 2007; Pérez-de-Luque 2013; Kuijt 2015; Kokla and Melnyk 2018; Wakatake et al. 2018). Irrespective of its origin, the general anatomy of the penetration peg is similar across most parasitic plants, being composed of multiple parenchyma cells. The main exception is the searching hyphae formed by Cuscuta, which are formed by single parenchyma cells that grow via cell tip elongation (Vaughn 2003). It is noteworthy that regular root hairs, which differentiate from epidermal cells, also grow via tip elongation (Miller et al. 1997). The searching hyphae of Cuscuta, however, originate from cortical cells and later differentiates into vascular cells (Shimizu and Aoki 2019).
The final stage of haustorium development involves the expansion and differentiation of penetrating structures into specialized tissues and cell types that promote connection between the vascular systems of parasite and host. In addition to the contact hyphae described above, such tissues also include cortical strands and haustorium flanges, which expand the parasite–host interface by spreading through the host bark and wood, respectively (Kuijt 1977; Condon and Kuijt 1994). Cell types other than tracheary elements are also common at the host–parasite interface, including transfer cells and flange cells (Fineran 1985; Fineran and Calvin 2000). This set of parasitic tissues embedded within the host body is then termed the endophyte (Teixeira-Costa and Ceccantini 2018). These and other peculiar structures of parasitic plants will be discussed in more detail in the following sections.
It is noteworthy that the three phases of haustorium development summarized above have not been observed for all parasitic plant lineages. Early haustorium development, including the phases of initiation and penetration, has yet to be described for seven of the 12 currently recognized parasitic plant lineages. This includes Apodanthaceae, Cynomoriaceae, Cytinaceae, Hydnoraceae, Lennoaceae, Mitrastamonaceae, and Rafflesiacae (Kuijt 1966; Heide-Jørgensen 2008). Among these groups, scanty information is available regarding seed germination (Heinricher 1917; Bolin et al. 2009; Wicaksono et al. 2020); still, no reports of how the parasite first penetrates the host are available. Nevertheless, the structure of the mature haustorium, including establishment of parasite–host phloem and xylem connections, has been described for species in all of these families. The following section discusses the structure and origin of these connections.
3 Structure of haustorium vascular connections
Haustorium vascular connections to the host xylem and eventually to the host phloem are classified as either direct, or indirect. Direct connections (Fig. 2a–d) occur when uninterrupted luminal/symplastic continuity is observed between tracheary/sieve elements of both plants (Hibberd and Jeschke 2001). Connections are classified as indirect (Fig. 2e–h) when mediated by parenchymatic tissue, often including specialized cells, such as flange and transfer cells (Pate et al. 1990; Fineran and Calvin 2000). A combination of both direct and indirect connections is also observed in many species (Fig. 2b, f) (Cameron and Seel 2007).
Examples of parasitic plant species and the types of vascular connections formed with host plants. a, b Helosis cayanensis (Sw.) Spreng. (Balanophoraceae, Santalales). a External morphology. b Light microscopy of the parasite-host interface showing direct xylem connection (dashed square) between vessel elements of parasite and host; notice parasite parenchyma cell abutting a host vessel (arrowhead). c, d Rafflesia cantleyi Solms (Rafflesiaceae). c External morphology (photograph by Charles C. Davis). d Fluorescence microscopy of the parasite-host interface showing direct phloem connection between parasite and host sieve elements (callose marked with aniline blue dye; dashed circles). e, f Scybalium fungiforme Schott & Endl. (Balanophoraceae, Santalales). e External morphology. f Fluorescence microscopy (autofluorescence) of the parasite-host interface showing indirect xylem connection between parenchyma cells of the parasite and vessel elements of the host; notice large nuclei of parasite parenchyma cells (arrowheads). g, h Bdallophytum americanum (R. Br.) Eichler ex Solms (Cytinaceae). g External morphology (photograph by Cyril H. Nelson). h. Fluorescence microscopy of the parasite-host interface showing indirect phloem connection between parasite parenchyma cells and host sieve elements (callose marked with aniline blue dye); notice large nuclei of parasite parenchyma cells (arrowheads). p parasite, h: host
Phloem and symplast connections
– Phloem differentiation in the haustorium of parasitic plants and the development of parasite–host phloem connections have been points of controversy in the parasitic plant literature for decades. The use of inadequate methods for the detection of phloem sieve elements might have contributed to this debate (Esau 1969; Dörr 1990). To date, direct phloem connections have been confirmed in few parasitic species. Sieve connections between certain species of Cuscuta (Israel et al. 1980) and Orobanche (Dörr and Kollmann 1995; Krupp et al. 2019) were revealed through the analysis of haustorium ultrastructure. More recently, phloem-mobile fluorescent dyes and in situ hybridization have also been used to detect the presence of sieve elements and the occurrence of direct phloem connections between Cuscuta species and their hosts (Birschwilks et al. 2006; Shimizu et al. 2018; Shimizu and Aoki 2019). Uninterrupted parasite–host phloem connections can also be detected by labeling the callose of sieve plates with specific fluorescent dyes (Angyalossy et al. 2016). Following this method, sieve elements of Apodanthaceae and Rafflesiaceae species were observed to connect directly to the sieve elements of host plants (Teixeira-Costa et al. in press).
It is crucial to note that the presence of sieve elements in the haustorium is not necessarily an indication of direct parasite–host phloem connections (Dörr 1990). Indeed, sieve elements and companion cells have been observed in the haustorium of hemiparasites with no connection to the host phloem (Calvin 1967; Kuijt and Dobbins 1971). At the same time, the endophytic tissue of holoparasites can also contain sieve elements that do not connect directly to the host phloem. This has been shown to be the case in Balanophoraceae, Cytinaceae, Hydnoraceae, and, more recently, Cynomoriaeae species (Hsiao et al. 1995; De Vega et al. 2007; Tennakoon et al. 2007; Fahmy and Hassan 2020). Furthermore, although transfer cells are observed at the interface between Balanophora species (Balanophoraceae) and their hosts, connections to host sieve elements were not detected (Gedalovich-Shedletzky and Kuijt 1990). Similarly, electron microscopy revealed no phloem connections between Boschniakia hookeri Walp. (Orobanchaceae) and its host (Kuijt and Toth 1985).
These observations suggest that direct phloem connections are either ephemeral or absent in several holoparasites, which would imply that these parasites obtain most (or all) of their nutrition by tapping into the host xylem. Considering holoparasites usually have low transpiration rates (Seel et al. 1992; Fahmy 1993), species without direct access to the host phloem would be expected to grow slow as a response to consequent low rates of resource uptake. This is observed in Balanophoraceae species, in which transpiration is also reduced to a minimum due to the absence of stomata (Moore 1940; Kuijt and Dong 1990). A similar situation could occur for Cytinaceae and Mitrastemonaceae species, which have been anecdotally reported to take several years to bloom for the first time (Watanabe 1933; Forstmeier et al. 1983). To a certain degree, the relation between slow growth and absence of direct sieve connections can be extended to Striga (Orobanhaceae). These annual hemiparasites remain underground for most of their life cycle, being fully dependent on the host for their carbon supply during this period (Spallek et al. 2013; Lambers and Oliveira 2019). The lack of parasite–host phloem connections (Dörr 1997) could then be an explanation to the delayed emergence of the above-ground stems of most Striga species.
