Abstract
The symptom similarities between training-overload (with or without an Overtraining Syndrome (OTS) diagnosis) and Relative Energy Deficiency in Sport (RED-S) are significant, with both initiating from a hypothalamic–pituitary origin, that can be influenced by low carbohydrate (CHO) and energy availability (EA). In this narrative review we wish to showcase that many of the negative outcomes of training-overload (with, or without an OTS diagnosis) may be primarily due to misdiagnosed under-fueling, or RED-S, via low EA and/or low CHO availability. Accordingly, we undertook an analysis of training-overload/OTS type studies that have also collected and analyzed for energy intake (EI), CHO, exercise energy expenditure (EEE) and/or EA. Eighteen of the 21 studies (86%) that met our criteria showed indications of an EA decrease or difference between two cohorts within a given study (n = 14 studies) or CHO availability decrease (n = 4 studies) during the training-overload/OTS period, resulting in both training-overload/OTS and RED-S symptom outcomes compared to control conditions. Furthermore, we demonstrate significantly similar symptom overlaps across much of the OTS (n = 57 studies) and RED-S/Female Athlete Triad (n = 88 studies) literature. It is important to note that the prevention of under-recovery is multi-factorial, but many aspects are based around EA and CHO availability. Herein we have demonstrated that OTS and RED-S have many shared pathways, symptoms, and diagnostic complexities. Substantial attention is required to increase the knowledge and awareness of RED-S, and to enhance the diagnostic accuracy of both OTS and RED-S, to allow clinicians to more accurately exclude LEA/RED-S from OTS diagnoses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Energy availability (EA) is defined as [Energy Intake (EI)—Exercise Energy Expenditure (EEE)] corrected for fat free mass, and is disturbed both by training-overload (e.g. excessive EEE) and/or inadequate EI. Accordingly the EA concept potentially provides an elegant model that can unify many of the symptoms that present with both RED-S and many training-overload/OTS situations. |
Both OTS and RED-S identification are based on a diagnosis of exclusion, with both lacking a single validated universal identifier but both syndromes feature progressive deterioration of quantitative and qualitative health and performance outcomes. |
Although under-recovery resulting in OTS is multi-factorial, there are significant and close parallels between OTS and RED-S, and in many instances the negative outcomes of training-overload (with, or without an OTS diagnosis) may primarily be due to misdiagnosed under-fueling via low EA. |
1 Introduction
Extreme training loads are required for elite-athlete success, especially in endurance sports [1,2,3,4,5,6,7,8]. At the elite/professional level, peak training volumes can exceed 30 h/week in weight-supported endurance sports such as swimming, cycling, triathlon and rowing [1,2,3,4], resulting in substantial exercise energy expenditures (EEE). Furthermore, performance constraints of many sports also favor an ideal power (or force) to weight ratio [9,10,11], incentivizing body mass/composition manipulations via adjustment of energy intake (EI) [12,13,14]. The prolonged combination of these pursuits creates a perfect storm for potentially adverse outcomes of training-overload [with or without the diagnoses of Overtraining Syndrome (OTS)] and/or under-fueling [with a potential diagnosis of Relative Energy Deficiency in Sport (RED-S)]. Accordingly, OTS and RED-S have many shared pathways and symptoms of under-recovery.
Undeniably, acute fatigue, caused by a single or series of training sessions, is part of a normal elite training program. However, when recovery is inadequate, this acute fatigue can eventually progress and manifest into a continuum of short-term performance decrements (days to weeks) ranging from Functional Overreaching (FOR), to unplanned fatigue termed Non-Functional Overreaching (NFOR), and then over time (weeks to months of performance decrements), to OTS [6]. Meeusen et al. [6] have defined OTS as follows: “an accumulation of training and/or non-training stress resulting in long-term decrement in performance capacity”. Thus, the defining factor between OTS and NFOR/FOR is the time needed for performance restoration, not the specific type of training or life stress causing the performance decrement in the first place. For this review we will use the term training-overload/OTS, unless FOR or NFOR are specified within the original paper; appreciating that prolonged OTS is much more clinically significant than FOR or NFOR. We note that, by definition, only studies that quantify a significant and sustained decline in performance outcomes can be termed FOR, NFOR or OTS studies. Therefore, the current review will differentiate between these studies and others that involve a significant increase in training load, but not necessarily OTS, which should be defined as training-overload studies.
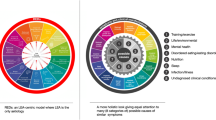
RED-S was first introduced by the International Olympic Committee (IOC) in 2014 [15] (updated by another IOC consensus in 2018 [16]). Within the RED-S umbrella, the negative health outcomes of low energy availability (LEA) on reproductive and bone health are known as the Triad [17]. RED-S has been defined as a complex syndrome caused by LEA that results in impaired physiological function, negatively impacting aspects of health and performance. The introduction of RED-S was made in response to emerging scientific literature supporting the premise that chronic LEA can result in negative health outcomes beyond the hypothalamic–pituitary–gonadal (HPG) axis and bone health, and because male athletes were also seen to suffer health and performance consequences. The IOC RED-S consensus authors have developed spoke and wheel descriptive figures [15, 16] to demonstrate how chronic LEA potentially affects various body systems resulting in negative health and performance outcomes and to exhibit the relationship to the Triad. It is relevant to note that psychological problems, specifically disordered eating or eating disorders, can precede LEA/RED-S, and also, in some athletes, LEA itself can result in psychological sequelae [15]. The cornerstone of treatment for RED-S is the restoration of EA, generally most optimally achieved through enhancing EI via nutritional interventions, but also potentially addressing EEE through exercise modifications (for review see: [18]).
The primary etiological factor underpinning RED-S is chronically poor energy availability (EA). EA is defined as [EI–EEE] corrected by fat-free mass (FFM; Fig. 1), and can be disturbed both by training-overload (e.g. excessive EEE) and/or inadequate EI. Regardless of source of LEA, chronic LEA has been shown to result in RED-S and many related OTS symptoms (Tables 1, 2). Certainly, the EA concept potentially provides an elegant model that can unify many of the symptoms that present with both RED-S and many training-overload/OTS situations (Fig. 1). To date, however, only a handful of the training-overload/OTS publications have specifically considered assessing EA in the underlying methodology [19,20,21,22], and despite a plethora of expert RED-S/Triad [23,24,25,26,27] and OTS [28,29,30,31] reviews over the previous decades, we are unaware of a single extensive review which conceptually highlights the many similarities of RED-S and OTS, as well as the diagnostic challenges of each syndrome. Furthermore, it is also important to note that despite diagnostic progress as elaborated below (Section 2), there is no singular validated diagnosis method/tool for either OTS (or FOR/NFOR) or RED-S–with both syndromes having a complex overlap of symptoms wherein a diagnosis of exclusion is recommended.
Energy availability as a theoretical unifying framework for both RED-S and potentially many training-overload/OTS symptoms, while identifying the specific underlying etiology for RED-S and OTS, once all appropriate diagnostic exclusion criteria are met. EA, energy availability; EEE, exercise energy expenditure; EI, energy intake; FFM, fat free mass; FOR, functional overreaching; HPA, hypothalamic-pituitary-adrenal; LCA, low carbohydrate availability; LEA, low energy availability; NFOR, non-functional overreaching; OTS, overtraining syndrome; RED-S, relative energy deficiency in sport
The premise of this narrative review is to showcase that many of the negative outcomes of training-overload (with, or without an OTS diagnosis) may be misdiagnosed due to a failure to recognize under-recovery from under-fueling, or a frank diagnosis of RED-S. Although under-recovery is clearly multifactorial (e.g. sleep, stress), this review will demonstrate that under-recovery specifically attributed to under-fueling, resulting in low EA and poor CHO availability, is especially prevalent and often overlooked and misdiagnosed in training-overload/OTS situations. We will showcase the complexity of diagnosis of OTS and RED-S due to the significant overlapping symptom similarities between training-overload/OTS and RED-S. Furthermore, we will establish that (1) humans do not always naturally increase ad libitum EI enough to match significantly increased EEE, resulting in many instances in inadvertent and non-intentional LEA; and (2) that there is a failure in some high- performance endurance athletes to intentionally achieve compensatory EI during chronically high EEEs. We propose that an analysis of the literature shows that many classic training-overload/OTS studies may be confounded with underlying LEA (due to inadequate EI (and primarily CHO macronutrients) coupled with high training loads), as many training-overload/OTS studies do not even assess for LEA or mention it as a confounding factor that should be a diagnosis exclusion criteria. A secondary aim is to show that the symptoms of RED-S and training-overload/OTS significantly overlap, and as such, a definitive diagnosis of training-overload/OTS or RED-S is fraught with challenges. A final aim was to create increased awareness regarding the commonalities between training-overload/OTS and RED-S amongst the athlete, coaching, medical, physiology, psychology, and nutrition communities, who tend to work independently, thus emphasizing the fact that a multi-practitioner integrated approach is required when diagnosing and treating both OTS/NFOR and RED-S.
2 OTS vs. RED-S: Symptom Overlap and Diagnosis Complexities
It is beyond the scope of the current review to systemically highlight the step by step exclusion and diagnostic assessments of both OTS and RED-S, which have been extensively articulated in previous consensus statements [6, 16, 32]. Instead, we will demonstrate below that OTS and RED-S share a significant overlap of symptoms (Table 1) and diagnoses of exclusion, resulting in considerable difficulties in accurate diagnosis of either syndrome, where elements of LEA can be especially challenging.
2.1 Diagnosis Complexities Related to Overtraining Syndrome (OTS)
Despite extensive study and review over the past 40 years [6, 29,30,31, 33,34,35,36], there is still no single, readily available and validated diagnostic tool to identify OTS. Like RED-S (Sect. 2.2 below), OTS features a diagnosis of exclusion and a multi-factorial etiology in which performance decline is coupled with a broad and individually variable palette of psychological/emotional, physiological, immunological, and neuroendocrine decrements (Table 1), of which exercise/training may not be the sole causative factor. As highlighted in the OTS consensus statement, “the etiology of OTS involves the exclusion of organic diseases or infections and factors such as dietary caloric restriction (negative energy balance) and insufficient carbohydrate and/or protein intake, iron deficiency, magnesium deficiency, allergies, etc., together with identification of initiating events or triggers [6].” Therefore, LEA (or RED-S) should be ruled out from an NFOR/OTS diagnosis and once all confounding factors have been excluded: strictly speaking, an NFOR/OTS diagnosis would ultimately be based on just outstanding psychogenic and lifestyle stress factors being responsible for the unexplained prolonged fatigued and decreased performance, as outlined in Fig. 1.
Nevertheless, significant OTS diagnostic progress has been made by the Meeusen research group utilizing a two-bout maximal exercise protocol (with 4 h of rest between) in athletes with NFOR/OTS to detect attenuated changes in the typical exercise-induced increases to the hypothalamic–pituitary–adrenal (HPA) axis-related hormones (cortisol, adrenocorticotrophic hormone (ACTH), prolactin and human growth hormone [37, 38]. Building upon this, the Meeusen group has recently undertaken s tatistical discriminate analysis of 100 athletes reporting excessive fatigue and/or poor performances and found attenuated ACTH and prolactin responses in athletes with OTS to a second exercise test, coupled with a psychological test, resulted in correct diagnosis of NFOR and OTS with 98% sensitivity [39]. Additional data are needed to further validate this two-bout test across various athletic populations as well as explore its potential utility, or differential diagnosis, in RED-S situations.
