Abstract
Background
In a previous meta-analysis including nine trials comparing aerobic interval training with aerobic continuous training in patients with coronary artery disease, we found a significant difference in peak oxygen uptake favoring aerobic interval training.
Objective
The objective of this study was to (1) update the original meta-analysis focussing on peak oxygen uptake and (2) evaluate the effect on secondary outcomes.
Methods
We conducted a systematic review with a meta-analysis by searching PubMed and SPORTDiscus databases up to March 2017. We included randomized trials comparing aerobic interval training and aerobic continuous training in patients with coronary artery disease or chronic heart failure. The primary outcome was change in peak oxygen uptake. Secondary outcomes included cardiorespiratory parameters, cardiovascular risk factors, cardiac and vascular function, and quality of life.
Results
Twenty-four papers were identified (n = 1080; mean age 60.7 ± 10.7 years). Aerobic interval training resulted in a higher increase in peak oxygen uptake compared with aerobic continuous training in all patients (1.40 mL/kg/min; p < 0.001), and in the subgroups of patients with coronary artery disease (1.25 mL/kg/min; p = 0.001) and patients with chronic heart failure with reduced ejection fraction (1.46 mL/kg/min; p = 0.03). Moreover, a larger increase of the first ventilatory threshold and peak heart rate was observed after aerobic interval training in all patients. Other cardiorespiratory parameters, cardiovascular risk factors, and quality of life were equally affected.
Conclusion
This meta-analysis adds further evidence to the clinically significant larger increase in peak oxygen uptake following aerobic interval training vs. aerobic continuous training in patients with coronary artery disease and chronic heart failure. More well-designed randomized controlled trials are needed to establish the safety of aerobic interval training and the sustainability of the training response over longer periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aerobic interval training is more beneficial compared with aerobic continuous training in increasing peak oxygen uptake in patients with coronary artery disease and chronic heart failure. |
A larger increase of the first ventilatory threshold and peak heart rate was observed after aerobic interval training compared with aerobic continuous training in all cardiac patients, while other cardiorespiratory parameters, cardiovascular risk factors, and quality of life were equally influenced. |
1 Background
Cardiovascular diseases are the main cause of death worldwide [1] and in Europe [2], accounting for 31.5% [1] and 45% [2] of all deaths, respectively. Exercise-based cardiac rehabilitation is a cornerstone in the secondary prevention of cardiovascular diseases, reducing cardiovascular mortality by 26% in patients with coronary artery disease (CAD) [3]. These improved survival rates are mediated amongst others through training-induced increases in peak oxygen uptake (peak VO2), which is an important prognostic parameter for all-cause and cardiovascular morbidity and mortality [4]. That is, an increase of 1 mL O2/kg/min has been shown to result in an almost 15% increase in survival [4].
Traditional training methods include continuous training (30–60 min) at moderate intensity (40–80% of peak VO2) [aerobic continuous training; ACT], leading to gains in peak VO2 of approximately 20% after 12 weeks of three-times-weekly exercise sessions [5]. Training at a higher intensity leads to higher increases in peak VO2 [6]. However, it is impossible to sustain higher intensities for longer periods. Therefore, to prolong the time that training could be sustained at higher intensities, interval training was suggested [7]. About 15 years ago, aerobic interval training (AIT) was introduced in cardiac rehabilitation in Norway. These first small studies reported that the increments in peak VO2 were significantly higher after AIT in both patients with CAD [8] and chronic heart failure (CHF) [9]. Since then, research investigating the potential superiority of AIT for improving peak VO2 in different cardiac patient groups has grown rapidly. Results of the individual studies were contradictory and inconclusive, while meta-analyses collating the results showed significantly higher increases after AIT ranging from 1.04 [10] to 2.14 mL/kg/min [11] in both patients with CAD [12,13,14,15] and CHF [10,11,12, 16].
Since the publication of our first meta-analysis in 2014 [15] involving only nine study groups and 206 patients, an increasing number of larger randomized clinical trials have been published comparing the efficacy of AIT and ACT in cardiac patients. This larger number of trials allows now for a more precise estimate of the effect on peak VO2 but also on other relevant secondary outcomes.
Therefore, our aim was to update the original meta-analysis [15] comparing the efficacy of AIT and ACT on peak VO2 in patients with CAD and CHF. Moreover, we focused on secondary outcomes including (1) cardiorespiratory parameters [peak heart rate (HR), oxygen pulse (O2 pulse), first ventilatory threshold (VT1), oxygen uptake efficiency slope (OUES), ventilatory efficiency slope (VE/VCO2 slope), heart rate recovery after 1 min of exercise (HRR 1 min)], (2) cardiovascular risk factors [body weight, systolic blood pressure and diastolic blood pressure (SBP and DBP), resting HR, blood lipids, and blood glucose], (3) cardiac function [left ventricular ejection fraction (LVEF)], (4) vascular function [flow-mediated dilation (FMD)] and (5) quality of life (QoL).
2 Methods
The systematic review and meta-analysis were conducted in accordance with the guidelines from Preferred Reporting for Systematic Review and Meta-Analysis [17].
2.1 Literature Search
We conducted a literature search in the electronic PubMed and SPORTDiscus databases from the earliest available date up to March 2017 with the following search terms (‘All field’ terms): [(Aerobic interval training OR high intensity interval training OR interval training OR intermittent training OR high intensity exercise) AND (coronary artery disease OR coronary heart disease OR heart failure OR myocardial infarction OR coronary artery bypass surgery OR ischemic heart disease OR angina pectoris OR percutaneous coronary intervention)], without any further limitations. Screening of all titles and abstracts was performed by two independent investigators (NP and RB). Results from both investigators were compared and papers were selected after consensus. The reference lists of retrieved papers were manually searched to identify other appropriate studies.