Xylem connections
– As with many aspects of haustorium development and functionality, the growth of parasitic penetration structures and subsequent differentiation of haustorium vasculature depend, to a certain extent, on the anatomy of host stems/roots. When infesting herbaceous species or other plants that do not undergo pronounced secondary growth, parasite penetration structures are able to continue growing and elongating until they have reached the host primary xylem. This has been illustrated and reviewed for both aerial (McLuckie 1924; MacLeod 1962) and root parasites (Pérez-de-Luque 2013). Conversely, contact with the host secondary xylem is usually achieved by a coordinated proliferation between the endophyte and the host cambium. When infesting woody roots/stems, centripetal growth of the penetration peg is halted once it reaches the host cambial zone (Fig. 3a) (Fineran 1965). At this point, the meristematic tip of the peg usually becomes flattened and promotes further circumferential growth against the surface of the host secondary xylem (Pate et al. 1990). Then, as the host cambium produces new xylem tissue, the parasitic endophyte becomes passively embedded within the host wood (Kuijt 1965).
Details of parasite–host xylem connections. Light (a, b, d, f, i), autofluorescence (c), and polarized light (g) microscopy shows longitudinal and cross sections through the host stem/root. a Penetration peg (white outline) of Phoradendron juniperinum (Santalaceae) halted at the host cambial zone (asterisks). b Endophyte (white outline) and sinker (diamond) of Bdallophytum americanum (Cytinaceae) penetrating through the host wood rays. c Parasite sinker (diamond) of Struthanthus martianus Dettke & Waechter (Loranthaceae, Santalales) branching to form vessel elements (white circle) within a host vessel. d Predominance of parenchymatic tissue at the interface between Pyrularia pubera (Santalaceae, Santalales) and its host; notice two areas of direct xylem connection (black circles). e Transmission electron microscopy showing transfer cell at the interface between Phoradendron perrottetii (DC.) Eichler (Santalaceae, Santalales) and its host; notice invaginations of the parasite cell wall (arrowheads). f Flange cells (white circle) at the interface between Phoradendron perrottetii (Santalaceae, Santalales) and its host. g Interface between Struthanthus flexicaulis (Mart. ex Schult. f.) Mart. (Loranthaceae, Santalales) and a conifer host indicating parenchymatic composition of the sinker (diamond) and endophyte (white outlines); notice parasite vessel elements (arrowhead) are absent in the sinker. h Transmission electron microscopy showing parenchymatic cell of the sinker formed by Struthanthus flexicaulis (Loranthaceae, Santalales) at the interface with a conifer host. i Direct vascular connection (white ellipse) at the interface formed by Arceuthobium americanum Nutt. ex Engelm. (Santalaceae, Santalales) and its conifer host; notice perforation plate (arrowhead). p parasite, h host
Alternatively, a few species can actively penetrate the host secondary xylem by growing in between radial cells (Fig. 3b) (Heil 1926; Dell et al. 1982; Kuijt et al. 1985; De Vega et al. 2007). Detection of this penetration strategy requires detailed examination of the transition between intrusion and conduction phases of haustorium development. For this reason, such strategy may still be underestimated among parasitic species. This is especially relevant in the case of root holoparasites, due to difficulties in sampling these plants during early developmental stages. The portion of the endophyte that extends radially into the host xylem is termed “sinker,” alluding to the sinking of parasitic cells deeper within the host (Kuijt 1977; Teixeira-Costa and Ceccantini 2018).
Sinkers are initially composed of parenchymatic cells (Fig. 3b), part of which later differentiate into tracheary elements (Fig. 3c, d) and other specialized conductive cells, such as transfer cells (Fig. 3e) and flange cells (Fig. 3f) (Fineran 1996; Hibberd and Jeschke 2001; Vaughn 2006). Oftentimes, parenchymatic cells of the sinker invade host vessels trough lateral pit apertures, in a process similar to what is observed in the formation of tyloses (Esau 1965; Kuijt 1977). Once within the host vessel, the process of programmed cell death leads to the formation parasitic vessels elements inside and in continuity with host vessels (Fig. 3c) (Toth and Kuijt 1977; Venturelli 1980; Heide-Jørgensen and Kuijt 1995; Cameron et al. 2006). In Orobanchaceae species, this type of parasitic vessel element is usually referred as “oscula” (Dörr 1997). Another peculiar cell type frequently observed in the haustorium of Orobanchaceae, Santalales, and Cassytha hemiparasites is known as graniferous tracheary element (Musselman and Dickison 1975; Fineran 1985; Calvin and Wilson 1996; Rajanna and Shivamurthy 2001). This is a xylem conduit that contains amylaceous or proteinaceous granules attached to its inner cell walls, which have been hypothesized to help regulate sap flow from host to parasite (Fineran and Bullock 1979; Joel 2013).
In several cases, however, direct connections between parasite and host tracheary elements can be extremely rare (Lambers and Oliveira 2019). This is especially observed in Santalalean root hemiparasites and a few mistletoes, in which direct xylem connections account for less than 10% of the parasite–host interface area (Fig. 3d) (Pate et al. 1990; Calvin and Wilson 1995). In that event, indirect parasite–host xylem connections often involve transfer cells (Fig. 3e), characterized by intricate wall labyrinths that amplify the surface area of the plasma membrane (Offler and Patrick 2020), and flange-type parenchyma cells (Fig. 3f), which show wall thickenings in the form of flanges (Fineran 1996). Both cell types are associated with intense transport of nutrients (Fineran 1996; Fineran and Calvin 2000; Offler and Patrick 2020).
Parenchyma cells can also play a crucial role in xylem connections of species with a broad host range, known as host–generalist parasites, that attack tracheid-bearing hosts, such as conifers and most ferns. In the sinker of the mistletoe Struthanthus flexicaulis (Loranthaceae), for instance, parenchyma cells usually differentiated into vessel elements. However, when infesting conifer hosts, the sinker remains parenchymatic, leading to the formation of indirect xylem connections only (Fig. 3g, h) (Ceccantini et al. 2019). On the other hand, in mistletoes that exclusively infest conifer species, such as Arceuthobium spp. (Santalaceae), although most of the parasite–host interface is comprised of parenchyma cells, direct xylem connections are achieved via tracheary pits (Fig. 3i). This comparison suggests that parasitic species with broad host ranges can recognize the surrounding cells of the host xylem and accommodate the structure of their sinkers accordingly. Furthermore, this comparison highlights haustorium plasticity and its ability to accommodate physiological and anatomical differences between the parasite itself and its host plants. Future investigation of this topic should focus on the molecular mechanisms behind this form of host recognition, which could provide insights into why some parasitic plants display a virtually unlimited host range. At the same time, understanding how a parasite can differentiate between distinct types of tracheary elements could broaden the general understanding molecular xylem development.
4 Proposal for a general haustorium bauplan
As the parasitic habit evolved multiple times independently, so did the haustorium. Considered as a homoplastic character, there would be no a priori reason to imagine all haustoria to be similar in their developmental origin or structural organization (Kuijt 1969). Indeed, haustorium morphology and anatomy may vary greatly when comparing species from distantly related lineages and different functional groups, such as endoparasites and the root hemiparasites. Differences are less pronounced when comparing species with similar life histories. Similarities in development and structure of the haustorium among and within root hemiparasitic clades, including Krameriaceae (Fig. 4a), Orobanchaceae (Fig. 4b), and Santalaceae, have been long recognized (Barber 1907; Musselman and Dickison 1975; Musselman 1977). Likewise, the convergence between Cassytha (Fig. 4c) and Cuscuta (Fig. 4d) has long been observed, and it extends from their lianescent form and rudimentary roots, to the general aspect of their haustorium (Kuijt 1969; Heide-Jørgensen 2008).