It is also worth noting that some sports feature significant mechanical forces (e.g. running or contact based sports) or excessive mechanical overuse repetition (e.g. elite rowers take > 30,000–40,000 strokes/week). Therefore, diagnostic aspects of ‘mechanical’ over-training [5, 40] and overuse injuries can occur irrespective of an athlete’s EA due to over-riding tissue strain/damage (e.g. muscle, connective tissue, bone) and biomechanical inefficiencies [40,41,42]. It is beyond the scope of this review to expand further on the diagnosis of mechanical over-training, but important to note the extensive body of evidence demonstrating chronic LEA can accelerate poor bone mineral density (BMD) that is certainly an important moderating factor of the mechanical training load-injury etiology [16, 17, 43,44,45,46,47,48].
2.2 Diagnosis Complexities Related to Relative Energy Deficiency in Sport (RED-S)
To aid with the screening, diagnosis and return to play, the IOC published a RED-S clinical assessment tool (RED-S CAT) to assist clinicians [32]. The RED-S CAT provides a framework for assessment of athletes by accumulated clinical parameters of risk (e.g. blood work, injury history, menstrual status, bone mineral density, etc.), many of which are highlighted in Table 1. These accumulating risk factors result in categorizing athletes as low (green; full training and competing), moderate (yellow; continued training, with supervision, some modifications, and regular assessments) or high risk (red; athlete removed from sport for health and safety reasons but clinically supported and reassessed regularly). The tool also provides a RED-S specific return-to-play framework to facilitate these decisions [32]. The Low Energy Availability in Females Questionnaire (LEAF-Q) is a diagnostic screening tool, validated in female endurance athletes and dancers, to facilitate the identification of athletes at risk for RED-S [49]. A LEAM-Q screening tool for male athletes is under development. The Brief Eating-Disorder in Athletes Questionnaire (BEDA-Q) is another validated tool to aid in the diagnosis of eating disorders in athletes, which may be an underlying cause of RED-S [50].
It has long been recognized that LEA is the underlying etiology of the Triad, and by extension the RED-S syndrome [44, 51]. However, it is important to note that despite a mathematically simple definition (Fig. 1), the accurate calculation of EA is challenged by methodological considerations that introduce risk for significant under- or overestimation of EI [52] and/or EEE [53] and confound accurate estimations of daily dietary CHO intake [45, 54]. Furthermore, the “low” EA threshold of ~ 30 kcal/kg FFM/day which was established by assessing luteinizing hormone (LH) pulsatility every 10–15 min for 24 h under strictly controlled laboratory conditions, along with other LEA indicators such as bone turnover markers and triiodothyronine (T3), in healthy sedentary females [55, 56], may not translate to menstrual disturbances in free-living female athletes and/or other athlete cohorts, obviously including males. Indeed, experimental evidence has not always shown a relationship between measured EA and menstrual disturbances [45, 54, 57, 58]. Furthermore, an EA threshold in males remains to be established, but is thought to be lower [45, 59,60,61,62,63], while EA thresholds in various classifications of para-athletes continue to be speculative [64,65,66]. These methodological or diagnostic challenges associated with the precise assessment of EA underpin the complexity associated with accurate RED-S diagnoses [54], and by extension many situations of LEA may go undetected and lead to a potential misdiagnosis of NFOR/OTS, instead of RED-S.
2.3 Extensive Symptom Commonalities Between OTS and RED-S Resulting In Challenging Diagnoses
Given that many NFOR/OTS outcomes are based on the HPA axis (primarily cortisol/testosterone imbalance with regard to OTS [6, 28, 29, 33, 67, 68]), while many of the RED-S outcomes are based on the HPG axis [15, 16, 69,70,71], and thus both initiating from a hypothalamic-pituitary origin, it should not be surprising that NFOR/OTS and RED-S have significant commonalities of symptoms. Table 1 provides a primary original study comparison of symptom similarities (as reported in each individual study) clustering around six performance and seven health-based groupings (n = 57 OTS studies and n = 88 RED-S/Triad studies found). It is beyond the scope of this review to extensively elaborate on the impact and/or directionality of every marker, but we direct the reader to several OTS [31, 33, 72] and RED-S/Triad [17, 69, 73] reviews on this subject. These aforementioned reviews highlight many overlapping symptoms of OTS and RED-S, but our understanding of a definitive set of symptoms to diagnose either syndrome remains poor.
Indeed, across these 13 general performance and health symptom outcome groupings, RED-S/Triad and OTS share reported symptom similarities for all but one of them (bone outcomes). The predominant RED-S diagnostic criteria centers around endocrinology (sex and metabolic hormones) and bone health outcomes leading to injury (Table 1), due to the strongly developed research spanning nearly 40 years on the Triad [17], which triangulates on EA, reproductive and bone health. Conversely, OTS studies have centered primarily on associated hormone and blood work markers which have been shown to be associated with endurance performance and training adaptation decrements. Furthermore, both OTS and RED-S can also be significantly impacted by psychogenic factors and lifestyle stress. It is also interesting to note that the 88 identified RED-S/Triad studies feature primarily female athletes/subjects [female n = 7400 (78%); Males n = 2105 (22%)]. Conversely, the opposite sex breakdown is found across the 57 various training-overload/OTS studies [females n = 210 (19%); males n = 880 (81%); data only reported for studies given subject and sex breakdown numbers].
Several previous OTS reviews have potentially misdiagnosed OTS by highlighting a potential caloric mismatch (LEA) or low CHO availability as a confounding factor involved in the development of OTS [5, 28, 29, 31, 33, 35, 67, 68], instead of the development of RED-S. As emphasized above, OTS and RED-S identification are both based on a diagnosis of exclusion, as they mutually lack a single validated universal identifier for diagnosis. However, as highlighted within the OTS consensus statement, negative energy balance and/or insufficient CHO should be excluded from the OTS diagnosis [6]. Nevertheless, both OTS and RED-S feature accumulating quantitative and qualitative symptoms resulting in more certainty of accurate identification [6, 32, 74]. However, excluding LEA/RED-S from an OTS diagnosis is incredibly challenging, given the significant challenges with accurate EI and EEE measurement leading to RED-S identification [54]. Furthermore, both OTS and RED-S are syndromes of “under-recovery” resulting in a constellation of symptom overlap between OTS and RED-S (Table 1 and Section 3 below), which again makes accurate diagnosis challenging. Importantly, the current OTS consensus statement, published in 2013, pre-dates the 2014 introduction of the RED-S syndrome and its recognition of a wider sequalae of outcomes of chronic LEA [15]. In addition, because the focus of LEA within the Triad may have prevented consideration of its presence in male athletes [62, 75, 76], the overlap with the significant prevalence of males (81%) in training-overload/OTS studies may have gone unnoticed. The wide-ranging and over-lapping OTS and RED-S/Triad symptoms (Table 1) span across nearly all sport science and medicine disciplines. Therefore, to adequately prevent, diagnose and/or treat the multi-factorial underpinnings of under-recovery (OTS/RED-S), the collaborative expertise within medical, nutrition, physiology and psychology are all particularly essential (Fig. 2) [77]. Unfortunately, many athletes/coaches do not have access to such an extensive multi-disciplinary expertise.
Undeniably, the close parallels of OTS and RED-S symptoms are remarkable, resulting in significant chance for misdiagnoses; thus, the pursuit of more clear and separate diagnostic criteria for both syndromes needs more scientific consideration. However, the further development of accurate diagnostics and validated assessment tools is also scientifically challenging, because they are typically reliant on the collection of the prevalence of a range of cohort symptoms in cross-sectional observational studies rather than the more scientifically robust intervention-based randomized clinical trial (RCT) designs. Generally, long-term interventional RCTs are less ethically feasible since one would need to induce full-blown OTS or RED-S for an accurate and comprehensive diagnoses. Accordingly, as outlined below, most of the intervention-based OTS literature are actually short-term studies inducing FOR/NFOR, rather than actual OTS.
3 Does Unappreciated Under-Fueling (LEA) and/or Low CHO Availability Confound Much of the Training-Overload/OTS Literature?
In this section, we hope to further demonstrate the hypothesis that many of the negative outcomes of training-overload (with, or without an OTS, NFOR or FOR diagnosis) may primarily be due to misdiagnosed under-recovery from under-fueling (LEA leading to RED-S). Evidence for this hypothesis is derived from published studies implementing training-overloads (many of which resulted in an OTS or NFOR diagnosis) that have also collected and analyzed for EI and potentially EEE, energy balance (EB) and/or EA during the training-overload period to enable analysis and comparison of EA between or within cohorts. Herein EB = EI – total daily energy expenditure (TDEE = Basal Metabolic Rate (BMR) + EEE + Non-Exercise Activity Thermogenesis (NEAT) + Thermic Effect of Food (TEF)). With these criteria, we identified 21 investigations summarized in Table 2 (Note: we have highlighted the extensive series of Endocrine and Metabolic Responses on Overtraining Syndrome (EROS) publications as a single analyzed investigation). Here, we made within- (n = 9 studies) and between-group (n = 12 studies) comparisons to demonstrate potential LEA where EEE has been reported or where training loads have otherwise been characterized (as increase/decrease compared to control condition). Two papers reported actual EA data [19, 22], while another four studies [78,79,80,81] reported EEE (along with EI and FFM; thus EA could be directly calculated) and two papers reported total daily EE [82, 83]. In four studies [84,85,86,87], we were able to estimate EEE using a metabolic equivalent of task (MET) approach [88] or for running data, utilizing the conversion factor 1 kcal/kg BM/km of running [89]. In addition to the two studies reporting EA data [19, 22], there were enough data to enable EA and/or CHO availability estimations in 9 of 21 studies [20, 78,79,80,81, 83,84,85, 87], but not in the remainder [21, 82, 90,91,92,93,94,95,96,97], so we examined the relative (increase/decrease/no change) differences and overall associated direction in EA and/or CHO availability.
3.1 Energy Availability Analysis
Fourteen of the 21 studies [19,20,21,22, 78,79,80,81, 83,84,85, 90,91,92, 97] showed indications of an EA decrease across time or difference between two cohorts within a given study during the training-overload/OTS period compared to control conditions (Table 2). Within these 14 studies, ten studies (71%; [19, 21, 22, 78, 83,84,85,86, 90, 91, 97, 98]) reported OTS or RED-S symptoms likely linked to lower energy or CHO availability (across time or compared to the control group); meanwhile, two investigations [79, 80] reported those symptoms in both groups (i.e. no between-group difference) while no REDS or OTS symptoms were shown in the last two studies [81, 92]. Only eight studies reported performance outcomes, with a performance impairment (relative to baseline or control group) evident in five studies [21, 22, 78, 86, 97], with the rest [79, 80, 85] showing either no change or no difference between groups.