2.2 Eligibility Criteria
We included (1) randomized clinical trials comparing the effects of supervised AIT with ACT in patients with CAD and/or CHF, (2) with a duration of at least 4 weeks, (3) reporting on peak VO2 (mean changes or pre- and post-intervention means and variability measures), and (4) published in a peer-reviewed journal up to March 2017. Exclusion criteria included any study not meeting any of the criteria listed above.
2.3 Measured Outcomes
The primary outcome for this meta-analysis was the change in peak VO2 in mL/min/kg. Secondary outcomes included (1) cardiorespiratory parameters (peak HR, O2 pulse, VT1, OUES, VE/VCO2 slope, HRR 1 min), (2) cardiovascular risk factors (body weight, SBP and DBP, resting HR, blood lipids, and blood glucose), (3) cardiac function (LVEF), (4) vascular function (FMD), and (5) QoL.
2.4 Data Extraction
The two main authors (NP and RB) independently extracted characteristics of patients and intervention, and point and variability data on primary and secondary outcomes, in a standardized form. Results were compared and discrepancies were resolved after mutual agreement or by consulting the senior author (VC). Cohen’s kappa was 0.83, showing a very good interrater agreement.
2.5 Study Quality
Papers were assessed for quality using the TESTEX-scale (Tool for the assEssment of Study qualiTy and reporting in EXercise) [18], which is a 14-point scale recently designed specifically for assessing study quality in exercise training studies. It includes data on eligibility criteria, random allocation, similarity of baseline values, blinding of investigators, key outcome obtained in at least 85% of subjects, reporting adverse events and adherence rates, intention-to-treat analysis, between-group differences, point and variability measures, activity monitoring in control groups, adaptation of relative intensity, and data on exercise characteristics and energy expenditure. A higher score reflects a better quality. No trials were excluded based on quality.
2.6 Statistical Analysis
Statistical analyses were performed using Review Manager Software (RevMan 5.3; Cochrane Collaboration, Oxford, UK). Descriptive data are reported as mean ± standard deviation (SD) or mean and 95% confidence interval (CI). Prior to the statistical analysis, outcome data of the included papers were converted to mean ± SD if necessary. The formula used to calculate SD from standard error (SE) was: SD = SE × √(n; the formula to calculate SD from 95% CI was: SD = [√(n) × (upper limit − lower limit)]/3.92. The mean baseline values were calculated by combining mean values from the intervention groups, weighted by the number of participants included in the final analysis in each study group.
Effect sizes were calculated by subtracting the pre-intervention value from the post-intervention value of each trial. The net treatment effect was then calculated by subtracting the change in the ACT group from the change in the AIT group. Review Manager Software calculated the variances from the inserted pooled SDs of the change in the intervention groups. However, some studies reported only the SDs or SEs at baseline and post-intervention. Therefore, missing change score SDs were calculated from pre- and post-SD values, using the following formula: SDchange = √(SDpre2 + SDpost2 − 2 × corr(pre, post) × SDpre × SDpost) [19], for which we used a correlation coefficient (corr) of 0.5 for each outcome. We used random-effects models to pool the data, given the small sample sizes of study groups and the differences in study populations. Each effect size was weighted by the inverse of its variance. The results are reported as weighted means and 95% CI. Two-sided tests for overall effects were considered significant at p ≤ 0.05.
Statistical heterogeneity among the trials was assessed using Cochrane’s Q statistic and an alpha value for statistical significance of 0.10 indicated significant heterogeneity. In addition, the I2 parameter was used to quantify inconsistency of treatment effects across trials (I2 = [Q − df] × 100%, where Q is the χ2 statistic and df are the degrees of freedom). A value for I2 > 50% has been considered to be substantial heterogeneity.
To examine the influence of each trial on the overall results of our primary outcome peak VO2, sensitivity analyses were performed with each study deleted from the model once. For the primary outcome peak VO2, different a priori-defined subgroup analyses were performed. Subgroups were compared according to pathology [patients with CAD vs. patients with CHF with reduced ejection fraction (HFrEF) vs. patients with CHF with preserved ejection fraction (HFpEF)], duration of the high-intensity interval (short ≤ 1 min, medium 1–3 min, long ≥ 4 min), intensity of the interval [vigorous effort (70–89% of peak HR; 60–84% of HRR; 60–79% of peak VO2) or very hard effort (≥ 90% of peak HR; ≥ 85% of HRR; ≥ 80% of peak VO2) (in case of training HR zones, the mean of the HR zone was used for classification) [20], training mode (cycle ergometer, treadmill) and total duration of the intervention (< 12 weeks vs. ≥ 12 weeks), energy expenditure (isocaloric vs. not iscaloric), and sample size (< 20 patients in at least one group, 20–50 patients in both groups, > 50 patients in both groups). Fixed-effects models were used to compare the subgroups. If p ≤ 0.05, we checked for a non-overlapping CI to identify which groups differed significantly. Finally, funnel plots of the effect size vs. the SE of the effect size were visually inspected to assess publication bias.