Similarities and differences in the haustorium of multiple parasitic plant lineages. a, b Cross section through host roots showing a similar form of penetration by the parasites a Krameria lappacea (Dombey) Burdet & B.B. Simpson (Krameriaceae; image provided by G. Brokamp and M. Weigend) and b Aureolaria pedicularia (L.) Raf. ex Pennell (Orobanchaceae). c, d Longitudinal and cross sections through host stems showing the general aspects of the haustorium in c Cassytha filiformis L. (Lauraceae) and d Cuscuta sp (Convolvulaceae); note searching hypha (black outline). e, f Longitudinal section through the host root and macroscopical image showing similarities of the interface formed by e Mitrastemon matudade Yamam. (Mitrastemonaceae) and f Rafflesia cantleyi Solms (Rafflesiaceae); note the presence of a cupule (cp) in both species. g Habit of the parasite Prosopanche caatingicola (Hydnoraceae) bearing lateral haustoria (black circles). h Habit of the parasite Scybalium fungiforme (Balanophoraceae) bearing a terminal haustorium (tuber). i. Habit of the parasite Scybalium glaziovii Eichler (removed) causing the formation of a placenta-like structure (black arrowhead) in the host root. p parasite, h host; white arrowheads: parasite xylem
Among mistletoes, the remarkable diversity in haustorium morphology has been recently shown to have a common developmental trajectory, one that is also partially shared with Santalalean root hemiparasites (Teixeira-Costa et al. 2020). Striking developmental similarities have also been observed among endoparasitic species of the families Apodanthaceae, Cytinaceae, Mitrastemonaceae (Fig. 4e), and Rafflesiaceae (Fig. 4F) (Teixeira-Costa et al. in press). On the other hand, root holoparasites are more diverse, forming haustorial systems with different origins and morphologies. For instance, Prosopanche caatingicola Machado & L.P. Queiroz (Hydnoraceae, Fig. 4g) forms multiple haustoria, which emerge laterally along the root system, while Scybalium fungiforme (Balanophoraceae, Fig. 4h) forms a single haustorium, which emerges at a terminal position. As expected, similarities have been more often recognized between root holoparasites with the same type of haustorium, such as in the case of the endophyte tissue of Hydnoraceae and Lennoaceae, both of which have lateral haustoria (Tennakoon et al. 2007). A similar endophyte is also observed in Cynomoriaceae (Fahmy and Hassan 2020). Among root holoparasites with a terminal haustorium, the same form of parasite-induced alteration to the host xylem, forming a “placenta-like” structure that accommodates parasite tissues (Fig. 4i), is observed in Orobanchaceae (Kuijt and Toth 1985; Baird and Riopel 1986) and Balanophoraceae (Holzapfel 2001). Curiously, this feature is also developed in the association of many mistletoe species and their hosts, being called a woodrose (Kuijt and Lye 2005).
The examples discussed above highlight the value of examining convergence, and more specifically, phenotypic convergence at multiple levels of biological organization. Similarity/difference at one hierarchical level not necessarily implies similarity/difference at another level (Rosenblum et al. 2014). Indeed, conservation or divergence in morphology can be influenced by developmental, genetic or structural constrains (Lau and Oakley 2020). Because haustorium development is a dynamic process that involves shifts in structure and functionality (Yoshida et al. 2016), tissues and cell types that are readily identified during one phase may not be distinguishable later in development. For instance, in Loranthaceae mistletoes such as Psittacanthus and Loranthus species, few sinkers can be observed at young stages of haustorium development, but become indistinguishable at a later, mature stage (Dzerefos and Witkowski 1997; Teixeira-Costa et al. 2020).
Based on a comparative analysis across parasitic plant lineages, a convergence in the topology of haustorium tissues emerges, suggesting a convergent haustorium bauplan. This shared body plan can be artificially divided in two parts: the upper haustorium and lower haustorium. The first lies external to the host body and, in most cases, originates from the appendages that aid in mechanical anchorage, which are formed during haustorium initiation. The lower haustorium comprises the endophyte and its diverse set of tissues and cell types, all of which derive from the penetration peg formed during haustorium intrusion. This includes tissues that establish vascular connections with the host phloem and/or xylem. The proposed haustorium bauplan is represented in Fig. 5, indicating the similar topology observed in both aerial (Fig. 5a) and root (Fig. 5b, c) parasites.
This schematic representation also illustrates the topology observed in the haustorium of Balanophoraceae species (Fig. 5d). It has been suggested that plants in this family have a unique type of haustorium in which the host vascular system is stimulated to differentiate new conductive cells toward the parasite (Mangenot 1947; Kuijt 1969). While this is indeed the case, stimulation and rearrangement of host phloem and/or xylem is not an exclusive feature of the Balanophoraceae haustorium. The extreme phenotype observed in Balanophoraceae is similar to what has been reported in other root holoparasites (Tate 1925), mistletoes (Aloni 2015), and endoparasites (García-Franco et al. 2007; do Amaral and Ceccantini 2011; Teixeira-Costa et al. in press). The frequent occurrence of host vascular cells converging toward the haustorium highlights another level of convergence among different parasitic plants. At a physiological level, these changes caused to host vascular development appear to be mediated by the same hormones released by the parasite at the interface with the host (Zhang et al. 2012; Hu et al. 2017; Spallek et al. 2017).
Another feature of the Balanophoraceae haustorium that deserves special mention is the highly modified chimeric structure that many of them develop with their hosts. The tuberous organ of Balanophora, Langsdorffia, and Thonningia species is traversed by chimeric vascular strands composed of both parasite and host cells (Fig. 5d) (Holzapfel 2001). In addition to xylem, phloem, and transfer cells, these chimeric strands contain both parasitic and host-derived meristematic cells at the apical region (Gedalovich-Shedletzky and Kuijt 1990; Hsiao et al. 1995). This close integration of meristematic cells is also observed in endoparasites such as Pilostyles thurberi A. Gray (Apodanthaceae; Rutherford 1970), Arceuthobium douglasii Engelm. (Santalaceae; Lye 2006), and at least temporarily in Tristerix aphyllus (DC.) Barlow (Loranthaceae; Mauseth et al. 1985). Moreover, endoparasites such as Rafflesiaceae and Mitrastemonaceae species develop another type chimeric structure known as cupule, which is part of the parasite haustorium (Fig. 4e, f, cp) and partially produced by the host as a response to the burst of parasite flower buds (Kuijt 1969; Nikolov et al. 2014).
It is noteworthy that, at their mature developmental stage, endoparasites lack a recognizable upper haustorium (Fig. 5e). In other parasites, the upper haustorium provides a link between the exterior parasite body (i.e., the exophyte) and the vascular connections formed with host tissues via the lower haustorium. Through the course of evolution, the increased specialization of the endophytic system, which acquired the function of giving rise to the main exophyte (Kuijt 1969; Těšitel 2016), could have coincided with a reduction of the upper haustorium, rendering it ultimately superfluous. In endoparasites with remnant photosynthesis, such as Tristerix aphyllus (Loranthaceae) and Viscum minimum Harv. (Santalaceae), a distinct upper haustorium is formed upon germination (Mauseth et al. 1985; Kuijt 1986). As development progresses, the shoot apex is aborted and the upper haustorium disintegrates (Mauseth et al. 1985; Kuijt 1986). A similar form of germination and initial development could also occur for other endoparasites.
5 New interpretation of haustorium organ identity
Questions of homology between parasitic plant structures, especially the haustorium, and other plant organs have long puzzled researchers and divided opinions. For instance, structures that foster the development of additional lateral haustoria, such as the epicortical roots of certain mistletoes, the pilot roots of Lennoaceae and Hydnoraceae species, and the runners of Balanophoraceae have all been initially identified as modified roots (Kuijt 1964, 1966, 1969). However, more recent publications have reopened the question of homology in all of these structures (Mauseth et al. 1992; Tennakoon et al. 2007; Kuijt 2015).