The impact of significantly increased EEE has on EA outcomes in OTS was first reported more than three decades ago, as Costill et al. [80] observed lowered EA in 12 male collegiate swimmers during a 10-day intensive training block. Based on subjective feelings of fatigue, the authors divided the athletes retrospectively into OTS and non-OTS (paper identified OTS distinction) and discovered an ad libitum EI of 4682 kcal/day in the non-OTS swimmers versus ~ 20% lower EI (3631 kcal/day) in the OTS group despite the same increase in EEE. We estimate EA to have been around 20 and 36 kcal/kg FFM/d for OTS and non-OTS, respectively. Accordingly, the authors suggest that OTS swimmers may have been in a ~ 1000 kcal/day energy deficit, which was accompanied by several training overload/OTS or RED-S related symptoms while noting that because this time period is likely too brief to induce a full-blown OTS case (nor is that ethically advisable in a study), subjects were probably NFOR. The only cross-sectional investigation included in this analysis, albeit one that resulted in a total of 13 publications (of which 3 were relevant for the purpose of our summary in Table 2, [90, 91, 98]), implemented an evaluation of 67 health parameters in athletes with OTS (n = 14), healthy athletes (n = 25), and healthy non-athlete controls (n = 12). Here, food diaries [91] indicated drastically lower EI in OTS vs healthy athletes (estimated at 30 vs 58 kcal/kg/day). Although no training data were provided, the nearly twofold greater EI of healthy versus OTS athletes is likely to translate into a significant difference in EA between groups. Several publications on various aspects of this single study support our hypothesis of the potentially confounding role that low energy and/or CHO intake may play in the misdiagnosis of OTS [90, 99]. We concede, however, that since the collection of dietary intake data in this study was implemented only after athletes had already presented with symptoms of OTS, the energy and CHO intakes reported in food diaries may not represent habitual intake, but rather a compensation to reduced training volumes. As no information is provided on training in any of the papers by the Cadegiani group, and as we point out in table 2, it is impossible to estimate EA for these athlete groups. Taken together, our analysis suggests between- and within-group differences in EA ranging from 15–100% and 18–28%, respectively (Table 2). Although a difference of 15–18% in EA between intervention groups may appear small, one study has demonstrated that this translates into a 450 kcal daily negative deviation from EB [83], with all other studies in our analysis demonstrating much greater energetic mis-matches than ~ 15 to 18%. Indeed, even small within-day energy deficits (only 300–400 kcal) have been associated with clinically meaningful RED-S symptom outcomes in cross-sectional study designs [100,101,102]. Although a one-off small 300 kcal deficit (either within day, or over an entire day) is most certainly not clinically impactful, when multiplied over months, these small mismatches in energy can become significant (e.g. energy deficit of 300 kcal/day over 1 year = 100,000+ kcal deficit).
3.2 CHO Availability Analysis
Four of the 21 studies [93,94,95,96] demonstrated an independent effect of CHO on OTS during isoenergetic conditions, and performance impairments were evident in all studies (relative to baseline or control condition). Here, CHO intake reached up to a 2-fold difference between groups (e.g. 4 vs 8 g/kg/day), which for a 65-kg athlete would translate into a difference of 260–325 g CHO/day. Further, all but one paper reporting a decrease in EI or EA in the previous section also reported a concomitant decrease in CHO (ranging from a difference of 1.4–6.0 g/kg/day, corresponding to a caloric difference of 364–1560 kcal/day for a 65-kg athlete). When considering the studies reporting a reduction in EA (Sect. 3.1), along with studies in this section, it appears that the reduction in EA from increased EEE is primarily driven via deficits in CHO intake, as observed in 14 out of 21 studies (Table 2) [19, 78,79,80,81, 84, 85, 90, 91, 93,94,95,96].
Indeed, poor chronic CHO availability, beyond or instead of LEA, is emerging as a potential mechanism also associated with some RED-S related outcomes (Table 1); although according to the current CAT would not strictly be diagnosed as RED-S. For example, it was recently shown that 3.5 weeks of extreme CHO restriction in a group of elite race walkers (a ketogenic LCHF diet; <50g CHO/day) impaired markers of bone remodeling despite adequate EA. Indeed, a control group implementing an isocaloric high CHO diet for the same time period experienced no change in bone markers [103]. Another recent investigation assessed the acute (< 24 h) effects of energy versus CHO availability on markers of bone remodeling in male participants [104]. In this study, it was shown that low CHO intake (3 g/kg/day) with either low (20 kcal/kg FFM/day) or adequate (60 kcal/kg FFM/day) EA led to a similar magnitude of increase in the marker of bone resorption as opposed to no change with a high energy, high CHO (60 kcal/kg FFM/day and 12 g/kg/day CHO) diet. Interestingly, the influence and impact of CHO availability has been shown in earlier research demonstrating that hormones associated with Triad outcomes, such as luteinizing hormone [56], T3 [105] and leptin [106], appear to be especially sensitive to changes in CHO availability. This energy-independent hormonal signaling role of CHO in the development of poor health outcomes is an intriguing finding and one that may exacerbate the LEA outcomes traditionally been linked to RED-S/Triad, but requires further research.
It is important to note that not all situations of training increases/overload result in decreased EA or CHO availability accompanied by RED-S related symptoms. Accordingly, three training-overload/OTS studies reporting impaired performance outcomes [82, 87, 107] and symptoms of OTS/REDS [82, 107] failed to support our hypothesis that increased EEE results in LEA and associated RED-S symptoms. Lehmann et al. [107] had 17 middle- and long-distance runners complete a 3-week overload protocol characterized by either increased training volume (ITV) or increased training intensity (ITI). Habitual dietary energy and CHO intakes were higher in ITV compared to ITI (58 vs 48 kcal/kg/day and 7.1 vs 5.4 g/kg/day, respectively). However, as training volume differed between groups (115–175 km for IVT and 63–85 km for ITI), we estimate EEE to have been lower in ITI and thus, EA at around 43 kcal/kg FFM/day for both groups. Rämson et al. [82] showed near-parallel increases in EI and TDEE during an intense training block (thereby assume unchanged EA), which was accompanied by impaired performance metrics. Both these studies failed to demonstrate clear differences in symptom outcomes between the study groups. Finally, a recent investigation has shown evidence for the role of muscle fiber typology (type I vs II fibers) in the development of FOR, without any indications of LEA [87]. In this 3-week investigation, the researchers showed that athletes with a higher gastrocnemius carnosine z-score (suggesting a higher proportion of type II fibers) were more likely to develop symptoms of FOR which were unrelated to indices of EA such as RMR, changes in body mass or composition, or blood hormone concentrations. No difference was shown in dietary intakes nor our estimations of EA between the two groups.
3.3 Methodological Challenges Associated with EA and CHO Availability Calculations
Collectively, we have many years of experience in the collection of EI and EA data, both in the laboratory and in the field, and while we acknowledge the difficulty in deriving accurate estimations of EA (Sect. 2.2), we also note that the main purpose of our analysis is to provide evidence for a notable difference in EA between two time points or groups (e.g. baseline vs fatigued; or OTS vs control athletes). Accordingly, within our analysis (Table 2) the potential confounding issues with EA and/or CHO availability calculations apply equally and randomly to all groups and time points. Nevertheless, 14 out of 21 studies demonstrated greater than 15% decrease in estimated EA between treatment groups or pre to post (with some studies showing as much as 100% difference). Taken together, 86% of the training-overload/OTS studies reporting dietary outcomes (18 out of 21) showed reduced EA (n = 14 studies) and/or CHO availability (n = 4) between treatment groups or between pre and post concurrent with symptoms consistent with both OTS and RED-S (Table 1). Indeed, in absolute terms, the magnitudes of change or difference in EA between treatment groups in our analysis (Table 2) [19, 22, 78,79,80,81, 84, 85] averaged ~ 10 kcal/kg FFM/day (range 6–18 kcal/kg FFM/day difference) which is in line with significant symptom differences demonstrated with an EA difference of just ~ 7 kcal/kg FFM/day shown by Schaal et al. [19].
Another key real-world elite athlete consideration is an appreciation of the absolute EEEs implemented by elite/professional endurance athletes compared to typical study designs, as highlighted in Table 2. For example, training volumes can approach ~ 30 h/week in endurance sports such as swimming, cycling, and rowing [1,2,3,4]. This volume of training can easily project (depending on body mass, exercise intensity and mode of exercise) to at least ~ 20,000–35,000 kcal/week for just EEE (~ 2500–5000 kcal/day of EEE at ~ 500–1000 kcal/h). Supporting this, studies in elite athletes have shown TDEE of ~ 6000 kcal/day in Tour de France cyclists [108], ~ 9000 kcal/day in a 10h Ironman [109] and ~ 11,000 kcal/day in a 5 day ultra-running event [110]. Our lab has recently assessed EA in professional cyclists over 8 days (including several race days) where we reported a range from ~ 4000 to 6000 kcal/day for just the EEE portion of TDEE [111]. Conversely, several of the studies included in our analysis (Table 2) implemented an increase in training load protocol resulting in a daily EEE of only 725 kcal [79] to ~ 1212 kcal/day [19]. Overall, the mean (± SD) reported or estimated daily EEE of investigations summarized in Table 2 (across groups and time points) was just 1639 ± 714 kcal/day (range 725–2800 kcal/day). We propose that if the moderate increases in training loads/EEE reported in the current literature result in a mismatch between EEE and EI and various subsequent OTS/RED-S symptoms outcomes, the likelihood for these mismatches to manifest in the real-life elite sporting training environment is significantly greater.
4 Evidence of Inadequate EI with Large Increases in EEE: What Limits Sustained Extreme EEE’s?
Most certainly, deliberate restriction from eating disorders (ED)/disordered eating can be an underlying cause of inadequate EI to sustain healthy EA, across a range of training loads. The reader interested in sport-related eating disorders is directed to these recent position statements [112, 113]. Nevertheless, we are unaware of studies examining NFOR/OTS prevalence rates, let alone the prevalence of eating disorders that would be diagnostically excluded in cases of NFOR/OTS. There is a higher prevalence of EDs in athletes than non-athletes [114] and a particularly high prevalence in aesthetic and weight class sports (i.e. sports favoring a high power to weight ratio [115]). Therefore, endurance sports might provide a risk for both symptoms of NFOR/OTS and prevalence of EDs; a hypothesis that requires scientific validation. Deliberate restriction or reduction of EI and/or CHO availability may be involved when manipulation of body composition or training adaptation (e.g. low CHO availability training [116, 117]) are desired outcomes of a training block. However, the phenomenon of an “inadvertent” mismatch between ad libitum EI and EEE is of most interest in situations of increased training loads/OTS.