3 Results
3.1 Study Selection
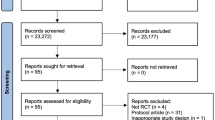
A Preferred Reporting for Systematic Review and Meta-Analysis flow diagram of our literature search and selection is presented in Fig. 1. The initial search identified 2592 potentially relevant studies of which 37 studies were retrieved for full-text review. From these, we excluded six studies that did not fulfill the inclusion criteria (training intervention < 4 weeks, [21] no randomization [22, 23], deviant exercise protocols, [24, 25], or no peak VO2 data) [26]. Seven studies produced more than one publication [27,28,29,30,31,32,33]. If these duplicate papers, however, contained data that were not reported in the main paper, they were included for quantitative analyses of the secondary outcomes [27, 28, 31, 32]. In total, 28 (14 in CAD; 12 in HFrEF; two in HFpEF) publications were included for quantitative analysis, of which 24 were used for the primary outcome peak VO2 [8, 9, 34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55].
3.2 Characteristics of the Participants and Study Design
A summary of the 24 studies including the primary outcome peak VO2 is presented in Table 1. A total of 1080 patients (mean age 60.7 ± 10.7 years) were randomized to either AIT (n = 540) or ACT (n = 540). The drop-out rate ranged from 0 to 48%, with a mean of 12.4%. Drop-out rates after AIT and ACT were 13.0% (range 0–50% [45]) and 11.9% (range 0–47% [45]), respectively. A total of 470 patients completed the AIT intervention (mean age 60.4 ± 11.3 years) and 476 completed the ACT intervention (mean age 61.1 ± 10.4 years). Main reasons for drop-out were low compliance with the study protocol or training sessions, patients’ withdrawal of consent, intolerance of imposed training protocol, or lack of data.
Six studies were randomized controlled trials [9, 41, 47, 49, 50, 55], 18 were randomized clinical trials. Two large multicenter studies were included: one in patients with CAD [51] and one in patients with HFrEF [55]. Three studies included only men [34, 42, 54]. and overall 83.9% of the analyzed participants were male. The New York Heart Association class ranged from I to III, with most patients being in class II. All of them had to be stable at the time of inclusion. In the studies with patients with CAD, all had normal to slightly reduced LVEF (lowest exclusion criterion on LVEF was > 30% [52]).
Studies were published between 2004 and 2017, of which 12 (50%) were published since our previous meta-analysis in 2014. Fourteen studies were performed in Europe, accounting for 72% of the study population [8, 9, 35,36,37, 42, 43, 45,46,47, 49, 51, 53, 55]. Seven studies were performed in North America [34, 40, 44, 48] and Latin America [38, 50, 54], two studies originated from Asia [41, 52] and one from Australia [39].
All patients were requested to take their medication as prescribed; beta-blockers, angiotensin converting enzyme inhibitors, statins, diuretics, and antiplatelet agents being the main drug categories. Two studies excluded patients with type II diabetes mellitus [45, 49], one study excluded smokers [40]. All studies stated that baseline characteristics were similar among the intervention groups.
Overall, study quality was good, with a median TESTEX-score of 10 (range 8–13) [Table S1 of the Electronic Supplementary Material (ESM)]. Shortcomings were unblinding of assessors, no intention-to-treat analysis, lack of activity monitoring in the control group, and no adaptation of the relative intensity according to the progress of the patients.
3.3 Outcome Assessment and Intervention Characteristics
The primary outcome for this meta-analysis was the change in peak VO2, which was measured during a cardiopulmonary exercise test until exhaustion on a treadmill [8, 9, 34, 36,37,38, 42,43,44, 46, 48, 50, 52, 54, 55] or cycle ergometer [35, 39,40,41, 45, 47, 49, 51, 53, 55] (Table 1). The exercise test was considered maximal if (1) the criteria of the American College of Sports Medicine or American Heart Association [56,57,58] were fulfilled [8, 41, 48,49,50], (2) patients were exhausted, defined by intolerable leg fatigue or dyspnea [35, 37, 39], (3) there was a leveling off in VO2 [9, 34, 54], and (4) the respiratory exchange ratio exceeded 1.10 [44, 53]. The mode of training was similar to the mode of exercise testing, except for one study, in which a combination of a treadmill, stair climber, and arm and leg cycle ergometer was performed during the training sessions and a treadmill for testing [34].
Median intervention duration was 12 weeks (range 4–24 weeks), with only four studies having a duration longer than 12 weeks [34, 39, 45, 50]. Median training frequency was three times a week (range 2–6 times a week), and at least two sessions were supervised. Three studies added one to three home-based training sessions per week to the supervised intervention (Table 1) [9, 34, 40].
Mean duration of the total training session (including warming up and cool down) was 42 min for AIT (range 30–74 min) and 46 min for ACT (range 30–65 min) (Table 1). The intensity of the interventions was prescribed as a percentage of peak HR in nine studies (AIT 80–95% of peak HR—ACT 60–75% of peak HR) [8, 9, 36, 46, 48, 50, 51, 54, 55], as a percentage of HR reserve in six studies (AIT 75–95% of HR reserve—ACT 60–85% of HR reserve) [34, 41,42,43,44, 52], as a percentage of the maximal workload in five studies [35, 37, 40, 45, 49], using the first (ACT) and second (AIT) ventilatory thresholds in three studies [37, 38, 45], as a percentage of peak VO2 in two studies [39, 47], or another method [53]. Twelve studies reported that the energy expenditure was equal for both interventions (Table 1) [8, 9, 34,35,36, 38, 39, 41, 42, 46, 52, 55]. In the other studies, the training protocols differed in total training work, with ACT expending more calories than AIT [40, 45, 51, 55], or did not report on energy expenditure [37, 43, 44, 47,48,49,50, 53, 54]. Overall, compliance with the training sessions and protocol was high, ranging from 75 to 100% of scheduled training sessions. However, patients with poor compliance were mostly excluded from the final analyses and counted as drop-outs. All studies reported similar compliance ratios between groups.