The most debated issue, however, is the question to whether haustoria are modified roots, stems, or an entirely different type of structure. Based on the evolutionary plasticity of plants, leading to a diverse array of morphologies, Kuijt (1969) claims that the haustorium “represents a root in function and evolutionary origin.” Considering haustorium anatomy is highly modified, showing no clear parallels with other organs, Goebel (1905) and Forstreuter (1988) have interpreted the haustorium as an organ sui generis, that is, a structure of its own kind. Finally, Weber (1987) avoided the “sui generis debate” by suggesting that a terminal haustorium could be interpreted as a type of lateral haustoria formed directly at the root apex due to extreme root reduction.
The analysis of multi-level convergence is an interesting approach that can also be helpful in the discussion of organ identity of parasitic plant haustoria. At the functional level, all different types of haustorium exert the same basic functions of a root system. In fact, parasitic plants depend upon their hosts in a similar way that non-parasitic plants depend on the soil (Calvin and Wilson 1998). Haustoria provide mechanical anchorage to the host and act in the uptake of water and mineral nutrients (Joel 2013). In some cases, haustoria might also be involved in mycorrhizal interactions (Baird and Riopel 1986; de Vega et al. 2010). At the molecular level, albeit data are still restricted to a few Cuscuta and Orobanchaceae species (Yang et al. 2015; Vogel et al. 2018; Yoshida et al. 2019), evidence suggests genes that control lateral root formation in non-parasitic plants have been coopted for haustorium development in parasite species (Yoshida et al. 2019). Similarities between hormonal control of haustorium formation and root development have also been described (Zhang et al. 2015, 2016). Nevertheless, at the developmental and anatomical levels, the message is not quite clear.
Four key features are used in comparative morphology and anatomy of seed plants to differentiate roots from shoots: (1) the organization of xylem and phloem tissues in alternating sectors (roots, Fig. 6a) versus same axial sectors (shoots, Fig. 6b); (2) endogenous (root, Fig. 6c) versus exogenous (shoot, Fig. 6d) origin of daughter axes; (3) presence (roots, Fig. 6e) versus absence (shoots, Fig. 6f) of a root cap; (4) absence (roots, Fig. 6g) versus presence (shoots, Fig. 6h) of exogenously formed leaves (Rutishauser and Isler 2001). Internal haustorium anatomy in the different lineages of parasitic plants is not organized in sectors and cannot be classified as either root-like, or stem-like (Fig. 6i; Bhandari and Mukerji 1993). In terms of their ontogenesis, terminal haustoria develop from the embryo root apex soon after germination, while lateral haustoria have an exogenous origin (Fig. 6j), developing from cells in the cortical region of roots or stems (Kuijt 1969; Heide-Jørgensen 2008). Despite their root-like origin, the root apical meristem of most parasites with a terminal haustorium lacks a root cap (Calvin 1966; Musselman and Dickison 1975; Lamont 1983). In parasites with lateral haustoria, a cap-like tissue can be present (Brokamp et al. 2012), however, with a different appearance, more similar to bark tissues (Fig. 6k). The underground structures of Balanophoraceae are regarded as not having a root (nor a shoot) organization (Hansen 2015).
Diagnostic anatomical features of roots (a, c, e, g) and stems (b, d, f, h) compared with aspects of haustorium (i–l) anatomy. a, b Organization of xylem (black arrowheads) and phloem (black outlines) tissues in alternating sectors (roots, a) versus same axial sectors (shoots, b); note the opposite direction of vessel element differentiation (curvy black arrows) in each organ. c, d Daughter axes (black outlines) with an endogenous (root, c) versus exogenous (shoot, d) origin; note the position of the vascular system in each organ (black star). e, f Apical meristem (black asterisks) showing presence (root, e) versus absence (shoot, f) of a root cap. g, h Absence (roots, g) versus presence (shoots, h) of exogenously formed leaves; note the position of the vascular system in each organ (black star) and the shape of the leaf gap in the stem (black outline). i Cross section through the haustorium of Krameria lappacea (Krameriaceae; image provided by G. Brokamp and M. Weigend); note the absence of phloem and presence of xylem (white arrowhead) tissues. j Haustorium initiation (white outline) in the cortex of Cuscuta americana L. (Convolvulaceae); note the position of the vascular system (white star). k Pre-haustorium of Krameria lappacea (Krameriaceae; image provided by G. Brokamp and M. Weigend); note the presence of a bark-like dermal tissue (white arrow) covering part of the structure. l Shoot apical meristem (white asterisk) developed from parenchyma cells in the haustorial root of Lennoa madreporoides (Lennoaceae)
Considering the exogenous development of lateral haustoria, a similar origin is mostly common for bud formation (Fig. 6d), developing from either pre-existing shoots (stem ramification or branching) or roots (root–shoots or root buds) (Esau 1965). On the other hand, lateral and adventitious root formation in angiosperms are both associated exclusively with an endogenous origin (Fig. 6c), i.e., developing from cells in the vascular system (Esau 1965). Development of structures that emerge directly from a haustorium occurs in few parasitic clades. In root holoparasites with a terminal haustorium, such as some Orobanchaceae and all Balanophoraceae, inflorescences develop from the tuberous haustorium, originating from parenchyma cells among the many vascular bundles (Schrenk 1894; Shivamurthy et al. 1981). In the haustorial roots of Lennoaceae species, stem apical meristems also develop from parenchyma cells among vascular bundles (Fig. 6l). In Loranthaceae mistletoes, basal epicortical roots develop at the base of the hypocotyl, in close proximity to the upper haustorium, but separate from it (Calvin and Wilson 2006). Finally, in the case of most endoparasites, such as Apodanthaceae, Cytinaceae, Mitrastemonaceae, and Rafflesiaceae species, flower/inflorescence axes also develop endogenously, from a secondary morphological surface formed internally to the reproductive meristem apex (Kuijt 1969; Nikolov et al. 2014).
In face of these multiple interpretations, all of which based on somehow conflicting evidence, the haustorium appears as a “misfit” in the sense of the classical morphology discipline (Bell 1991). Often used in reference to plants such as river-weeds (Podostemaceae) and bladderworts (Lentibulariaceae), the term “morphological misfit” has been applied to label a variety of natural deviations to the norm of a root–shoot axis with independent, non-overlapping structures (Rutishauser 2016). Using these peculiar plants as subjects and examples, several plant morphologists, philosophers, and developmental geneticists (e.g., Arber 1950; Sattler 1996; Sinha 1999; Rutishauser and Isler 2001) have argued in favor of a complementary approach to the classical morphology framework. Known as Continuum Morphology, or Fuzzy Arberian Morphology, in homage to the pioneer work of Agnes Arber (1950), this complementary approach understands plant structures as processes, highlighting that drastic evolutionary changes to the basic root–shoot program may require fuzzy, rather than clear-cut concepts of organ identity (Rutishauser 2020).
Under this framework, the haustorium of parasitic flowering plants would be better interpreted as a root–shoot mosaic, as it has been suggested for the underground structures of Utricularia and Pinguicula (Lentibulariaceae) (Rutishauser and Isler 2001). Using the mosaic as a metaphor, a terminal haustorium could then be interpreted as composed mostly of “root-like tiles,” while lateral haustoria would be composed of roughly equal parts of “root-like tiles” and “stem-like tiles.” This interpretation provides more than a resolution to the conflict of haustorium homology and organ identity, opening up new research avenues for the comparison between parasitic plants and other morphological misfits, especially in terms of their evolutionary development. The continuum morphology approach complements the interpretations based on classical morphology, providing a more comprehensive framework for the comparison and investigation of the haustorium across the multiple angiosperm lineages. Finally, this mosaic interpretation reinforces that, despite being a homoplastic character, the haustoria of the different functional and taxonomic groups of parasitic plants are more similar to each other, than they are similar to other plant organs. The shared developmental trajectory of the different types of haustoria could then be due to homologous regulatory genes expressed in a similar manner in all different lineages of parasitic flowering plants.