Without a mismatch between EI and EEE, our hypothesis that many situations of training-overload resulting in symptoms of OTS/NFOR are a result of LEA would not be supported. Therefore, it is important to include an examination of the EI, satiety and exercise literature within our review. Overall, systematic reviews of studies of EI across populations with different physical activity levels, or changes in EI in response to the initiation of an exercise program, have found only a loose relationship between EI and physical activity, and little evidence of consistent increases in EI to compensate for increased EEE [118, 119]. Across these two systematic reviews, ~ 75% of the studies (n = 173 studies) demonstrate a lack of a compensatory increase in EI with an acute increase in EEE; this aligns with our analysis of 84% studies demonstrating either a clear decrease in EA (n = 14 studies) or CHO availability decrease (n = 4 studies) during the training-overload/OTS period (Sect. 3/Table 2). However, studies focused on the role that exercise has in determining appetite and EI suggest a lag of days or even weeks between an increase in EEE and adjustments to EI [120, 121]. Indeed, short-term appetite regulation following an acute bout of exercise appears to reduce appetite in an intensity-dependent manner, with increasing intensity leading to a greater suppression of orexigenic signals and greater stimulation of anorexigenic signals [122, 123]. There is also great variability in individual responses to changes in EI with exercise [118,119,120,121,122,123] and this is complicated in highly active individuals due to the effect of spontaneous physical activity or NEAT [124] as well as recent evidence demonstrating the excretion of energy in feces from non-digested food components [125]. To add further complication, emerging evidence from gut microbiota research has demonstrated dysbiosis (imbalances between pathogenic and symbiotic species; lack of diversity) potentially contributing to dysfunction between the gut–brain axis and the pathophysiological development of EDs [126]. This complexity if further compounded, as EI on its own may not represent fully bioavailable energy as healthy gut microbiota diversity appears required to fully assimilate and digest food (energy). It is also important to recognize that an individual’s eating behaviour and EI is determined by a complex interaction of characteristics, including factors of non-biological origin. For example, the Eating Motivation Survey [127] identifies 78 main motives which can be divided in 15 themes (such as: Habit, Need and Hunger, Convenience, Pleasure, etc., etc.) [128]. This illustrates the complexity of eating behaviours and encourages identification of sport- and/or individual-specific environmental and social/cultural factors that occur specific to the athlete’s lifestyle. Here, the availability of food/drinks and the opportunity to consume them, coupled with a sport or training group’s culture around eating, are key considerations in determining EI, particularly during the substantial proportion of an athlete’s “waking hours” which involve preparing for, undertaking, or recovering from large volumes of training. Nevertheless, the topics present here are rife for further scientific investigation, as there is a paucity of similar data (impact of increased EEE on subsequent EI) and potential mechanisms in elite athletes, especially athletes with > 20 h week of training volume. A full account of the neurohumoral control of appetite and energy homeostasis is beyond the scope of the current paper; readers are referred to excellent summaries of various models and information on the complexity of orexigenic (appetite-stimulating) and anorexigenic (appetite-inhibiting) hormones [129,130,131].
The logistics of eating or drinking during exercise vary according to the exercise mode (e.g. rowing or swimming versus running or cycling), as some are more permissive for EI during training. The availability and palatability of nutrition is clearly important, while rules and sport-specific cultural traditions that promote eating/drinking within some sports assist the athlete to match their EI to high energy demands. By way of illustration, professional road cycling involves sports scientists/nutritionists and chefs within the team support personnel, team busses equipped with mobile kitchens, a “coms-linked” network of feed zones and team cars during races to supply riders with drink bottles and musette bags individually composed for riders with diverse tastes and texture to reduce “flavor fatigue”. Cyclists even wear jerseys with pockets to carry foods, gels, and bottles and have bottle cages on their bikes. Furthermore, peloton traditions such as allowing periods within a race in which riders can eat without their competitors “attacking” and designating domestique riders to ferry food/drink supplies to the team leader also promote energy intake. Such features might explain the consistency of reports from stage races in which professional cyclists are observed to consume high mean daily intakes of energy [5600–6000 kcal (23–25 MJ)] and CHO [12–13 g/kg BM] to achieve a remarkably close match of TDEE, with impressive proportions of daily EA consumed while racing [108, 132, 133]. Obviously, whether these reports of EB found during racing can be matched during daily high-volume training situations in cycling and other sports, where athletes tend to be much less professionally supported, remain to be fully elucidated. However, despite this nutrition support, both training-overload/OTS and RED-S do occur in professional cycling, and without staff support, training-overload/OTS and RED-S is even more likely in recreational/national class athletes.
Food access and eating convenience vary for different sport settings. Professional sports teams are congregated many hours per day and supported via catered “training tables”. In contrast, availability of suitable foods may limit EI in other sports or individual athletes, especially during solo-training situations. Indeed, food insecurity and limited finances are a challenge for many athletes, while traditional eating patterns, which include features such as reliance on food choices with low energy density or a limited number of eating occasions in a day, may also restrict total EI. Finally, there is speculation that there is a finite capacity of the gastrointestinal system to absorb and process foods, which sets an upper limit to sustained EI, and, as a by-product, sustainable EEE (from a health perspective). Although observations of sporting and recreational activities involving high rates of EEE in humans first suggested an upper ceiling equivalent to 4–5 × BMR over moderate time courses of several days to weeks [134, 135], the appreciation of a curvilinear relationship with event duration now suggests a chronic limit of approximately 2.5 × BMR [136]. It should be noted, surpassing and sustaining beyond 2.5 × BMR is theoretically routine in some elite endurance athletes, in that many accumulate 25–30h/week of training over extended periods [– 4, 8].
5 Conclusions and Future Directions
We have presented evidence to suggest that decreased EA and CHO availability may actually be a confounding factor in a significant number of training-overload/OTS studies (Table 2), resulting in misdiagnoses of training-overload/OTS, instead of LEA leading to RED-S. Fortunately, not all athletes who increase EEE manifest with OTS/NFOR and/or RED-S. Consequently, many athletes seem to, (un)consciously, be able to enhance the multi-factorial approach of recovery during sustained and intense periods of training/competition, including aspects of sleep [137,138,139], hydrotherapy [140], psycho-social-emotional factors [141,142,143], along with optimization of nutrition/hydration [144,145,146,147]. Many athletes appear to be able to periodize EI: increasing EI to meet large and sustained EEE during training phases and decreasing EI to meet body composition and performance goals at competition phases [12, 13]. Indeed, recent data from a single study suggest that full-blown OTS diagnoses are rare, as only 15% of an athletic endurance-based population who were already reporting under-performance and fatigue (n = 100) were diagnosed with OTS [39]. However, the overall prevalence rates (either seasonally or over a career and across many different types of sports and between sexes) of OTS, NFOR or FOR in athletes are unknown and can also impact training and performance outcomes. Nevertheless, the prevalence rates of at least one RED-S component range from ~ 30 to 90% (depending on the sport, type and level of athlete, sex of the athlete, and diagnostic tool [148,149,150,151]). Our analysis showed that 84% (n = 18 studies; Table 2) of training-overload/OTS studies show indications of either LEA and/or low CHO availability with subsequent OTS/RED-S symptoms (n = 14 or 67% of papers; [19, 21, 22, 78, 83,84,85,86, 90, 91, 93, 95, 97, 98]) or impaired sports performance (n = 9 or 43% of papers; [21, 22, 78, 86, 93,94,95,96,97]). These findings are not surprising as they are in line with the RED-S prevalence rates in the literature. Indeed, we have highlighted the challenges of achieving accurate diagnoses of LEA/RED-S increases the potential for a failure to exclude RED-S within an NFOR/OTS diagnosis.
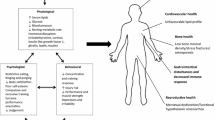
Accordingly, Fig. 3 is a hypothetical, but clinically relevant (authors’ observations) OTS/RED-S framework that captures many of the concepts highlighted in this review. Indeed, and especially during the junior development phases, increases in training load (EEE) [1,2,3,4, 8] tends to initially result in improvements in performance, potentially through a combination of increased training adaptations and/or initial changes in body composition outcomes [9,10,11] (Fig. 3). This can, in some athletes and coaches, “feed-forward” the misconception that even more training and/or more decreases in extreme body composition metrics might further drive positive performance outcomes. This combination of pursuits (long-term large EEE and/or EI manipulation) creates a perfect storm for potential LEA, which can result in an ever increasing risk of adverse health and performance outcomes [148,149,150,151].
An OTS/RED-S framework demonstrating the approximate time course of performance outcomes to OTS/RED-S symptoms linked to increased EEE with no appreciable change in EI resulting in low(er) EA, and possible drops in body composition/body mass metrics. LEA may contribute to many, but not all, FOR/NFOR/OTS symptoms. EEE, exercise energy expenditure; LEA, low energy availability; OTS, overtraining syndrome; RED-S, relative energy deficiency in sport
The prevention of either overtraining/under-fueling (OTS/RED-S) should be primarily based on awareness and monitoring. RED-S prognosis has been shown to be linked to duration of illness/dysfunction, and thus early recognition (awareness) and early intervention, when symptoms are still minor, is desired [77]. This is probably true of OTS as well. Nevertheless, RED-S/Triad awareness remains poor, as less than 50% of physicians, coaches, physiotherapists and athletic trainers were able to identify the three components of the Triad [152,153,154]. We are unaware of studies examining OTS awareness among athletes, coaches, and sport-practitioners. Nonetheless, as authors with decades of experience in the field, we observe that the conceptual awareness and appreciation of nutritional under-recovery/under-fueling (LEA) is low, many times resulting in the potential for misdiagnosis of OTS/NFOR, instead of RED-S. This poor awareness of RED-S is especially concerning given the data presented in this review demonstrating that the insufficient recovery underpinning many OTS/NFOR studies and clinical diagnoses may actually be primarily due to LEA and/or low CHO availability, and instead actually be RED-S. We hope this review creates more awareness of the close symptomatic parallels and diagnostic challenges for both OTS and RED-S. We have highlighted that in many instances the negative outcomes of training-overload (with, or without an OTS diagnosis) may primarily be due to under-fueling and thus actually RED-S, which could be avoided by emphasizing adequate nutrition support for the increased metabolic demands of exercise during periods of training overload.
References
Schumacher YO, Mueller P. The 4000-m team pursuit cycling world record: theoretical and practical aspects. Med Sci Sports Exerc. 2002;34(6):1029–36.
Stoggl TL, Sperlich B. The training intensity distribution among well-trained and elite endurance athletes. Front Physiol. 2015;6:295.
Hellard P, Avalos-Fernandes M, Lefort G, et al. Elite Swimmers’ training patterns in the 25 weeks prior to their season’s best performances: insights into periodization from a 20-years cohort. Front Physiol. 2019;10:363.
Fiskerstrand A, Seiler KS. Training and performance characteristics among Norwegian international rowers 1970–2001. Scand J Med Sci Sports. 2004;14(5):303–10.
Kuipers H. How much is too much? Performance aspects of overtraining. Res Q Exerc Sport. 1996;67(3):65–9.
Meeusen R, Duclos M, Foster C, et al. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc. 2013;45(1):186–205.
Casado A, Hanley B, Santos-Concejero J, et al. World-class long-distance running performances are best predicted by volume of easy runs and deliberate practice of short-interval and tempo runs. J Strength Cond Res. 2019. https://doi.org/10.1519/JSC.0000000000003176.
Stellingwerff T. Case study: nutrition and training periodization in three elite marathon runners. Int J Sport Nutr Exerc Metab. 2012;22(5):392–400.
Faria EW, Parker DL, Faria IE. The science of cycling: factors affecting performance—part 2. Sports Med. 2005;35(4):313–37.
Weyand PG, Davis JA. Running performance has a structural basis. J Exp Biol. 2005;208(Pt 14):2625–31.
Hoogkamer W, Kram R, Arellano CJ. How biomechanical improvements in running economy could break the 2-hour marathon barrier. Sports Med. 2017. https://doi.org/10.1007/s40279-017-0708-0.
Stellingwerff T. Case study: body composition periodization in an olympic-level female middle-distance runner over a 9-year career. Int J Sport Nutr Exerc Metab. 2018;28(4):428–33.
Heydenreich J, Kayser B, Schutz Y, et al. Total energy expenditure, energy intake, and body composition in endurance athletes across the training season: a systematic review. Sports Med Open. 2017;3(1):8.
Heikura IA, Stellingwerff T, Mero AA, et al. A mismatch between athlete practice and current sports nutrition guidelines among elite female and male middle- and long-distance athletes. Int J Sport Nutr Exerc Metab. 2017;27(4):351–60. https://doi.org/10.1123/ijsnem.2016-0316.
Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the Female Athlete Triad-Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2014;48(7):491–7.
Mountjoy M, Sundgot-Borgen JK, Burke LM, et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med. 2018;52(11):687–97.
Joy E, De Souza MJ, Nattiv A, et al. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep. 2014;13(4):219–32.
Kuikman M, Mountjoy M, Stellingwerff T, et al. A narrative review of non-pharmacological strategies in the treatment of relative energy deficiency in sport. Int J Sport Nutr Exerc Metab. 2020. https://doi.org/10.1123/ijsnem.2020-0211.
Schaal K, Tiollier E, Le Meur Y, et al. Elite synchronized swimmers display decreased energy availability during intensified training. Scand J Med Sci Sports. 2017;27(9):925–34.
Woods AL, Garvican-Lewis LA, Lundy B, et al. New approaches to determine fatigue in elite athletes during intensified training: resting metabolic rate and pacing profile. PLoS ONE. 2017;12(3):e0173807.
Woods AL, Rice AJ, Garvican-Lewis LA, et al. The effects of intensified training on resting metabolic rate (RMR), body composition and performance in trained cyclists. PLoS ONE. 2018;13(2):e0191644.
Schaal K, VanLoan MD, Hausswirth C, et al. Decreased energy availability during training overload is associated with non-functional overreaching and suppressed ovarian function in female runners. Appl Physiol Nutr Metab. 2021. https://doi.org/10.1139/apnm-2020-0880.
Loucks AB. Energy balance and body composition in sports and exercise. J Sports Sci. 2004;22(1):1–14.
Loucks AB, Kiens B, Wright HH. Energy availability in athletes. J Sports Sci. 2011;29(Suppl 1):S7-15.
Manore MM, Kam LC, Loucks AB, et al. The female athlete triad: components, nutrition issues, and health consequences. J Sports Sci. 2007;25(Suppl 1):S61-71.
De Souza MJ, Koltun KJ, Etter CV, et al. Current status of the female athlete triad: update and future directions. Curr Osteoporos Rep. 2017;15(6):577–87.
Nattiv A, Lynch L. The female athlete triad. Phys Sportsmed. 1994;22(1):60–8.
Bellinger P. Functional overreaching in endurance athletes: a necessity or cause for concern? Sports Med. 2020. https://doi.org/10.1007/s40279-020-01269-w.
Fry RW, Morton AR, Keast D. Overtraining in athletes. An update Sports Med. 1991;12(1):32–65.
Halson SL, Jeukendrup AE. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med. 2004;34(14):967–81.
Urhausen A, Gabriel H, Kindermann W. Blood hormones as markers of training stress and overtraining. Sports Med. 1995;20(4):251–76.
Mountjoy M, Sundgot-Borgen J, Burke L, et al. RED-S CAT. relative energy deficiency in sport (RED-S) clinical assessment tool (CAT). Br J Sports Med. 2015;49(7):421–3.
Cadegiani FA, Kater CE. Hormonal aspects of overtraining syndrome: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:14.
Lehmann M, Foster C, Keul J. Overtraining in endurance athletes: a brief review. Med Sci Sports Exerc. 1993;25(7):854–62.
Kuipers H, Keizer HA. Overtraining in elite athletes. Review and directions for the future. Sports Med. 1988;6(2):79–92.
Kreider RB, Fry AC, O'Toole ML, editors. Overtraining in sport. Champaign: Human Kinetics; 1998.
Meeusen R, Nederhof E, Buyse L, et al. Diagnosing overtraining in athletes using the two-bout exercise protocol. Br J Sports Med. 2010;44(9):642–8.
Meeusen R, Piacentini MF, Busschaert B, et al. Hormonal responses in athletes: the use of a two bout exercise protocol to detect subtle differences in (over)training status. Eur J Appl Physiol. 2004;91(2–3):140–6.
Buyse L, Decroix L, Timmermans N, et al. Improving the diagnosis of nonfunctional overreaching and overtraining syndrome. Med Sci Sports Exerc. 2019;51(12):2524–30.
Edwards WB. Modeling overuse injuries in sport as a mechanical fatigue phenomenon. Exerc Sport Sci Rev. 2018;46(4):224–31.
Paquette MR, Napier C, Willy RW, et al. Moving beyond weekly “distance”: optimizing quantification of training load in runners. J Orthop Sports Phys Ther. 2020;50(10):564–9.
Bertelsen ML, Hulme A, Petersen J, et al. A framework for the etiology of running-related injuries. Scand J Med Sci Sports. 2017;27(11):1170–80.
Drinkwater BL, Bruemner B, Chesnut CH 3rd. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263(4):545–8.
Drinkwater BL, Nilson K, Chesnut CH 3rd, et al. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med. 1984;311(5):277–81.
Heikura IA, Uusitalo ALT, Stellingwerff T, et al. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):403–11.
Tenforde AS, Carlson JL, Chang A, et al. Association of the female athlete triad risk assessment stratification to the development of bone stress injuries in collegiate athletes. Am J Sports Med. 2017;45(2):302–10.
Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19(8):1231–40.
Ackerman KE, Cano Sokoloff N, G DENM, et al. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc. 2015;47(8):1577–86.
Melin A, Tornberg AB, Skouby S, et al. The LEAF questionnaire: a screening tool for the identification of female athletes at risk for the female athlete triad. Br J Sports Med. 2014;48(7):540–5.
Martinsen M, Holme I, Pensgaard AM, et al. The development of the brief eating disorder in athletes questionnaire. Med Sci Sports Exerc. 2014;46(8):1666–75.
Drinkwater BL, Nilson K, Ott S, et al. Bone mineral density after resumption of menses in amenorrheic athletes. JAMA. 1986;256(3):380–2.
Capling L, Beck KL, Gifford JA, et al. Validity of dietary assessment in athletes: a systematic review. Nutrients. 2017;9(12):1313. https://doi.org/10.3390/nu9121313.
O’Driscoll R, Turicchi J, Beaulieu K, et al. How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br J Sports Med. 2020;54(6):332–40.
Burke LM, Lundy B, Fahrenholtz IL, et al. Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):350–63.
Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88(1):297–311.
Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84(1):37–46.
Lieberman JL, MJ DES, Wagstaff DA, et al. Menstrual disruption with exercise is not linked to an energy availability threshold. Med Sci Sports Exerc. 2018;50(3):551–61.
Williams NI, Leidy HJ, Hill BR, et al. Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. Am J Physiol Endocrinol Metab. 2015;308(1):E29-39.
Schofield KL, Thorpe H, Sims ST. Where are all the men? Low energy availability in male cyclists: a review. Eur J Sport Sci. 2020. https://doi.org/10.1080/17461391.2020.1842510.
Heikura IA, Burke LM, Bergland D, et al. Impact of energy availability, health, and sex on hemoglobin-mass responses following live-high-train-high altitude training in elite female and male distance athletes. Int J Sports Physiol Perform. 2018;13(8):1090–6.
Keay N, Francis G, Entwistle I, et al. Clinical evaluation of education relating to nutrition and skeletal loading in competitive male road cyclists at risk of relative energy deficiency in sports (RED-S): 6-month randomised controlled trial. BMJ Open Sport Exerc Med. 2019;5(1):e000523.
Burke LM, Close GL, Lundy B, et al. Relative energy deficiency in sport in male athletes: a commentary on its presentation among selected groups of male athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):364–74.
Langan-Evans C, Germaine M, Artukovic M, et al. The psychological and physiological consequences of low energy availability in a male combat sport athlete. Med Sci Sports Exerc. 2020. https://doi.org/10.1249/MSS.0000000000002519.
Blauwet CA, Brook EM, Tenforde AS, et al. Low energy availability, menstrual dysfunction, and low bone mineral density in individuals with a disability: implications for the para athlete population. Sports Med. 2017;47(9):1697–708.
Brook EM, Tenforde AS, Broad EM, et al. Low energy availability, menstrual dysfunction, and impaired bone health: a survey of elite para athletes. Scand J Med Sci Sports. 2019;29(5):678–85.
Pritchett K, DiFolco A, Glasgow S, et al. Risk of low energy availability in national and international level paralympic athletes: an exploratory investigation. Nutrients. 2021;13(3):979.
Jurimae J, Maestu J, Jurimae T, et al. Peripheral signals of energy homeostasis as possible markers of training stress in athletes: a review. Metabolism. 2011;60(3):335–50.
Cadegiani FA, Kater CE. Hypothalamic-pituitary-adrenal (HPA) Axis functioning in overtraining syndrome: findings from endocrine and metabolic responses on overtraining syndrome (EROS)-EROS-HPA Axis. Sports Med Open. 2017;3(1):45.
Elliott-Sale KJ, Tenforde AS, Parziale AL, et al. Endocrine effects of relative energy deficiency in sport. Int J Sport Nutr Exerc Metab. 2018;28(4):335–49.
Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413–39.
Cano Sokoloff N, Misra M, Ackerman KE. Exercise, training, and the hypothalamic-pituitary-gonadal axis in men and women. Front Horm Res. 2016;47:27–43.
Urhausen A, Kindermann W. Diagnosis of overtraining: what tools do we have? Sports Med. 2002;32(2):95–102.
Heikura IA, Stellingwerff T, Areta JL. Low energy availability in female athletes: from the lab to the field. Eur J Sport Sci. 2021. https://doi.org/10.1080/17461391.2021.1915391.
Mountjoy M, Andersen LB, Armstrong N, et al. International Olympic Committee consensus statement on the health and fitness of young people through physical activity and sport. Br J Sports Med. 2011;45(11):839–48.
Torstveit MK, Fahrenholtz IL, Lichtenstein MB, et al. Exercise dependence, eating disorder symptoms and biomarkers of relative energy deficiency in sports (RED-S) among male endurance athletes. BMJ Open Sport Exerc Med. 2019;5(1):e000439.
De Souza MJ, Koltun KJ, Williams NI. The role of energy availability in reproductive function in the female athlete triad and extension of its effects to men: an initial working model of a similar syndrome in male athletes. Sports Med. 2019;49(Suppl 2):125–37.
Joy EA, Wilson C, Varechok S. The multidisciplinary team approach to the outpatient treatment of disordered eating. Curr Sports Med Rep. 2003;2(6):331–6.
Poffe C, Ramaekers M, Van Thienen R, et al. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol. 2019. https://doi.org/10.1113/JP277831.
Svendsen IS, Killer SC, Carter JM, et al. Impact of intensified training and carbohydrate supplementation on immunity and markers of overreaching in highly trained cyclists. Eur J Appl Physiol. 2016;116(5):867–77.
Costill DL, Flynn MG, Kirwan JP, et al. Effects of repeated days of intensified training on muscle glycogen and swimming performance. Med Sci Sports Exerc. 1988;20(3):249–54.
Barr SI, Costill DL. Effect of increased training volume on nutrient intake of male collegiate swimmers. Int J Sports Med. 1992;13(1):47–51.
Ramson R, Jurimae J, Jurimae T, et al. The effect of 4-week training period on plasma neuropeptide Y, leptin and ghrelin responses in male rowers. Eur J Appl Physiol. 2012;112(5):1873–80.
Ramson R, Jurimae J, Jurimae T, et al. The influence of increased training volume on cytokines and ghrelin concentration in college level male rowers. Eur J Appl Physiol. 2008;104(5):839–46.