3.4 Primary Outcome
As shown in Fig. 2, a significantly larger improvement was observed after AIT compared with ACT in the total group (+ 1.40 mL/kg/min; 95% CI 0.69–2.11; p < 0.001), and in the subgroups of patients with CAD (+ 1.25 mL/kg/min, 95% CI 0.49–2.02; p = 0.001) and patients with HFrEF (+ 1.46 mL/kg/min; 95% CI 0.10–2.82; p = 0.03). No difference was found between both interventions in the small group of patients with HFpEF (+ 0.37 mL/kg/min; 95% CI − 1.59–2.32; p = 0.71). The subgroup analysis showed no differences between patients with CAD, HFrEF, and HFpEF (p = 0.65; I2 = 0%) (Fig. 2). With each trial deleted from the model once, significance did not change in the patients with CAD. When we excluded the studies of Freyssin et al. (p = 0.08) [37], Fu et al. (p = 0.08) [41], or Wisløff et al. (p = 0.28) [9] in the HFrEF group, differences between both interventions were no longer significant.
Forest plot of peak oxygen uptake according to pathology. ACT aerobic continuous training, AIT aerobic interval training, CADpEF coronary artery disease with preserved ejection fraction, CI confidence interval, HFrEF heart failure with reduced ejection fraction, HFpEF heart failure with preserved ejection fraction, IV intervention, SD standard deviation
Subgroup analyses on the duration of the peak load of AIT (Fig. S1 of the ESM), the duration of the total intervention (Fig. S2 of the ESM), and the training mode (Fig. S3 of the ESM) revealed no significant subgroup differences on peak VO2 (test for subgroup differences, p = 0.71, p = 0.66, p = 0.11, respectively). Studies prescribing a vigorous effort during the interval showed only a trend for larger increases after AIT compared with ACT (+ 1.06 mL/kg/min; − 0.03 to 2.16; p = 0.06), while studies with intervals at very hard effort were in favor of AIT (+ 1.52 mL/kg/min; 0.48–2.56; p = 0.004) [test for subgroup differences, p = 0.55] (Fig. S4 of the ESM). Studies with a sample size of > 50 patients in each group showed smaller differences between both interventions compared with studies with < 20 patients in each group (test for subgroup differences, p = 0.01) (Fig. 3). In addition, studies stating that they were comparing isocaloric AIT and ACT programs showed that AIT was more effective for improving peak VO2 (+ 2.08 mL/kg/min; 95% CI 0.91–3.25; p < 0.001) while interventions with a lower energy expenditure in the AIT group showed similar effects (+ 0.21; 95% CI − 0.48–0.90; p = 0.55) [subgroup difference, p = 0.007] (Fig. 4).
3.5 Secondary Outcomes
In Table 2, meta-analytic results of the secondary outcomes are presented. Analyses were performed for the total group and patients with CAD and CHF separately, the latter including both patients with HFrEF and HFpEF.
For the total group, a significantly higher increase in VT1 (0.88 mL/kg/min; 95% CI 0.16–1.60; p = 0.02) and peak HR (3.78 beats per minute; 95% CI 1.10–6.45; p = 0.006) was observed after AIT compared with ACT. In addition, peak O2 pulse (p = 0.07), OUES (p = 0.10), LVEF (p = 0.07), and FMD (p = 0.09) tended to improve more after AIT compared with ACT.
In the subgroup of patients with CAD, peak HR increased more after AIT compared with ACT (5.11 beats per minute; 95% CI 1.94–8.28; p = 0.002). In patients with CHF (HFpEF and HFrEF), LVEF was significantly more improved after AIT compared with ACT (2.76%; 95% CI 0.98–4.55; p = 0.002). Excluding the papers of Angadi et al. [48] and Aksoy et al. [47] with patients with HFpEF did not change the results (p = 0.02). Similarly, in patients with CHF, VT1 (p = 0.10) and FMD (p = 0.05) tended to increase more after AIT compared with ACT. For FMD, however, results were no longer significant (p = 0.20) after excluding patients with HFpEF [48].
All other secondary outcomes responded similarly to both training interventions (p > 0.05 for all) (Table 2). Thirteen studies reported on QoL using a variety of questionnaires. Some papers used a generic QoL questionnaire including the 12-Item [51] or 36-Item Short Form Health Survey [39, 41, 45, 47, 49, 53, 54]. Other trials used disease-specific questionnaires such as the MacNew Heart Disease Health Related Quality of Life Questionnaire [9, 36, 46, 53] or the Minnesota Living with Heart Failure Questionnaire [39, 41, 45, 49, 54]. Other questionnaires that were reported less frequently were the Cardiac Depression Scale [39], the Hospital Anxiety and Depression Scale [37, 55], the 36-Item Left Ventricular Dysfunction questionnaire [47], the Kansas City Cardiomyopathy Questionnaire [55], the Global Mood Scale [55], and the Type D Scale [55]. Given this large variety in questionnaires, only a qualitative analysis was performed for QoL.
The 12-Item and 36-Item Short Form Health Surveys showed similar effects after AIT and ACT, with some trials reporting improvements in one or more subscales [39, 47, 51, 53, 54] and others showing no changes in any of the subscales [45, 49]. In the paper of Fu et al., AIT increased the physical and mental score of the 36-Item Short Form Health Survey while ACT did not [41].