References
Aloni R (2015) Ecophysiological implications of vascular differentiation and plant evolution. Trees 29:1–16. https://doi.org/10.1007/s00468-014-1070-6
Angyalossy V, Pace MR, Evert RF et al (2016) IAWA list of microscopic bark features. IAWA J 37:517–615. https://doi.org/10.1163/22941932-20160151
Arber A (1950) The natural philosophy of plant form. Cambridge University Press, Cambridge
Aukema JE (2003) Vectors, viscin, and Viscaceae: mistletoes as parasites, mutualists, and resources. Front Ecol Environ 1:212–219
Baird WV, Riopel JL (1986) Life history studies of Conopholis americana (Orobanchaceae). Am Midl Nat 116:140–151
Baldauf SL (2008) An overview of the phytogeny and diversity of eukaryotes. J Syst Evol 46:263–273. https://doi.org/10.3724/SP.J.1002.2008.08060
Barber CA (1907) Parasitic trees in Southern India. Proc Camb Philos Soc Math Phys Sci 14:246–255
Bell AD (1991) An illustrated guide to flowering plant morphology. Oxford University Press, Oxford
Bell TL, Adams MA (2011) Attack on all fronts: functional relationships between aerial and root parasitic plants and their woody hosts and consequences for ecosystems. Tree Physiol 31:3–15. https://doi.org/10.1093/treephys/tpq108
Bhandari NN, Mukerji KG (1993) The haustorium. Research studies in botany and related applied fields. Research Studies Press, Taunton
Birschwilks M, Haupt S, Hofius D, Neumann S (2006) Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J Exp Bot 57:911–921. https://doi.org/10.1093/jxb/erj076
Bolin JF, Maass E, Tennakoon KU, Musselman LJ (2009) Host-specific germination of the root holoparasite Hydnora triceps (Hydnoraceae). Botany 87:1250–1254. https://doi.org/10.1139/B09-078
Brokamp G, Dostert N, Cáceres-H F, Weigend M (2012) Parasitism and haustorium anatomy of Krameria lappacea (Dombey) Burdet & B.B. Simpson (Krameriaceae), an endangered medicinal plant from the Andean deserts. J Arid Environ 83:94–100. https://doi.org/10.1016/j.jaridenv.2012.03.004
Bromham L, Cowman PF, Lanfear R (2013) Parasitic plants have increased rates of molecular evolution across all three genomes. BMC Evol Biol 13:1–11. https://doi.org/10.1186/1471-2148-13-126
Calvin CL (1966) Anatomy of mistletoe (Phoradendron flavescens) seedlings grown in culture. Bot Gaz 127:171–183. https://doi.org/10.1086/336360
Calvin CL (1967) Anatomy of the endophytic system of the mistletoe, Phoradendron flavescens. Bot Gaz 128:117–137
Calvin CL, Wilson CA (1995) Relationship of the mistletoe Phoradendron macrophyllum (Viscaceae) to the wood of its host. IAWA J 16:33–45. https://doi.org/10.1163/22941932-90001386
Calvin CL, Wilson CA (1996) Endophytic system. In: Hawksworth FG, Wiens D (eds) Dwarf mistletoes: biology, pathology, and systematics. United States Department of Agriculture Forest Service, Washington, DC, p 429
Calvin C, Wilson CA (1998) Comparative morphology of haustoria within African Loranthaceae. In: Polhill R, Wiens D (eds) Mistletoes of Africa. Royal Botanic Gardens, Kew, London, pp 17–317
Calvin CL, Wilson CA (2006) Comparative morphology of epicortical roots in old and new world Loranthaceae with reference to root types, origin, patterns of longitudinal extension and potential for clonal growth. Flora Morphol Distrib Funct Ecol Plants 201:51–64. https://doi.org/10.1016/j.flora.2005.03.001
Cameron DD, Seel WE (2007) Functional anatomy of haustoria formed by Rhinanthus minor: linking evidence from histology and isotope tracing. New Phytol 174:412–419
Cameron DD, Coats AM, Seel WE (2006) Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Ann Bot 98:1289–1299. https://doi.org/10.1093/aob/mcl218
Ceccantini G, Oliveira-da-Silva M, Ángeles G, Teixeira-Costa L (2019) Unfitting pipes! Patterns of connection between mistletoes and their hosts: anatomical and hydraulic consequences for angiosperms parasitizing conifers. In: 15th world congress on parasitic plants, Amsterdam, The Netherlands, p 126
Cesarino I, Dello IR, Kirschner GK et al (2020) Plant science’s next top models. Ann Bot 126:1–23. https://doi.org/10.1093/aob/mcaa063
Clarke CR, Timko MP, Yoder JI et al (2019) Molecular dialog between parasitic plants and their hosts. Annu Rev Phytopathol 57:279–299. https://doi.org/10.1146/annurev-phyto-082718-100043
Combes C (2001) Parasitism: the ecology and evolution of intimate interactions. University of Chicago Press, Chicago
Condon J, Kuijt J (1994) Anatomy and ultrastructure of the primary endophyte of Ileostylus micranthus (Loranthaceae). Int J Plant Sci 155:350–364
Cui S, Wakatake T, Hashimoto K et al (2016) Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant, Phtheirospermum japonicum. Plant Physiol 170:1492–1503. https://doi.org/10.1104/pp.15.01786
De Vega C, Ortiz PL, Arista M, Talavera S (2007) The endophytic system of Mediterranean Cytinus (Cytinaceae) developing on five host Cistaceae species. Ann Bot 100:1209–1217. https://doi.org/10.1093/aob/mcm217
de Vega C, Arista M, Ortiz PL, Talavera S (2010) Anatomical relations among endophytic holoparasitic angiosperms, autotrophic host plants and mycorrhizal fungi: a novel tripartite interaction. Am J Bot 97:730–737. https://doi.org/10.3732/ajb.0900147
Dell B, Kuo J, Burbidge AH (1982) Anatomy of Pilostyles hamiltonii C. A. Gardner (Rafflesiaceae) in stems of Daviesia. Aust J Bot 30:1–9. https://doi.org/10.1071/BT9820001
do Amaral MM, Ceccantini G (2011) The endoparasite Pilostyles ulei (Apodanthaceae - Cucurbitales) influences wood structure in three host species of Mimosa. IAWA J 32:1–13
Dörr I (1990) Sieve elements in haustoria of parasitic angiosperms. In: Behnke H-D, Sjolund RD (eds) Sieve elements: comparative structure, induction and development. Springer, Berlin, p 305
Dörr I (1997) How Striga parasitizes its host: a TEM and SEM study. Ann Bot 79:463–472
Dörr I, Kollmann R (1995) Symplasmic sieve element continuity between Orobanche and its host. Bot Acta 108:47–55. https://doi.org/10.1111/j.1438-8677.1995.tb00830.x
Dzerefos CM, Witkowski ETF (1997) Development and anatomy of the attachment structure of woodrose-producing mistletoes. S Afr J Bot 63:416–420. https://doi.org/10.1016/S0254-6299(15)30794-8
Esau K (1965) Plant anatomy, 2nd edn. Wiley, New York
Esau K (1969) The Phloem: Handbuch der Pflanzenanatomie, vol V. Te. Gebrüder Borntraeger, Berlin
Fahmy GM (1993) Transpiration and dry matter allocation in the angiosperm root parasite Cynomorium coccineum L. and two of its halophytic hosts. Biol Plant 35:603–608. https://doi.org/10.1007/BF02928038
Fahmy GM, Hassan AERH (2020) Haustorial structure of the holoparasitic angiosperm Cynomorium coccineum L. invading host roots. Flora. https://doi.org/10.1016/j.flora.2020.151731