Costa RJS, Jones GE, Lamb KL, et al. The effects of a high carbohydrate diet on cortisol and salivary immunoglobulin A (s-IgA) during a period of increase exercise workload amongst Olympic and Ironman triathletes. Int J Sports Med. 2005;26(10):880–5.
Killer SC, Svendsen IS, Jeukendrup AE, et al. Evidence of disturbed sleep and mood state in well-trained athletes during short-term intensified training with and without a high carbohydrate nutritional intervention. J Sports Sci. 2017;35(14):1402–10.
Woods AL, Garvican-Lewis LA, Rice A, et al. 12 days of altitude exposure at 1800 m does not increase resting metabolic rate in elite rowers. Appl Physiol Nutr Metab. 2017;42(6):672–6.
Bellinger P, Desbrow B, Derave W, et al. Muscle fiber typology is associated with the incidence of overreaching in response to overload training. J Appl Physiol (1985). 2020. https://doi.org/10.1152/japplphysiol.00314.2020.
Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
Margaria R, Cerretelli P, Aghemo P, et al. Energy cost of running. J Appl Physiol. 1963;18:367–70.
Cadegiani FA, Kater CE. Novel insights of overtraining syndrome discovered from the EROS study. BMJ Open Sport Exerc Med. 2019;5(1):e000542.
Cadegiani FA, Kater CE. Body composition, metabolism, sleep, psychological and eating patterns of overtraining syndrome: results of the EROS study (EROS-PROFILE). J Sports Sci. 2018;36(16):1902–10.
Noland RC, Baker JT, Boudreau SR, et al. Effect of intense training on plasma leptin in male and female swimmers. Med Sci Sports Exerc. 2001;33(2):227–31.
Achten J, Halson SL, Moseley L, et al. Higher dietary carbohydrate content during intensified running training results in better maintenance of performance and mood state. J Appl Physiol. 2004;96(4):1331–40.
Kirwan JP, Costill DL, Mitchell JB, et al. Carbohydrate balance in competitive runners during successive days of intense training. J Appl Physiol. 1988;65(6):2601–6.
Mujika I. Case study: long-term low-carbohydrate, high-fat diet impairs performance and subjective well-being in a world-class vegetarian long-distance triathlete. Int J Sport Nutr Exerc Metab. 2019;29(3):339–44.
Simonsen JC, Sherman WM, Lamb DR, et al. Dietary carbohydrate, muscle glycogen, and power output during rowing training. J Appl Physiol (1985). 1991;70(4):1500–5.
Snyder AC, Kuipers H, Cheng B, et al. Overtraining following intensified training with normal muscle glycogen. Med Sci Sports Exerc. 1995;27(7):1063–70.
Anderson T, Wideman L, Cadegiani FA, et al. Effects of overtraining status on the cortisol awakening response-endocrine and metabolic responses on overtraining syndrome (EROS-CAR). Int J Sports Physiol Perform. 2021. https://doi.org/10.1123/ijspp.2020-0205.
Cadegiani FA, Kater CE, Gazola M. Clinical and biochemical characteristics of high-intensity functional training (HIFT) and overtraining syndrome: findings from the EROS study (The EROS-HIFT). J Sports Sci. 2019;37(11):1296–307.
Fahrenholtz IL, Sjodin A, Benardot D, et al. Within-day energy deficiency and reproductive function in female endurance athletes. Scand J Med Sci Sports. 2018;28(3):1139–46.
Deutz RC, Benardot D, Martin DE, et al. Relationship between energy deficits and body composition in elite female gymnasts and runners. Med Sci Sports Exerc. 2000;32(3):659–68.
Torstveit MK, Fahrenholtz I, Stenqvist TB, et al. Within-day energy deficiency and metabolic perturbation in male endurance athletes. Int J Sport Nutr Exerc Metab. 2018. https://doi.org/10.1123/ijsnem.2017-0337.
Heikura IA, Burke LM, Hawley JA, et al. A short-term ketogenic diet impairs markers of bone health in response to exercise. Front Endocrinol (Lausanne). 2019;10:880.
Hammond KM, Sale C, Fraser W, et al. Post-exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: implications for training adaptation. J Physiol. 2019;597(18):4779–96.
Spaulding SW, Chopra IJ, Sherwin RS, et al. Effect of caloric restriction and dietary composition of serum T3 and reverse T3 in man. J Clin Endocrinol Metab. 1976;42(1):197–200.
Jenkins AB, Markovic TP, Fleury A, et al. Carbohydrate intake and short-term regulation of leptin in humans. Diabetologia. 1997;40(3):348–51.
Lehmann M, Gastmann U, Petersen KG, et al. Training-overtraining: performance, and hormone levels, after a defined increase in training volume versus intensity in experienced middle- and long-distance runners. Br J Sports Med. 1992;26(4):233–42.
Saris WH, van Erp-Baart MA, Brouns F, et al. Study on food intake and energy expenditure during extreme sustained exercise: the Tour de France. Int J Sports Med. 1989;10(Suppl 1):S26-31.
Cuddy JS, Slivka DR, Hailes WS, et al. Metabolic profile of the Ironman World Championships: a case study. Int J Sports Physiol Perform. 2010;5(4):570–6.
Rontoyannis GP, Skoulis T, Pavlou KN. Energy balance in ultramarathon running. Am J Clin Nutr. 1989;49(5 Suppl):976–9.
Heikura IA, Quod M, Strobel N, et al. Alternate-day low energy availability during spring classics in professional cyclists. Int J Sports Physiol Perform. 2019. https://doi.org/10.1123/ijspp.2018-0842.
Wells KR, Jeacocke NA, Appaneal R, et al. The Australian Institute of Sport (AIS) and National Eating Disorders Collaboration (NEDC) position statement on disordered eating in high performance sport. Br J Sports Med. 2020;54(21):1247–58.
Bonci CM, Bonci LJ, Granger LR, et al. National athletic trainers’ association position statement: preventing, detecting, and managing disordered eating in athletes. J Athl Train. 2008;43(1):80–108.
Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14(1):25–32.
Sundgot-Borgen J, Garthe I. Elite athletes in aesthetic and Olympic weight-class sports and the challenge of body weight and body compositions. J Sports Sci. 2011;29(Suppl 1):S101–14.
Impey SG, Hearris MA, Hammond KM, et al. Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 2018;48(5):1031–48.
Burke LM, Hawley JA, Jeukendrup A, et al. Toward a common understanding of diet-exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int J Sport Nutr Exerc Metab. 2018;28(5):451–63.
Blundell JE, King NA. Physical activity and regulation of food intake: current evidence. Med Sci Sports Exerc. 1999;31(11 Suppl):S573–83.
Donnelly JE, Herrmann SD, Lambourne K, et al. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS ONE. 2014;9(1):e83498.
Blundell JE, Gibbons C, Caudwell P, et al. Appetite control and energy balance: impact of exercise. Obes Rev. 2015;16(Suppl 1):67–76.
Blundell JE, Stubbs RJ, Hughes DA, et al. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc Nutr Soc. 2003;62(3):651–61.
Dorling J, Broom DR, Burns SF, et al. Acute and chronic effects of exercise on appetite, energy intake, and appetite-related hormones: the modulating effect of adiposity, sex, and habitual physical activity. Nutrients. 2018. https://doi.org/10.3390/nu10091140.
Hazell TJ, Islam H, Townsend LK, et al. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite. 2016;98:80–8.
Thompson JL, Manore MM, Skinner JS, et al. Daily energy expenditure in male endurance athletes with differing energy intakes. Med Sci Sports Exerc. 1995;27(3):347–54.
Lund J, Gerhart-Hines Z, Clemmensen C. Role of energy excretion in human body weight regulation. Trends Endocrinol Metab. 2020;31(10):705–8.
Carbone EA, D'Amato P, Vicchio G, et al. A systematic review on the role of microbiota in the pathogenesis and treatment of eating disorders. Eur Psychiatry. 2020;64(1):e2.
Renner B, Sproesser G, Strohbach S, et al. Why we eat what we eat. The Eating Motivation Survey (TEMS). Appetite. 2012;59(1):117–28.
Wahl DR, Villinger K, Blumenschein M, et al. Why we eat what we eat: assessing dispositional and in-the-moment eating motives by using ecological momentary assessment. JMIR Mhealth Uhealth. 2020;8(1):e13191.
Hall KD, Heymsfield SB, Kemnitz JW, et al. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012;95(4):989–94.
MacLean PS, Blundell JE, Mennella JA, et al. Biological control of appetite: a daunting complexity. Obesity (Silver Spring). 2017;25(Suppl 1):S8–16.
Muller MJ, Geisler C, Heymsfield SB, et al. Recent advances in understanding body weight homeostasis in humans. F1000Research. 2018. https://doi.org/10.12688/f1000research.14151.1.
Muros JJ, Sanchez-Munoz C, Hoyos J, et al. Nutritional intake and body composition changes in a UCI World Tour cycling team during the Tour of Spain. Eur J Sport Sci. 2019;19(1):86–94.
Garcia-Roves PM, Terrados N, Fernandez SF, et al. Macronutrients intake of top level cyclists during continuous competition–change in the feeding pattern. Int J Sports Med. 1998;19(1):61–7.
Cooper JA, Nguyen DD, Ruby BC, et al. Maximal sustained levels of energy expenditure in humans during exercise. Med Sci Sports Exerc. 2011;43(12):2359–67.
Peterson CC, Nagy KA, Diamond J. Sustained metabolic scope. Proc Natl Acad Sci USA. 1990;87(6):2324–8.
Thurber C, Dugas LR, Ocobock C, et al. Extreme events reveal an alimentary limit on sustained maximal human energy expenditure. Sci Adv. 2019;5(6):eaaw0341.
Bonnar D, Bartel K, Kakoschke N, et al. Sleep interventions designed to improve athletic performance and recovery: a systematic review of current approaches. Sports Med. 2018;48(3):683–703.
Halson SL. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014;44(Suppl 1):S13-23.
Hausswirth C, Louis J, Aubry A, et al. Evidence of disturbed sleep and increased illness in overreached endurance athletes. Med Sci Sports Exerc. 2013. https://doi.org/10.1249/MSS.0000000000000177.
Halson SL, Bartram J, West N, et al. Does hydrotherapy help or hinder adaptation to training in competitive cyclists? Med Sci Sports Exerc. 2014;46(8):1631–9.
te Wierike SC, van der Sluis A, van den Akker-Scheek I, et al. Psychosocial factors influencing the recovery of athletes with anterior cruciate ligament injury: a systematic review. Scand J Med Sci Sports. 2013;23(5):527–40.
Stults-Kolehmainen MA, Bartholomew JB. Psychological stress impairs short-term muscular recovery from resistance exercise. Med Sci Sports Exerc. 2012;44(11):2220–7.
Pauli SA, Berga SL. Athletic amenorrhea: energy deficit or psychogenic challenge? Ann N Y Acad Sci. 2010;1205:33–8.
Burke LM, Hawley JA, Wong SH, et al. Carbohydrates for training and competition. J Sports Sci. 2011. https://doi.org/10.1080/02640414.2011.585473.
Burke LM, Kiens B, Ivy JL. Carbohydrates and fat for training and recovery. J Sports Sci. 2004;22(1):15–30.
Phillips SM, Van Loon LJ. Dietary protein for athletes: From requirements to optimum adaptation. J Sports Sci. 2011;29(Suppl 1):S29-38.