For the MacNew Heart Disease Health Related Quality of Life Questionnaire all trials reported significant improvements following both AIT and ACT [9, 36, 46, 53]. In only one study, the improvements were larger after AIT compared with ACT [9].
The Minnesota Living with Heart Failure Questionnaire was used in five trials [39, 41, 45, 49, 54]. Two trials reported significant improvements in the total score after AIT [41, 54] and three trials after ACT [39, 41, 54]. There were no significant group differences in any of the studies.
Six studies did not report on adverse events [35, 38, 41, 42, 49, 50], while 15 studies reported no records of training-related adverse events [8, 34, 36, 37, 39, 40, 43, 44, 46,47,48, 51,52,53,54]. In three studies, adverse events did occur [9, 45, 55]. Wisløff et al. reported one death in the ACT arm, but it was unrelated to exercise [9]. Next, Ellingsen et al. reported three serious adverse events within 3 h of supervised exercise in the AIT group (resuscitation after ventricular arrhythmia; inappropriate implantable cardioverter defibrillator discharge; dizziness without detectable cardiovascular cause) [55]. Finally, Koufaki et al. reported one syncope during AIT and one anxiety/panic attack in the ACT group [45].
3.6 Publication Bias
The funnel plot for our primary outcome peak VO2 showed a likelihood of small publication bias (Fig. S5 of the ESM) because of an asymmetric relationship between treatment effect and SE of the treatment effect. The papers by Wisloff et al. [9] and Ellingsen et al. [55] were outside the inverted funnel.
4 Discussion
This systematic review with a meta-analysis compared the effects of AIT with ACT in patients with CAD and CHF (HFrEF and HFpEF). In this updated meta-analysis, we were able to include 15 additional trials and five times more participants, allowing us to make more precise estimates on the effects on peak VO2 and a number of secondary outcomes.
4.1 Primary Outcome
The pooling of the results of 24 trials showed a larger increase in peak VO2 in favor of AIT (1.40 mL/min/kg; p < 0.001) confirming the results of our previous report [15]. Although small, the magnitude of this mean difference in peak VO2 between both interventions is not only statistically significant but is likely to be also clinically relevant as it has been established previously that for every 1-mL/min/kg increase in peak VO2, an approximate 15% reduction can be expected in all-cause mortality [4]. The larger number of trials and participants enabled us to perform some subgroup analyses based on underlying pathology, duration of the high-intensity interval, study duration, sample size, training mode, and energy expenditure. We found that AIT was also superior in the subgroups of patients with CAD (1.25 mL/min/kg; p = 0.001) and patients with HFrEF (1.46 mL/min/kg; p = 0.03). No significant difference in effect size was observed in the subgroup of patients with HFpEF, but the current paucity of published studies warrants further research [47, 48]. Our overall results are in concordance with most previous meta-analyses performed in study populations of patients with CAD [12,13,14,15] or CHF [10,11,12], all showing significant mean effect sizes ranging from 1.04 to 2.14 mL/kg/min in favor of AIT; but our results are in contrast to Cornelis et al. [16], who reported no significant difference among patients randomized to AIT vs. ACT. The latter might be explained by the inclusion of trials of shorter duration and some low-quality studies (i.e., non-randomized) [16].
Though meta-analyses show a benefit from AIT over ACT for peak VO2, a large variety exists among training protocols. To date, it is unknown which AIT protocol is the best. In our meta-analysis, the intervals differed in duration ranging from 20 s to 4 min, and peak load ranging from 50 to 75% of peak VO2 [39, 47] up to ≥ 90% of peak HR [8, 9, 34, 35, 51, 54]. Subgroup analysis revealed no difference with regard to the duration of AIT intervals. However, previous research showed a dose–response relationship [6] between increases in peak VO2 and the intensity of the intervals, with higher intensities (> 92% of peak HR) resulting in a 2-mL/kg/min larger increase compared with lower intensities (< 88% of peak HR). Therefore, it seems that not the duration of the interval but the intensity is of major importance for effectiveness. Indeed, we found that studies with AIT protocols performed at high intensities only tended to improve peak VO2 more compared with ACT (p = 0.06), while extremely high-intensity AIT protocols found significant results in favor of AIT (p = 0.004). Yet, there was no significant subgroup difference (p = 0.55). Finally, two other exercise characteristics that could potentially influence the magnitude of the effect size are the training mode, which was either cycling [35, 39,40,41, 45, 47, 49, 51, 53] or walking/running [8, 9, 36, 38, 42,43,44, 46, 48, 50, 52, 54], and energy expenditure. No subgroup differences in effect size could be found according to training mode (p = 0.11). In contrast, studies comparing isocaloric programs of AIT and ACT found more pronounced increases following AIT, but if energy expenditure of the AIT intervention was less compared with ACT, the effect sizes were similar. This suggests that AIT could be seen as a more time-efficient intervention resulting in similar improvements with less time.
Subgroups according to the duration of the total intervention (< 12 weeks vs. ≥ 12 weeks) found no differences between short and longer duration training periods, although it should be highlighted that the maximal duration was only 6 months [45]. In addition, the two largest multicenter studies [51, 55] found similar effects after AIT and ACT, while pooled results of smaller studies (< 20 patients per group or 20–50 patients per group) were in favor of AIT. It must be mentioned that these two multicenter studies prescribed isocaloric programs but reported that intensity of AIT was decreased and the intensity of ACT was increased to satisfy the subjective feelings of the patients. These two multicenter trials also investigated longer term effects after supervised AIT and ACT [55, 59]. While Pattyn et al. reported a maintenance of the peak VO2 in patients with CAD 9 months after finishing the training intervention [59], Ellingsen et al. reported that improvements were not maintained in patients with CHF [55]. Future research is needed to develop effective cardiac rehabilitation programs and follow-up strategies to improve the lifelong physically active lifestyle in cardiac patients.