Feild TS, Brodribb TJ (2005) A unique mode of parasitism in the conifer coral tree Parasitaxus ustus (Podocarpaceae). Plant Cell Environ 28:1316–1325. https://doi.org/10.1111/j.1365-3040.2005.01378.x
Fineran BA (1965) Studies on the root parasitism of Exocarpus bidwillii Hook. F. - V Early development of the haustorium. Phytomorphology 15:10–25
Fineran BA (1985) Graniferous tracheary elements in haustoria of root parasitic angiosperms. Bot Rev 51:389–441. https://doi.org/10.1007/BF02860969
Fineran BA (1996) Flange-type parenchyma cells: occurrence and structure in the haustorium of the dwarf mistletoe Korthalsella (Viscaceae). Protoplasma 194:40–53. https://doi.org/10.1007/BF01273166
Fineran BA, Bullock S (1979) Ultrastructure of graniferous tracheary elements in the haustorium of Exocarpus bidwillii, a root hemi-parasite of the Santalaceae. Proc R Soc Lond Ser B Biol Sci 204:329–343
Fineran BA, Calvin CL (2000) Transfer cells and flange cells in sinkers of the mistletoe Phoradendron macrophyllum (Viscaceae), and their novel combination. Protoplasma 211:76–93. https://doi.org/10.1007/BF01279901
Forstreuter W (1988) Zur morphologie, anatomie und okologie von Tripodanthus acutifolius (Ruiz et Pav.) Tiegh. (Loranthaceae). Philipps-Universitat Marburg
García-Franco JG, López-Portillo J, Ángeles G (2007) The holoparasitic endophyte Bdallophyton americanum affects root water conductivity of the tree Bursera simaruba. Trees Struct Funct 21:215–220. https://doi.org/10.1007/s00468-006-0113-z
Gedalovich-Shedletzky E, Kuijt J (1990) An ultrastructural study of the tuber strands of Balanophora (Balanophoraceae). Can J Bot 68:1271–1279
Goebel K (1905) Organography of plants, part II, special organography, (authorize. Clarendon Press, Cambridge
Goyet V, Wada S, Cui S et al (2019) Haustorium inducing factors for parasitic Orobanchaceae. Front Plant Sci 10:1–8. https://doi.org/10.3389/fpls.2019.01056
Hansen B (2015) Balanophorales. In: Kubitzki K (ed) The families and genera of vascular plants. Volume XII flowering plants. Eudicots. Santalales, Balanophorales. Springer, Heidelberg, pp 192–208
Hawksworth FG (1983) Mistletoes as forest parasites. In: Calder M, Bernhardt P (eds) The biology of mistletoes. Academic Press, Sidney, p 348
Heide-Jørgensen HS (1991) Anatomy and ultrastructure of the haustorium of Cassytha pubescens R. Br. I. The adhesive disk. Bot Gaz 152:321–334
Heide-Jørgensen HS (2008) Parasitic flowering plants. Brill, Leiden
Heide-Jørgensen HS (2013) Introduction: the parasitic syndrome in higher plants. In: Joel DM, Gressel J, Musselman LJ (eds) Parasitic Orobanchaceae: parasitic mechanisms and control strategies. Springer, Berlin, p 518
Heide-Jørgensen HS, Kuijt J (1995) The haustorium of the root parasite Triphysaria (Scrophulariaceae), with special reference to xylem bridge ultrastructure. Am J Bot 82:782–797
Heil H (1926) Haustorialstudien an Struthanthusarten. Flora oder Allg Bot Zeitung 121:40–76. https://doi.org/10.1016/S0367-1615(17)31101-1
Heinricher E (1917) Die erste Aufzucht einer Rafflesiaceae, Cytinus hypocistis L., aus Samen. Ber Dtsch Bot Ges 35:505–512. https://doi.org/10.1007/bf01547069
Hibberd JM, Jeschke WD (2001) Solute flux into parasitic plants. J Exp Bot 52:2043–2049
Holzapfel S (2001) Studies of the New Zealand root-parasite Dactylanthus taylorii (Balanophoraceae). Englera 22:7–176
Hsiao S-C, Mauseth JD, Peng C-I (1995) Composite bundles, the host/parasite interface in the holoparasitic angiosperms Langsdorffia and Balanophora (Balanophoraceae). Am J Bot 82:81–91
Hu B, Sakakibara H, Takebayashi Y et al (2017) Mistletoe infestation mediates alteration of the phytohormone profile and anti-oxidative metabolism in bark and wood of its host Pinus sylvestris. Tree Physiol 37:676–691. https://doi.org/10.1093/treephys/tpx006
Irving LJ, Cameron DD (2009) You are what you eat: interactions between root parasitic plants and their hosts. Adv Bot Res 50:87–138. https://doi.org/10.1016/S0065-2296(08)00803-3
Israel S, Dörr I, Kollmann R (1980) Das Phloem der Haustorien von Cuscuta. Protoplasma 103:309–321
Joel DM (2013) Functional structure of the mature haustorium. In: Joel DM, Gressel J, Musselman LJ (eds) Parasitic Orobanchaceae: parasitic mechanisms and control strategies. Springer, Berlin, p 518
Joel DM, Losner-Goshen D (1994) The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Can J Bot 72:564–574. https://doi.org/10.1139/b94-075
Kokla A, Melnyk CW (2018) Developing a thief: Haustoria formation in parasitic plants. Dev Biol 442:53–59. https://doi.org/10.1016/j.ydbio.2018.06.013
Krupp A, Heller A, Spring O (2019) Development of phloem connection between the parasitic plant Orobanche cumana and its host sunflower. Protoplasma 256:1385–1397. https://doi.org/10.1007/s00709-019-01393-z
Kuijt J (1964) Critical observations on the parasitism of New World mistletoes. Can J Bot 42:1243–1278
Kuijt J (1965) On the nature and action of the Santalalean haustorium, as exemplified by Phthirusa and Antidaphne (Loranthaceae). Acta Bot Neerl 14:278–307
Kuijt J (1966) Parasitism in Pholisma (Lennoaceae). I. external morphology of subterranean organs. Am J Bot 53:82–86
Kuijt J (1969) The biology of parasitic flowering plants. University of California Press, Berkeley
Kuijt J (1977) Haustoria of phanerogamic parasites. Annu Rev Phytopathol 17:91–118
Kuijt J (1986) Observations on establishment and early shoot emergence of Viscum minimum (Viscaceae). Acta Bot Neerl 35:449–456
Kuijt J (2015) Santalales. In: Kubitzki K (ed) The families and genera of vascular plants. Volume XII flowering plants. Eudicots. Santalales, Balanophorales. Springer, Berlin, p 209
Kuijt J, Dobbins DR (1971) Phloem in the haustorium of Castilleja (Scrophulariaceae). Can J Bot 49:1735–1736
Kuijt J, Dong W (1990) Surface features of the leaves of Balanophoraceae—a family without stomata ? Plant Syst Evol 170:29–35
Kuijt J, Lye D (2005) Gross xylem structure of the interface of Psittacanthus ramiflorus (Loranthaceae) with its hosts and with a hyperparasite. Bot J Linn Soc 147:197–201. https://doi.org/10.1111/j.1095-8339.2005.00370.x
Kuijt J, Toth R (1985) Structure of the host-parasite interface of Boschniakia hookeri Walpers (Orobanchaceae). Acta Bot Neerl 34:257–270. https://doi.org/10.1111/j.1438-8677.1985.tb01918.x
Kuijt J, Bray D, Olson AR (1985) Anatomy and ultrastructure of the endophytic system of Pilostyles thurberi (Rafflesiaceae). Can J Bot 63:1231–1240
Kusano SR (1902) Studies on the parasitism of Buckleya and on the structure of its haustorium. J Coll Sci 17:1–42
Lambers H, Oliveira RS (2019) Biotic influences: parasitic associations. In: Lambers H, Oliveira RS (eds) Plant physiological ecology, 3rd edn. Springer, Cham, pp 597–613