Shirreffs SM, Sawka MN. Fluid and electrolyte needs for training, competition, and recovery. J Sports Sci. 2011;29(Suppl 1):S39-46.
Ackerman KE, Holtzman B, Cooper KM, et al. Low energy availability surrogates correlate with health and performance consequences of Relative Energy Deficiency in Sport. Br J Sports Med. 2019;53(10):628–33.
Sygo J, Coates AM, Sesbreno E, et al. Prevalence of indicators of low energy availability in elite female sprinters. Int J Sport Nutr Exerc Metab. 2018. https://doi.org/10.1123/ijsnem.2017-0397.
Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports. 2015;25(5):610–22.
Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc. 2013;45(5):985–96.
Curry EJ, Logan C, Ackerman K, et al. Female athlete triad awareness among multispecialty physicians. Sports Med Open. 2015;1(1):38.
Pantano KJ. Knowledge, attitude, and skill of high school coaches with regard to the female athlete triad. J Pediatr Adolesc Gynecol. 2017;30(5):540–5.
Brown KN, Wengreen HJ, Beals KA. Knowledge of the female athlete triad, and prevalence of triad risk factors among female high school athletes and their coaches. J Pediatr Adolesc Gynecol. 2014;27(5):278–82.
Halson SL, Bridge MW, Meeusen R, et al. Time course of performance changes and fatigue markers during intensified training in trained cyclists. J Appl Physiol. 2002;93(3):947–56.
Halson SL, Lancaster GI, Achten J, et al. Effects of carbohydrate supplementation on performance and carbohydrate oxidation after intensified cycling training. J Appl Physiol (1985). 2004;97(4):1245–53.
Jeukendrup AE, Hesselink MK, Snyder AC, et al. Physiological changes in male competitive cyclists after two weeks of intensified training. Int J Sports Med. 1992;13(7):534–41.
Le Meur Y, Louis J, Aubry A, et al. Maximal exercise limitation in functionally overreached triathletes: role of cardiac adrenergic stimulation. J Appl Physiol (1985). 2014;117(3):214–22.
Coutts AJ, Wallace LK, Slattery KM. Monitoring changes in performance, physiology, biochemistry, and psychology during overreaching and recovery in triathletes. Int J Sports Med. 2007;28(2):125–34.
Halson SL, Lancaster GI, Jeukendrup AE, et al. Immunological responses to overreaching in cyclists. Med Sci Sports Exerc. 2003;35(5):854–61.
Urhausen A, Gabriel HH, Kindermann W. Impaired pituitary hormonal response to exhaustive exercise in overtrained endurance athletes. Med Sci Sports Exerc. 1998;30(3):407–14.
Urhausen A, Gabriel HH, Weiler B, et al. Ergometric and psychological findings during overtraining: a long-term follow-up study in endurance athletes. Int J Sports Med. 1998;19(2):114–20.
Uusitalo AL, Uusitalo AJ, Rusko HK. Exhaustive endurance training for 6–9 weeks did not induce changes in intrinsic heart rate and cardiac autonomic modulation in female athletes. Int J Sports Med. 1998;19(8):532–40.
Uusitalo AL, Uusitalo AJ, Rusko HK. Heart rate and blood pressure variability during heavy training and overtraining in the female athlete. Int J Sports Med. 2000;21(1):45–53.
Vanheest JL, Rodgers CD, Mahoney CE, et al. Ovarian suppression impairs sport performance in junior elite female swimmers. Med Sci Sports Exerc. 2014;46(1):156–66.
Tornberg AB, Melin A, Koivula FM, et al. Reduced neuromuscular performance in amenorrheic elite endurance athletes. Med Sci Sports Exerc. 2017;49(12):2478–85.
Coutts A, Reaburn P, Piva TJ, et al. Changes in selected biochemical, muscular strength, power, and endurance measures during deliberate overreaching and tapering in rugby league players. Int J Sports Med. 2007;28(2):116–24.
Steinacker JM, Lormes W, Kellmann M, et al. Training of junior rowers before world championships. Effects on performance, mood state and selected hormonal and metabolic responses. J Sports Med Phys Fitness. 2000;40(4):327–35.
Fry AC, Schilling BK, Weiss LW, et al. beta2-Adrenergic receptor downregulation and performance decrements during high-intensity resistance exercise overtraining. J Appl Physiol (1985). 2006;101(6):1664–72.
Moore CA, Fry AC. Nonfunctional overreaching during off-season training for skill position players in collegiate American football. J Strength Cond Res. 2007;21(3):793–800.
Wilson G, Hawken MB, Poole I, et al. Rapid weight-loss impairs simulated riding performance and strength in jockeys: implications for making-weight. J Sports Sci. 2014;32(4):383–91.
Harber VJ, Petersen SR, Chilibeck PD. Thyroid hormone concentrations and muscle metabolism in amenorrheic and eumenorrheic athletes. Can J Appl Physiol. 1998;23(3):293–306.
Kasper AM, Crighton B, Langan-Evans C, et al. Case study: extreme weight making causes relative energy deficiency, dehydration, and acute kidney injury in a male mixed martial arts athlete. Int J Sport Nutr Exerc Metab. 2019;29(3):331–8.
Gleeson M, McDonald WA, Cripps AW, et al. The effect on immunity of long-term intensive training in elite swimmers. Clin Exp Immunol. 1995;102(1):210–6.
Gleeson M, McDonald WA, Pyne DB, et al. Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc. 1999;31(1):67–73.
Lancaster GI, Halson SL, Khan Q, et al. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. 2004;10:91–106.
Main LC, Landers GJ, Grove JR, et al. Training patterns and negative health outcomes in triathlon: longitudinal observations across a full competitive season. J Sports Med Phys Fitness. 2010;50(4):475–85.
Morgado JM, Rama L, Silva I, et al. Cytokine production by monocytes, neutrophils, and dendritic cells is hampered by long-term intensive training in elite swimmers. Eur J Appl Physiol. 2012;112(2):471–82.
Morgado JP, Matias CN, Reis JF, et al. The cellular composition of the innate and adaptive immune system is changed in blood in response to long-term swimming training. Front Physiol. 2020;11:471.
Tiollier E, Gomez-Merino D, Burnat P, et al. Intense training: mucosal immunity and incidence of respiratory infections. Eur J Appl Physiol. 2005;93(4):421–8.
Hootman JM, Macera CA, Ainsworth BE, et al. Predictors of lower extremity injury among recreationally active adults. Clin J Sport Med. 2002;12(2):99–106.
Psaila M, Ranson C. Risk factors for lower leg, ankle and foot injuries during basic military training in the Maltese Armed Forces. Phys Ther Sport. 2017;24:7–12.
Sharma J, Greeves JP, Byers M, et al. Musculoskeletal injuries in British Army recruits: a prospective study of diagnosis-specific incidence and rehabilitation times. BMC Musculoskelet Disord. 2015;16:106.
Ackerman KE, Putman M, Guereca G, et al. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012;51(4):680–7.
Ackerman KE, Nazem T, Chapko D, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011;96(10):3123–33.
Drew MK, Vlahovich N, Hughes D, et al. A multifactorial evaluation of illness risk factors in athletes preparing for the Summer Olympic Games. J Sci Med Sport. 2017;20(8):745–50.
Drew M, Vlahovich N, Hughes D, et al. Prevalence of illness, poor mental health and sleep quality and low energy availability prior to the 2016 Summer Olympic Games. Br J Sports Med. 2018;52(1):47–53.
Barrack MT, Gibbs JC, De Souza MJ, et al. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: a prospective multisite study of exercising girls and women. Am J Sports Med. 2014;42(4):949–58.
Gibbs JC, Nattiv A, Barrack MT, et al. Low bone density risk is higher in exercising women with multiple triad risk factors. Med Sci Sports Exerc. 2014;46(1):167–76.
Tenforde AS, Fredericson M, Sayres LC, et al. Identifying sex-specific risk factors for low bone mineral density in adolescent runners. Am J Sports Med. 2015;43(6):1494–504.
Tenforde AS, Parziale AL, Popp KL, et al. Low bone mineral density in male athletes is associated with bone stress injuries at anatomic sites with greater trabecular composition. Am J Sports Med. 2018;46(1):30–6.
De Souza MJ, West SL, Jamal SA, et al. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone. 2008;43(1):140–8.
Hagmar M, Berglund B, Brismar K, et al. Body composition and endocrine profile of male Olympic athletes striving for leanness. Clin J Sport Med. 2013;23(3):197–201.
Hind K, Truscott JG, Evans JA. Low lumbar spine bone mineral density in both male and female endurance runners. Bone. 2006;39(4):880–5.
Rauh MJ, Nichols JF, Barrack MT. Relationships among injury and disordered eating, menstrual dysfunction, and low bone mineral density in high school athletes: a prospective study. J Athl Train. 2010;45(3):243–52.
Thein-Nissenbaum JM, Carr KE, Hetzel S, et al. Disordered eating, menstrual irregularity, and musculoskeletal injury in high school athletes: a comparison of oral contraceptive pill users and nonusers. Sports Health. 2014;6(4):313–20.
Warrington G, Dolan E, McGoldrick A, et al. Chronic weight control impacts on physiological function and bone health in elite jockeys. J Sports Sci. 2009;27(6):543–50.
Tenforde AS, Carlson JL, Sainani KL, et al. Sport and triad risk factors influence bone mineral density in collegiate athletes. Med Sci Sports Exerc. 2018;50(12):2536–43.
Kraus E, Tenforde AS, Nattiv A, et al. Bone stress injuries in male distance runners: higher modified Female Athlete Triad Cumulative Risk Assessment scores predict increased rates of injury. Br J Sports Med. 2019;53(4):237–42.
Nose-Ogura S, Yoshino O, Dohi M, et al. Risk factors of stress fractures due to the female athlete triad: differences in teens and twenties. Scand J Med Sci Sports. 2019;29(10):1501–10.
Lane AR, Hackney AC, Smith-Ryan A, et al. Prevalence of low energy availability in competitively trained male endurance athletes. Medicina (Kaunas). 2019;55(10).
Logue DM, Madigan SM, Melin A, et al. Self-reported reproductive health of athletic and recreationally active males in Ireland: potential health effects interfering with performance. Eur J Sport Sci. 2020:1-10. https://doi.org/10.1080/17461391.2020.1748116.
Logue DM, Madigan SM, Heinen M, et al. Screening for risk of low energy availability in athletic and recreationally active females in Ireland. Eur J Sport Sci. 2019;19(1):112–22.
Aubry A, Hausswirth C, Louis J, et al. Functional overreaching: the key to peak performance during the taper? Med Sci Sports Exerc. 2014;46(9):1769–77.
Areta JL, Burke LM, Camera DM, et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab. 2014;306(8):E989–97.
Ishibashi A, Kojima C, Tanabe Y, et al. Effect of low energy availability during three consecutive days of endurance training on iron metabolism in male long distance runners. Physiol Rep. 2020;8(12):e14494.
Kojima C, Ishibashi A, Tanabe Y, et al. Muscle glycogen content during endurance training under low energy availability. Med Sci Sports Exerc. 2020;52(1):187–95.
Rietjens GJ, Kuipers H, Adam JJ, et al. Physiological, biochemical and psychological markers of strenuous training-induced fatigue. Int J Sports Med. 2005;26(1):16–26.