4.2 Secondary Outcomes
A significantly larger increase in VT1 (3.78 mL/kg/min; p = 0.006) was found after AIT compared to ACT, with the effect size being more than double the effect size of peak VO2. This improved ability to use oxygen aerobically at higher intensities is likely to translate into improved performance of daily living activities. Yet, it should be acknowledged that the majority of studies observed no differences in QoL among AIT and ACT patients [9, 36, 39, 46, 47, 51, 53, 54]. Based on the Fick principle [VO2 = Q * (CaO2 − CvO2), where Q is the cardiac output (HR * stroke volume) and CaO2 − CvO2 the arteriovenous O2 difference], the larger improvement following AIT might partly be explained by the ability to reach higher peak HR (5.11 beats per minute; p < 0.01) and an improved stroke volume, as assessed by peak O2 pulse. It seems that higher exercise intensities challenge the cardiac muscle more to provide the working muscles with oxygen, resulting in an increased stroke volume and HR. Furthermore, increases in peak VO2 can be mediated through improvements in cardiac function and vascular function. Both tended to increase more after AIT in the total group (LVEF, p = 0.07; FMD, p = 0.09), which was driven by the results in the subgroups of patients with CHF (LVEF, 2.76%, p = 0.002; FMD, 2.27%, p = 0.05). The results of LVEF are in line with the meta-analyses of Haykowsky et al. (patients with CHF; five studies; + 3.29%; 95% CI − 0.7–7.28; p = 0.11) [11] and Cornelis et al. [16] (patients with CHF; six studies; + 3.39%; CI 1.62–5.16, p < 0.001). Even though this 2.76% larger effect is significantly different, it might not be clinically relevant. For FMD (2.27%, p = 0.05), our results are in line with a previous meta-analysis of Ramos et al. [60] including seven studies with both healthy and diseased individuals (2.26%, p < 0.001). This difference in favor of AIT might be clinically relevant because a 1% increase in FMD is associated with a 13% reduction in the risk of cardiovascular events [61, 62].
In line with the work of Xie et al. [12], we found no differences in any other cardiorespiratory parameter (VE/VCO2 slope, HRR 1 min) or cardiovascular risk factor (body weight, resting HR, SBP, DBP, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, fasting glucose). This might be explained by the optimal pharmacological management of blood pressure, cholesterol, and diabetes mellitus in the included cardiac patients. An additional effect of exercise training is therefore often absent or very small.
4.3 Safety
Even though higher intensity AIT results in more beneficial effects on peak VO2, it may also lead to an acute and transient increased risk for severe cardiac events or even sudden cardiac death [63, 64]. Epidemiological data suggested earlier that sedentary healthy individuals have a 50% increased risk for myocardial infarction during or following a bout of physical activity at high intensity compared with individuals who are used to performing high-intensity physical activity on a regular basis [65]. In the current meta-analysis, 18 studies (n = 775) reported on adverse events [8, 9, 34, 36, 37, 40, 43,44,45,46,47,48, 51,52,53,54,55]. Whereas the majority of these studies reported no adverse events, the largest multicenter study comparing AIT and ACT in patients with CHF found three adverse events, all within 3 h after AIT (resuscitation after ventricular arrhythmia; inappropriate implantable cardioverter defibrillator discharge; dizziness without detectable cardiovascular cause) [55]. In addition, Koufaki et al. reported one event of dizziness during an AIT session [45]. However, it remains difficult to derive conclusions based on these data. Using retrospective data, Rognmo and colleagues found a somewhat higher risk following AIT (i.e., 1/23,182 AIT exercise hours vs. 1/129,456 ACT hours) [66]. However, they also provided a power calculation showing that for an adequately powered randomized trial, we would require > 20,500 patients (and generate > 750,000 exercise hours) to determine the safety of AIT. To be able to draw unified conclusions about the safety of AIT, more and larger studies are needed.
4.4 Strengths and Limitations
This is the first systematic review and meta-analysis including both patients with CAD and CHF, focusing on secondary outcomes including cardiorespiratory parameters, cardiovascular risk factors, cardiac and vascular function, and QoL. In addition, we are the first to analyze the data on peak VO2 depending on interval duration, total study duration, sample size, training mode, and energy expenditure of the training protocols. However, there are some limitations. Only two large multicenter trials were identified for inclusion [51, 55], while the other studies had quite small sample sizes. Moreover, there was a large heterogeneity between study protocols and in the treatment effects. Therefore, random-effects models were used. The funnel plot showed minimal publication bias.
5 Conclusion
This meta-analysis further reinforces that peak VO2 benefits more from AIT compared with ACT in both patients with CAD and CHF. For secondary outcomes, AIT was more beneficial in increasing VT1 and peak HR, and tended to increase LVEF, peak O2 pulse, OUES, and FMD more compared with ACT. Other cardiorespiratory parameters, cardiovascular risk factors, and QoL were equally influenced by both methods of exercise training. Further, AIT seems to be a time-efficient strategy to improve peak VO2, while ACT can be equally effective if the training volume is increased.
References
Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128.