Lamont B (1983) Germination of mistletoes. In: Calder DM, Bernhardt P (eds) The biology of mistletoes. Academic Press, Melbourne, p 348
Lau ES, Oakley TH (2020) Multi-level convergence of complex traits and the evolution of bioluminescence. Biol Rev 13:1–40. https://doi.org/10.1111/brv.12672
Lee KB (2007) Structure and development of the upper haustorium in the parasitic flowering plant Cuscuta japonica (Convolvulaceae). Am J Bot 94:737–745. https://doi.org/10.3732/ajb.94.5.737
Losner-Goshen D, Portnoy VH, Mayer AM, Joel DM (1998) Pectolytic activity by the haustorium of the parasitic plant Orobanche L. (Orobanchaceae) in host roots. Ann Bot 81:319–326. https://doi.org/10.1006/anbo.1997.0563
Lye D (2006) Charting the isophasic endophyte of dwarf mistletoe Arceuthobium douglasii (Viscaceae) in host apical buds. Ann Bot 97:953–963. https://doi.org/10.1093/aob/mcl051
MacLeod DG (1962) Some anatomical and physiological observations on two species of Cuscuta. Trans Bot Soc Edinb 39:302–315. https://doi.org/10.1080/13594866209441713
Mangenot MG (1947) Sur les galles de Thonningia coccinea. Comptes rendus l’Académie des Sci 224:665–666
Mauseth JD, Rezaei K (2013) Morphogenesis in the parasitic plant Viscum minimum (Viscaceae) is highly altered, having apical meristems but lacking roots, stems, and leaves. Int J Plant Sci 174:791–801. https://doi.org/10.1086/669926
Mauseth JD, Montenegro G, Walckowiak AM (1985) Host infection and flower formation by the parasite Tristerix aphyllus (Loranthaceae). Can J Bot 63:567–581
Mauseth JD, Hsiao S-C, Montenegro G (1992) Vegetative body of the parasitic angiosperm Ombrophytum subterraneum (Balanophoraceae). Bull Torrey Bot Club 119:407–417
McLuckie J (1924) Studies in parasitism. I. A contribution to the physiology of the genus Cassytha. Proc Linn Soc N S W 49:55–78. https://doi.org/10.1177/1947603511410419
Merckx VSFT (2013) Mycoheterotrophy. The biology of plants living on fungi. Springer, Leiden
Merckx V, Bidartondo MI, Hynson NA (2009) Myco-heterotrophy: when fungi host plants. Ann Bot 104:1255–1261. https://doi.org/10.1093/aob/mcp235
Miller DD, de Ruijter NCA, Emons AMC (1997) From signal to form: aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J Exp Bot 48:1881–1896. https://doi.org/10.1093/jxb/48.11.1881
Molina J, Hazzouri KM, Nickrent D et al (2014) Possible loss of the chloroplast genome in the parasitic flowering plant Rafflesia lagascae (Rafflesiaceae). Mol Biol Evol 31:793–803. https://doi.org/10.1093/molbev/msu051
Moore LB (1940) The structure and life history of the root parasite Dactylanthus taylor Hook. f. N Z J Sci Technol 21:206–224
Musselman LJ (1977) Seed germination and seedlings of Krameria lanceolata (Krameriaceae). Sida 7:224–225
Musselman LJ, Dickison WC (1975) The structure and development of the haustorium in parasitic Scrophulariaceae. Bot J Linn Soc 70:183–212. https://doi.org/10.1139/b89-381
Musselman LJ, Press MC (1995) Introduction to parasitic plants. In: Press MC, Graves JD (eds) Parasitic plants. Chapman & Hall, London, p 292
Nagar R, Singh M, Sanwal GG (1984) Cell wall degrading enzymes in Cuscuta reflexa and its hosts. J Exp Bot 35:1104–1112
Nickrent DL (2020) Parasitic angiosperms: how often and how many? Taxon 69:5–27. https://doi.org/10.1002/tax.12195
Nikolov LA, Tomlinson PB, Manickam S et al (2014) Holoparasitic Rafflesiaceae possess the most reduced endophytes and yet give rise to the world’s largest flowers. Ann Bot 114:233–242. https://doi.org/10.1093/aob/mcu114
Oborník M (2019) Endosymbiotic evolution of algae, secondary heterotrophy and parasitism. Biomolecules 9:1–10. https://doi.org/10.3390/biom9070266
Offler CE, Patrick JW (2020) Transfer cells: what regulates the development of their intricate wall labyrinths ? New Phytol 228:427–444. https://doi.org/10.1111/nph.16707
Ouyang Y, Zhang X, Chen Y et al (2016) Growth, photosynthesis and haustorial development of semiparasitic Santalum album L. penetrating into roots of three hosts: a comparative study. Trees Struct Funct 30:317–328. https://doi.org/10.1007/s00468-015-1303-3
Pate JS, Kuo J, Davidson NJ (1990) Morphology and anatomy of the haustorium of the root hemiparasite Olax phyllanthi (Olacaceae), with special reference to the haustorial interface. Ann Bot 65:425–436
Pérez-de-Luque A (2013) Haustorium invasion into host tissues. In: Joel DM, Gressel J, Musselman LJ (eds) Parasitic Orobanchaceae: parasitic mechanisms and control strategies. Springer, Heidelberg, pp 75–86
Rajanna L, Shivamurthy GR (2001) Occurrence of graniferous tracheary elements in the haustorium of Cassytha filiformis Linn., a stem parasite of Lauraceae. Taiwania 46:40–48
Riopel JL, Timko MP (1995) Haustorial initiation and differentiation. In: Press MC, Graves JD (eds) Parasitic plants. Chapman & Hall, London, pp 39–79
Rosenblum EB, Parent CE, Brandt EE (2014) The molecular basis of phenotypic convergence. Annu Rev Ecol Evol Syst 45:203–226. https://doi.org/10.1146/annurev-ecolsys-120213-091851
Rutherford RJ (1970) The anatomy and cytology of Pilostyles Thurberi Gray (Rafflesiaceae). Aliso 7:263–288
Rutishauser R (2016) Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): a pictorial report at the interface of developmental biology and morphological diversification. Ann Bot 117:811–832. https://doi.org/10.1093/aob/mcv172
Rutishauser R (2020) EvoDevo: past and future of continuum and process plant morphology. Philosophies 5:41. https://doi.org/10.3390/philosophies5040041
Rutishauser R, Isler B (2001) Developmental genetics and morphological evolution of flowering plants, especially bladderworts (Utricularia): fuzzy Arberian morphology complements classical morphology. Ann Bot 88:1173–1202. https://doi.org/10.1006/anbo.2001.1498
Sallé G (1983) Germination and establishment of Viscum album L. In: Calder MC, Bernhardt P (eds) The biology of mistletoes. Academic Press, Sydney, pp 145–159
Sattler R (1996) Classical morphology and continuum morphology: opposition and continuum. Ann Bot 78:577–581. https://doi.org/10.1006/anbo.1996.0163
Schrenk H (1894) Parasitism of Epiphegus Virginiana (Broom Rape, Cancer Root.). Proc Am Microsc Soc 15:91–127
Seel WE, Cechin I, Vincent CA, Press MC (1992) Carbon partitioning in parasitic angiosperms and their hosts. In: Pollock CJ, Farrar JF, Gordon AJ (eds) Carbon partitioning within and between organisms. BIOS Scientific Publishers Ltd, Oxford
Shimizu K, Aoki K (2019) Development of parasitic organs of a stem holoparasitic plant in genus Cuscuta. Front Plant Sci 10:1–11. https://doi.org/10.3389/fpls.2019.01435