Nederhof E, Lemmink K, Zwerver J, et al. The effect of high load training on psychomotor speed. Int J Sports Med. 2007;28(7):595–601.
Nederhof E, Zwerver J, Brink M, et al. Different diagnostic tools in nonfunctional overreaching. Int J Sports Med. 2008;29(7):590–7.
Silva MR, Paiva T. Poor precompetitive sleep habits, nutrients’ deficiencies, inappropriate body composition and athletic performance in elite gymnasts. Eur J Sport Sci. 2016;16(6):726–35.
Baskaran C, Plessow F, Ackerman KE, et al. A cross-sectional analysis of verbal memory and executive control across athletes with varying menstrual status and non-athletes. Psychiatry Res. 2017;258:605–6.
Lane AR, Duke JW, Hackney AC. Influence of dietary carbohydrate intake on the free testosterone: cortisol ratio responses to short-term intensive exercise training. Eur J Appl Physiol. 2010;108(6):1125–31.
Lehmann M, Knizia K, Gastmann U, et al. Influence of 6-week, 6 days per week, training on pituitary function in recreational athletes. Br J Sports Med. 1993;27(3):186–92.
Arce JC, De Souza MJ, Pescatello LS, et al. Subclinical alterations in hormone and semen profile in athletes. Fertil Steril. 1993;59(2):398–404.
Hackney AC, Sinning WE, Bruot BC. Reproductive hormonal profiles of endurance-trained and untrained males. Med Sci Sports Exerc. 1988;20(1):60–5.
McColl EM, Wheeler GD, Gomes P, et al. The effects of acute exercise on pulsatile LH release in high-mileage male runners. Clin Endocrinol. 1989;31(5):617–21.
Christo K, Cord J, Mendes N, et al. Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: a cross-sectional study. Clin Endocrinol. 2008;69(4):628–33.
Hackney AC, Fahrner CL, Gulledge TP. Basal reproductive hormonal profiles are altered in endurance trained men. J Sports Med Phys Fitness. 1998;38(2):138–41.
Hooper DR, Kraemer WJ, Saenz C, et al. The presence of symptoms of testosterone deficiency in the exercise-hypogonadal male condition and the role of nutrition. Eur J Appl Physiol. 2017;117(7):1349–57.
Miller KK, Lawson EA, Mathur V, et al. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2007;92(4):1334–9.
Rickenlund A, Thoren M, Carlstrom K, et al. Diurnal profiles of testosterone and pituitary hormones suggest different mechanisms for menstrual disturbances in endurance athletes. J Clin Endocrinol Metab. 2004;89(2):702–7.
Keay N, Francis G, Hind K. Low energy availability assessed by a sport-specific questionnaire and clinical interview indicative of bone health, endocrine profile and cycling performance in competitive male cyclists. BMJ Open Sport Exerc Med. 2018;4(1):e000424.
Civil R, Lamb A, Loosmore D, et al. Assessment of dietary intake, energy status, and factors associated with RED-S in vocational female ballet students. Front Nutr. 2018;5:136.
Shimizu Y, Mutsuzaki H, Tachibana K, et al. Investigation of the female athlete triad in japanese elite wheelchair basketball players. Medicina (Kaunas). 2019. https://doi.org/10.3390/medicina56010010.
Mathisen TF, Heia J, Raustol M, et al. Physical health and symptoms of relative energy deficiency in female fitness athletes. Scand J Med Sci Sports. 2020;30(1):135–47.
Meng K, Qiu J, Benardot D, et al. The risk of low energy availability in Chinese elite and recreational female aesthetic sports athletes. J Int Soc Sports Nutr. 2020;17(1):13.
Stenqvist TB, Torstveit MK, Faber J, et al. Impact of a 4-week intensified endurance training intervention on markers of relative energy deficiency in sport (RED-S) and performance among well-trained male cyclists. Front Endocrinol (Lausanne). 2020;11:512365.
Mackinnon LT, Hooper SL, Jones S, et al. Hormonal, immunological, and hematological responses to intensified training in elite swimmers. Med Sci Sports Exerc. 1997;29(12):1637–45.
O’Connor PJ, Morgan WP, Raglin JS, et al. Mood state and salivary cortisol levels following overtraining in female swimmers. Psychoneuroendocrinology. 1989;14(4):303–10.
Simsch C, Lormes W, Petersen KG, et al. Training intensity influences leptin and thyroid hormones in highly trained rowers. Int J Sports Med. 2002;23(6):422–7.
Schaal K, Van Loan MD, Casazza GA. Reduced catecholamine response to exercise in amenorrheic athletes. Med Sci Sports Exerc. 2011;43(1):34–43.
MacConnie SE, Barkan A, Lampman RM, et al. Decreased hypothalamic gonadotropin-releasing hormone secretion in male marathon runners. N Engl J Med. 1986;315(7):411–7.
Koehler K, Hoerner NR, Gibbs JC, et al. Low energy availability in exercising men is associated with reduced leptin and insulin but not with changes in other metabolic hormones. J Sports Sci. 2016;34(20):1921–9.
Loucks AB, Heath EM. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol. 1994;266(3 Pt 2):R817–23.
Geesmann B, Gibbs JC, Mester J, et al. Association between energy balance and metabolic hormone suppression during ultraendurance exercise. Int J Sports Physiol Perform. 2017;12(7):984–9.
Berg U, Enqvist JK, Mattsson CM, et al. Lack of sex differences in the IGF-IGFBP response to ultra endurance exercise. Scand J Med Sci Sports. 2008;18(6):706–14.
Loucks AB, Mortola JF, Girton L, et al. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68(2):402–11.
Loucks AB, Callister R. Induction and prevention of low-T3 syndrome in exercising women. Am J Physiol. 1993;264(5 Pt 2):R924–30.
Russell M, Stark J, Nayak S, et al. Peptide YY in adolescent athletes with amenorrhea, eumenorrheic athletes and non-athletic controls. Bone. 2009;45(1):104–9.
Strock NCA, Koltun KJ, Southmayd EA, et al. Indices of resting metabolic rate accurately reflect energy deficiency in exercising women. Int J Sport Nutr Exerc Metab. 2020. https://doi.org/10.1123/ijsnem.2019-0199.
Strock NCA, De Souza MJ, Williams NI. Eating behaviours related to psychological stress are associated with functional hypothalamic amenorrhoea in exercising women. J Sports Sci. 2020;38(21):2396–406.
Snyder AC, Jeukendrup AE, Hesselink MK, et al. A physiological/psychological indicator of over-reaching during intensive training. Int J Sports Med. 1993;14(1):29–32.
De Souza MJ, Hontscharuk R, Olmsted M, et al. Drive for thinness score is a proxy indicator of energy deficiency in exercising women. Appetite. 2007;48(3):359–67.
Papillard-Marechal S, Sznajder M, Hurtado-Nedelec M, et al. Iron metabolism in patients with anorexia nervosa: elevated serum hepcidin concentrations in the absence of inflammation. Am J Clin Nutr. 2012;95(3):548–54.
Pasiakos SM, Margolis LM, Murphy NE, et al. Effects of exercise mode, energy, and macronutrient interventions on inflammation during military training. Physiol Rep. 2016. https://doi.org/10.14814/phy2.12820.
Rowbottom DG, Keast D, Goodman C, et al. The haematological, biochemical and immunological profile of athletes suffering from the overtraining syndrome. Eur J Appl Physiol Occup Physiol. 1995;70(6):502–9.
Smith DJ, Norris SR. Changes in glutamine and glutamate concentrations for tracking training tolerance. Med Sci Sports Exerc. 2000;32(3):684–9.
Parry-Billings M, Budgett R, Koutedakis Y, et al. Plasma amino acid concentrations in the overtraining syndrome: possible effects on the immune system. Med Sci Sports Exerc. 1992;24(12):1353–8.
Mackinnon LT, Hooper S. Mucosal (secretory) immune system responses to exercise of varying intensity and during overtraining. Int J Sports Med. 1994;15(Suppl 3):S179–83.
Shimizu K, Suzuki N, Nakamura M, et al. Mucosal immune function comparison between amenorrheic and eumenorrheic distance runners. J Strength Cond Res. 2012;26(5):1402–6.
Le Meur Y, Pichon A, Schaal K, et al. Evidence of parasympathetic hyperactivity in functionally overreached athletes. Med Sci Sports Exerc. 2013;45(11):2061–71.
Wilson G, Hill J, Sale C, et al. Elite male Flat jockeys display lower bone density and lower resting metabolic rate than their female counterparts: implications for athlete welfare. Appl Physiol Nutr Metab. 2015;40(12):1318–20.
Koehler K, De Souza MJ, Williams NI. Less-than-expected weight loss in normal-weight women undergoing caloric restriction and exercise is accompanied by preservation of fat-free mass and metabolic adaptations. Eur J Clin Nutr. 2017;71(3):365–71.
Zabriskie HA, Currier BS, Harty PS, et al. Energy status and body composition across a collegiate women's Lacrosse season. Nutrients. 2019. https://doi.org/10.3390/nu11020470.
Papageorgiou M, Elliott-Sale KJ, Parsons A, et al. Effects of reduced energy availability on bone metabolism in women and men. Bone. 2017;105:191–9.
Viner RT, Harris M, Berning JR, et al. Energy availability and dietary patterns of adult male and female competitive cyclists with lower than expected bone mineral density. Int J Sport Nutr Exerc Metab. 2015;25(6):594–602.
Bilanin JE, Blanchard MS, Russek-Cohen E. Lower vertebral bone density in male long distance runners. Med Sci Sports Exerc. 1989;21(1):66–70.
Barrack MT, Fredericson M, Tenforde AS, et al. Evidence of a cumulative effect for risk factors predicting low bone mass among male adolescent athletes. Br J Sports Med. 2017;51(3):200–5.
Morgan WP, Costill DL, Flynn MG, et al. Mood disturbance following increased training in swimmers. Med Sci Sports Exerc. 1988;20(4):408–14.
Petrie TA, Greenleaf C, Reel JJ, et al. An examination of psychosocial correlates of eating disorders among female collegiate athletes. Res Q Exerc Sport. 2009;80(3):621–32.
Petrie TA, Greenleaf C, Reel J, et al. Personality and psychological factors as predictors of disordered eating among female collegiate athletes. Eat Disord. 2009;17(4):302–21.
Greenleaf C, Petrie TA, Carter J, et al. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489–95.
Krentz EM, Warschburger P. A longitudinal investigation of sports-related risk factors for disordered eating in aesthetic sports. Scand J Med Sci Sports. 2013;23(3):303–10.
Keay N, Overseas A, Francis G. Indicators and correlates of low energy availability in male and female dancers. BMJ Open Sport Exerc Med. 2020;6(1):e000906.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
All authors declare that they have no conflict of interest as it relates to the material associated with this review.
Author contributions
Development and formulation of concept and primary authors: TS and IH; Original draft authors: TS, IH, MM and LB; Critical feedback and revisions: RM, SB, SS. All authors have read and approved the final version.
Rights and permissions
About this article
Cite this article
Stellingwerff, T., Heikura, I.A., Meeusen, R. et al. Overtraining Syndrome (OTS) and Relative Energy Deficiency in Sport (RED-S): Shared Pathways, Symptoms and Complexities. Sports Med 51, 2251–2280 (2021). https://doi.org/10.1007/s40279-021-01491-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01491-0