Wilkins E, Wilson L, Wickramasinghe K, et al. European cardiovascular disease statistics: 2017 edition. Available from: http://www.ehnheart.org/cvd-statistics.html. Accessed 25 May 2017.
Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67(1):1–12.
Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35.
Vanhees L, Stevens A, Schepers D, et al. Determinants of the effects of physical training and of the complications requiring resuscitation during exercise in patients with cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2004;11(4):304–12.
Moholdt T, Madssen E, Rognmo Ø, et al. The higher the better? Interval training intensity in coronary heart disease. J Sci Med Sport. 2014;17(5):506–10.
Mezzani A, Hamm LF, Jones AM, et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol. 2013;20(3):442–67.
Rognmo Ø, Hetland E, Helgerud J, et al. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11:2016–22.
Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–94.
Smart NA, Dieberg G, Giallauria F. Intermittent versus continuous exercise training in chronic heart failure: a meta-analysis. Int J Cardiol. 2013;166:352–8.
Haykowsky MJ, Timmons MP, Kruger C, et al. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fraction. Am J Cardiol. 2013;111:1466–9.
Xie B, Yan X, Cai X, et al. Effects of high-intensity training on aerobic capacity in cardiac patients: a systematic review with meta-analysis. Biomed Res Int. 2017;2017:5420840.
Liou K, Ho S, Fildes J, et al. High intensity interval versus moderate intensity continuous training in patients with coronary artery disease: a meta-analysis of physiological and clinical parameters. Heart Lung Circ. 2016;25:166–74.
Elliot AD, Rajopadhyaya K, Bentley DJ, et al. Interval training versus continuous exercise in patient with coronary artery disease: a meta-analysis. Heart Lung Circ. 2015;24:149–57.
Pattyn N, Coeckelberghs E, Buys R, et al. Aerobic interval training vs. moderate continuous training in coronary artery disease patients: a systematic review and meta-analysis. Sports Med. 2014;44:687–700.
Cornelis J, Beckers P, Taeymans J, et al. Comparing exercise training modalities in heart failure: a systematic review and meta-analysis. Int J Cardiol. 2016;221:867–76.
Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Smart NA, Waldron M, Ismail H, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Health. 2015;13(1):9–18.
Follman D, Elliott P, Suh I, et al. Variance imputation for overviews of clinical trials with continuous response. Clin Epidemiol. 1992;45(7):769–73.
Vanhees L, Geladas N, Hansen D, et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: recommendations from the EACPR. Part II. Eur J Prev Cardiol. 2012;19(5):1005–33.
Nechwatal RM, Duck C, Gruber G. Physical training as interval or continuous training in chronic heart failure for improving functional capacity, hemodynamics and quality of life: a controlled study. Z Kardiol. 2002;91(4):328–37.
Gremeaux M, Hannequin A, Laurent Y, et al. Usefulness of the 6-minute walk test and the 200-metre fast walk test to individualize high intensity interval and continuous exercise training in coronary artery disease patients after acute coronary syndrome: a pilot controlled clinical study. Clin Rehabil. 2011;25(9):844–55.
Kavanagh T, Shephard RJ. Conditioning of postcoronary patients: comparison of continuous and interval training. Arch Phys Med Rehabil. 1975;56(2):72–6.
Gaeini AA, Fallah AA, Kazemi F. Effects of aerobic continuous and interval training on rate-pressure product in patients after CABG surgery. J Sports Med Phys Fitness. 2015;55(1–2):76–83.
Möbius-Winckler S, Uhlemann M, Adams V, et al. Coronary collateral growth induced by physical exercise: results of the impact of intensive exercise training on coronary collateral circulation in patients with stable coronary artery disease (EXCITE) trial. Circulation. 2016;133(15):1438–48.
Tschentscher M, Eichinger J, Egger A, et al. High-intensity interval training is not superior to other forms of endurance training during cardiac rehabilitation. Eur J Prev Cardiol. 2016;23(1):14–20.
Prado DML, Rocco EA, Silva AG, et al. Effects of continuous vs interval exercise training on oxygen uptake efficiency slope in patients with coronary artery disease. Braz J Med Biol Res. 2016;49(2):e4890.
Yaylalı YT, Fındıkoğlu G, Yurtdaş M, et al. The effects of baseline heart rate recovery normality and exercise training protocol on heart rate recovery in patients with heart failure. Anatol J Cardiol. 2015;15(9):727–34.
Iellamo F, Manzi V, Caminiti G, et al. Dose-response relationship of baroreflex sensitivity and heart rate variability to individually-tailored exercise in patients with heart failure. Int J Cardiol. 2013;166:334–9.
Iellamo F, Manzi V, Caminiti G, et al. Validation of rate of perceived exertion-based exercise training in patients with heart failure: Insights from autonomic nervous system adaptations. Int J Cardiol. 2014;176(2):394–8.
Currie KD, Rosen LM, Millar PJ, et al. Heart rate recovery and heart rate variability are unchanged in patients with coronary artery disease following 12 weeks of high-intensity interval and moderate-intensity endurance exercise training. Appl Physiol Nutr Metab. 2013;38(6):644–50.
Amundsen BH, Rognmo Ø, Hatlen-Rebhan G, et al. High-intensity aerobic exercise improves diastolic function in coronary artery disease. Scand Cardiovasc J. 2008;42(2):110–7.
Roditis P, Dimopoulos S, Sakellariou D, et al. The effects of exercise training on the kinetics of oxygen uptake in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2007;14(2):304–11.