Shimizu K, Hozumi A, Aoki K (2018) Organization of vascular cells in the haustorium of the parasitic flowering plant Cuscuta japonica. Plant Cell Physiol 59:720–728. https://doi.org/10.1093/pcp/pcx197
Shivamurthy GR, Arekal D, Swamy BGL (1981) Establishment, structure and morphology of the tuber of Balanophora. Ann Bot 47:735–745
Sinha NR (1999) Leaf development in angiosperms. Annu Rev Plant Physiol Plant Mol Biol 50:419–446. https://doi.org/10.1016/B978-0-12-394807-6.00111-8
Spallek T, Mutuku M, Shirasu K (2013) The genus Striga: a witch profile. Mol Plant Pathol 14:861–869. https://doi.org/10.1111/mpp.12058
Spallek T, Melnyk CW, Wakatake T et al (2017) Interspecies hormonal control of host root morphology by parasitic plants. Proc Natl Acad Sci 114:5283–5288. https://doi.org/10.1073/pnas.1619078114
Tate P (1925) On the anatomy of Orobanche hederae Duby, and its attachment to the host. New Phytol 24:284–293. https://doi.org/10.1111/j.1469-8137.1925.tb06671.x
Teixeira-Costa L, Ceccantini G (2018) Haustório, haustor, apressório, extensor: Glossário ilustrado sobre plantas parasitas e a problemática das homologias das estruturas de conexão parasita-hospedeira. Bol Botânica da Univ São Paulo 36:103–120. https://doi.org/10.11606/issn.2316-9052.v36ip103-120
Teixeira-Costa L, Davis CC, Ceccantini G. Striking developmental convergence in angiosperm endoparasites. Am J Bot (in press)
Teixeira-Costa L, Ocampo G, Ceccantini G (2020) Morphogenesis and evolution of mistletoes’ haustoria. In: Demarco D (ed) Plant ontogeny. Nova Science Publishers Inc, New York, pp 107–157
Tennakoon KU, Bolin JF, Musselman LJ, Maass E (2007) Structural attributes of the hypogeous holoparasite Hydnora triceps Drège & Meyer (Hydnoraceae). Am J Bot 94:1439–1449
Těšitel J (2016) Functional biology of parasitic plants: a review. Plant Ecol Evol 149:5–20. https://doi.org/10.5091/plecevo.2016.1097
Thoday D (1951) The haustorial system of Viscum album. J Exp Bot 2:1–19. https://doi.org/10.1093/jxb/2.1.1-a
Toth R, Kuijt J (1977) Anatomy and ultrastructure of the haustorium in Comandra (Santalaceae). Can J Bot 55:455–469. https://doi.org/10.1139/b77-055
Vaughn KC (2002) Attachment of the parasitic weed dodder to the host. Protoplasma 219:227–237. https://doi.org/10.1007/s007090200024
Vaughn KC (2003) Dodder hyphae invade the host: a structural and immunocytochemical characterization. Protoplasma 220:189–200. https://doi.org/10.1007/s00709-002-0038-3
Vaughn KC (2006) Conversion of the searching hyphae of dodder into xylic and phloic hyphae: a cytochemical and immunocytochemical investigation. Int J Plant Sci 167:1099–1114
Venturelli M (1980) Desenvolvimento anatômico do haustório primário de Struthanthus vulgaris Mart. Bol Botânica da Univ São Paulo 8:47–64
Vogel A, Schwacke R, Denton AK et al (2018) Footprints of parasitism in the genome of the parasitic flowering plant Cuscuta campestris. Nat Commun 9:2515. https://doi.org/10.1038/s41467-018-04344-z
von Forstmeier L, Weberling F, Weber HC (1983) Zum Parasitismus von Cytinus hypocistis L. (Rafflesiaceae). Beiträge zur Bioiogie der Pflanz 58:299–311
Wakatake T, Yoshida S, Shirasu K (2018) Induced cell fate transitions at multiple cell layers configure haustorium development in parasitic plants. Development. https://doi.org/10.1242/dev.164848RESEARCH
Watanabe K (1933) Ungeschlechtliche Fortpflanzung von Mitrastemon Yamamotoi. Proc Imp Acad Japan 9:412–415
Watson DM, Cook M, Fadini RF (2020) Towards best-practice management of mistletoes in horticulture. Botany. https://doi.org/10.1139/cjb-2019-0205
Weber HC (1987) Evolution of the secondary haustoria to a primary haustorium in the parasitic Scrophulariaceae/Orobanchaceae. Plant Syst Evol 156:127–131
Westwood JH, Yoder JI, Timko MP, dePamphilis CW (2010) The evolution of parasitism in plants. Trends Plant Sci 15:227–235. https://doi.org/10.1016/j.tplants.2010.01.004
Wicaksono A, Mursidawati S, Molina J (2020) A plant within a plant: insights on the development of the Rafflesia endophyte within its host. Bot Rev. https://doi.org/10.1007/s12229-020-09236-w
Yang Z, Wafula EK, Honaas LA et al (2015) Comparative transcriptome analyses reveal core parasitism genes and suggest gene duplication and repurposing as sources of structural novelty. Mol Biol Evol 32:767–790. https://doi.org/10.1093/molbev/msu343
Yoshida S, Cui S, Ichihashi Y, Shirasu K (2016) The haustorium, a specialized invasive organ in parasitic plants. Annu Rev Plant Biol 67:643–667. https://doi.org/10.1146/annurev-arplant-043015-111702
Yoshida S, Kim S, Wafula EK et al (2019) Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr Biol 29:3041–3052. https://doi.org/10.1016/j.cub.2019.07.086
Zhang X, Teixeira da Silva JA, Duan J et al (2012) Endogenous hormone levels and anatomical characters of haustoria in Santalum album L. seedlings before and after attachment to the host. J Plant Physiol 169:859–866. https://doi.org/10.1016/j.jplph.2012.02.010
Zhang X, Berkowitz O, Teixeira da Silva JA et al (2015) RNA-Seq analysis identifies key genes associated with haustorial development in the root hemiparasite Santalum album. Front Plant Sci 6:661. https://doi.org/10.3389/fpls.2015.00661
Zhang X, Liu B, Guo Q et al (2016) Construction of a haustorium development associated SSH library in Thesium chinense and analysis of specific ESTs included by Imperata cylindrica. Biochem Syst Ecol 64:46–52. https://doi.org/10.1016/j.bse.2015.11.007
Acknowledgements
I would like to thank Dr. Gregorio Ceccantini for the support, mentoring, and fruitful discussions during many years of learning and collaborations. Dr. Fernanda Oliveira provided valuable suggestions to earlier versions of this work. Dr. Rafael Cruz provided excellent discussions about evolutionary developmental biology, identity of plant organs, and morphological misfits. I am also thankful to colleagues Dr. Charles Davis and Dr. Maximilian Weigend who kindly provided some of the photographs used in this work. The image used in Fig. 2g is under a Creative Commons, Non-Commercial Share-Alike 3.0 type of license (https://creativecommons.org/licenses/by-nc-sa/3.0/legalcode). I also thank the Harvard University Herbaria of Harvard University for allowing the capturing of images from specimens in the anatomical slide collection (Fig. 6a–h). Funding was provided by the Harvard University Herbaria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teixeira-Costa, L. A living bridge between two enemies: haustorium structure and evolution across parasitic flowering plants. Braz. J. Bot 44, 165–178 (2021). https://doi.org/10.1007/s40415-021-00704-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-021-00704-0