Warburton DE, McKenzie DC, Haykowsky MJ, et al. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol. 2005;95:1080–4.
Dimopoulos S, Anastasiou-Nana M, Sakellariou D, et al. Effects of exercise rehabilitation program on heart rate recovery in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:67–73.
Moholdt TT, Amundsen BH, Rustad LA, et al. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J. 2009;158:1031–7.
Freyssin C, Verkindt C, Prieur F, et al. Cardiac rehabilitation in chronic heart failure: effects of an 8-week, high-intensity training versus continuous training. Arch Phys Med Rehabil. 2012;93:1359–64.
Rocco EA, Prado DM, Silva AG, et al. Effects of continuous and interval exercise training on PETCO2 response during graded exercise test in patients with coronary artery disease. Clinics. 2012;67:623–8.
Smart NA, Steele M. A comparison of 16 weeks of continuous vs intermittent exercise training in chronic heart failure patients. Congest Heart Fail. 2012;18:205–11.
Currie KD, Dubberley JB, McKelvie RS, et al. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc. 2013;45:1436–42.
Fu TC, Wang CH, Lin PS, et al. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. 2013;167:41–50.
Iellamo F, Manzi V, Caminiti G, et al. Matched dose interval and continuous exercise training induce similar cardiorespiratory and metabolic adaptations in patients with heart failure. Int J Cardiol. 2013;167:2561–5.
Iellamo F, Caminiti G, Sposato B, et al. Effect of high-intensity interval training versus moderate continuous training on 24-h blood pressure profile and insulin resistance in patients with chronic heart failure. Intern Emerg Med. 2014;9(5):547–52.
Keteyian SJ, Hibner BA, Bronsteen K, et al. Greater improvements in cardiorespiratory fitness using higher-intensity interval training in the standard cardiac rehabilitation setting. J Cardiopulm Rehabil Prev. 2014;34:98–105.
Koufaki PK, Mercer TH, George KP, et al. Low-volume high-intensity interval training vs continuous aerobic cycling in patients with chronic heart failure: a pragmatic randomised clinical trial of feasibility and effectiveness. J Rehabil Med. 2014;46:348–56.
Madssen E, Moholdt T, Videm V, et al. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am J Cardiol. 2014;114:1504–11.
Aksoy S, Findikoglu G, Ardic F, et al. Effect of 10-week supervised moderate-intensity vs. continuous aerobic exercise programs on vascular adhesion molecules in patients with heart failure. Am J Phys Med Rehabil. 2015;94:898–911.
Angadi SS, Mookadam F, Lee CD, et al. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J App Physiol. 2015;119:753–8.
Benda NM, Seeger JP, Stevens GG, et al. Effects of high-intensity training versus continuous training on physical fitness, cardiovascular function and quality of life in heart failure patients. PloS One. 2015;10:e0141256.
Cardozo GG, Oliveira RB, Farinatti PT. Effects of high intensity interval versus moderate continuous training on markers of ventilatory and cardiac efficiency in coronary heart disease patients. Sci World J. 2015;2015:192472.
Conraads VM, Pattyn N, De Maeyer C, et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol. 2015;179:203–10.
Kim C, Choi HE, Lim MH. Effect of high interval training in acute myocardial infarction patients with drug-eluting stent. Am J Phys Med Rehabil. 2015;94:879–86.
Jaureguizar KV, Vicente-Campos D, Bautista LR, et al. Effect of high-intensity interval versus continuous exercise training on functional capacity and quality of life in patients with coronary artery disease: a randomized controlled trial. J Cardiopulm Rehabil Prev. 2016;36:96–105.
Ulbrich AZ, Angarten VG, Netto AS, et al. Comparative effects of high intensity interval training versus moderate intensity continuous training on quality of life in patients with hearts failure: study protocol for a randomized controlled trial. Clin Trials Regul Sci Cardiol. 2016;13:21–8.
Ellingsen Ø, Halle M, Conraads V, et al. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. 2017;135:839–49.
Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standard for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694–740.
Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934.
Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA guidelines for exercise testing: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Exercise Testing). Circulation. 1997;96:345–54.
Pattyn N, Vanhees L, Cornelissen VA, et al. The long-term effects of a randomized trial comparing aerobic interval versus continuous training in coronary artery disease patients: 1-year data from the SAINTEX-CAD study. Eur J Prev Cardiol. 2016;23(11):1154–64.
Ramos JS, Dalleck LC, Tjonna AE, et al. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015;45:679–92.
Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–40.
Ras RT, Streppel MT, Draijer R, et al. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168(1):344–51.
Thompson PD, Franklin BA, Balady GJ, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–68.
Williams PT, Thompson PD. Increased cardiovascular disease mortality associated with excessive exercise in heart attack survivors. Mayo Clin Proc. 2014;89(9):1187–94.
Mittleman MA, Maclure M, Tofler GH, et al. Triggering of acute myocardial infarction by heavy physical exertion: protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329(23):1677–83.
Rognmo O, Moholdt T, Bakken H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126(12):1436–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Nele Pattyn, Randy Beulque, and Véronique Cornelissen have no conflicts of interest directly relevant to the content of this review.
Authors’ responsibilities
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pattyn, N., Beulque, R. & Cornelissen, V. Aerobic Interval vs. Continuous Training in Patients with Coronary Artery Disease or Heart Failure: An Updated Systematic Review and Meta-Analysis with a Focus on Secondary Outcomes. Sports Med 48, 1189–1205 (2018). https://doi.org/10.1007/s40279-018-0885-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-018-0885-5