Abstract
Aerobic training (AT) has been the primary mode of exercise training in cardiac rehabilitation. Historically, the reason for the prescription of AT was that it was speculated that although RT may be beneficial for some clinical outcomes, it may have an adverse effect on ventricular structure and function. However, RT has now made its way into current cardiac rehabilitation guidelines, including those directed towards patients with HF, albeit differences exist across institutions and guidelines. A systematic search of PubMed, EMBASE and Cochrane Trials Register on April 30, 2021, was conducted for exercise-based rehabilitation trials in HF. Randomised and controlled trials that reported on resistance training versus usual care or trials that directly compared RT to an AT intervention were included. Resistance training versus controls improves parameters of lower (SMD 0.76 (95%CI 0.26, 1.25, p = 0.003] and upper extremity muscle strength (SMD 0.85 (95%CI 0.35, 1.35), p = 0.0009], both key parameters of physical function throughout the lifespan. Importantly, RT in isolation, versus control, improves VO2peak [MD: 2.64 ml/kg/min (95%CI 1.67, 3.60), p < 0.00001] and 6MWD [MD: 49.94 m (95%CI 34.59, 65.29), p < 0.00001], without any detrimental effect on left ventricular parameters. Resistance training in HF patients is safe and improves parameters of physical function and quality of life. Where people with HF are unable to, or are not inclined to, partake in aerobic activity, RT alone is appropriate to elicit meaningful benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise training is now widely accepted as an important adjunct therapy in heart failure (HF). While the underlying mechanisms for improvements may differ between heart failure reduced ejection fraction (HFrEF) and heart failure preserved ejection fraction (HFpEF), exercise training in HF patients provides numerous benefits including improvements in exercise capacity [1, 2], quality of life [1, 3], diastolic and autonomic function [4, 5], and endothelial function [6]. Specifically, aerobic training (AT) which has been the mainstay of cardiac rehabilitation programmes improves VO2peak [7] and left ventricular ejection fraction (LVEF) [8]. In heart failure, skeletal muscle abnormalities (e.g. muscle atrophy, mitochondrial volume and fibre type) exist irrespective of ejection fraction [9] and low skeletal muscle mass predicts outcomes [10, 11]. In individuals with HFrEF, in which reduced axial skeletal muscle mass occurs, the loss of muscle is considered clinically significant [12]. In HFpEF, loss of lean body mass and decreased quality of the skeletal muscle contribute to reductions in VO2peak [13]. Sarcopenia, a loss of muscle mass and strength, often leads to physical impairment and frailty and worsening of HF. The recent REHAB-HF Trail [14] in older individuals with acute decompensated HF noted 97% of individuals were frail or prefrail, and in addition to mobility and balance issues, one third of individuals had severe leg weakness. As resistance training (RT) is widely accepted as the most effective training method to increase muscle mass (hypertrophy), and strength [15], for HF patients, this may be an important strategy in the prevention of sarcopenia, maintenance, and increase in the muscle [12] and in improving exercise tolerance [9, 13].
Muscular strengthening or RT exercise is endorsed in physical activity guidelines and exercise recommendations in adults [16,17,18,19], albeit only in the last two decades. Even in the very elderly older adult, RT increases muscle strength and size [20]. Furthermore, RT is recommended across a range of pre-clinical and clinical populations [21,22,23] due to the wide-ranging health benefits derived. Resistance training improves cardiometabolic health and reduces CVD risk [24], increases muscle mass and strength [25] leading to improved mobility [26] and improves mental health [27], to name a few of the many benefits. Furthermore, the benefits of RT in some circumstances confer greater health benefits than AT. Additionally, while AT is accepted as reducing the risk of mortality across the general population, RT is also associated with lower mortality, with Saedifard and colleagues (2019) [28] reporting that muscle strengthening was independently associated with a 21% lower risk of all-cause mortality.
In the cardiac patient, RT was once thought to be dangerous due to the possibility that the high-pressure load during lifting may lead to hemodynamic abnormalities and unfavourable cardiac remodelling [29]. Aerobic training was therefore the more favoured or safer mode for exercise-based cardiac rehabilitation, in addition to being the type of training most likely to confer improvements in aerobic capacity. However, in a small 1995 study, McKelvie and colleagues [30] compared the hemodynamic response to cycling and RT in congestive heart failure secondary to ischemic cardiomyopathy, finding that RT using a unilateral leg press did not adversely affect left ventricular function. Additionally, studies that have directly compared VO2peak improvements in AT and RT have found improvements from both modes of training [31, 32]. Resistance training was first recommended in cardiac rehabilitation guidelines in 2000, and these recommendations were cautious in the use of RT [33]. However, over several decades, the evidence for the safety and efficacy of RT in cardiac rehabilitation has grown. In addition to AT, current cardiac rehabilitation guidelines and position statements, including in individuals with HF [34,35,36,37], provide recommendations for RT. The most recent guidelines published by the American Physical Therapy Association (APTA) on the management of HF advocate for the inclusion of RT [35]. Specifically, the guideline provides a strong recommendation for the inclusion of RT for stable HFrEF (classes I–III) [35].
Meta-analyses in 2016 [38] and 2017 [39] reported on the isolated effects of RT, with improvements in VO2peak, quality of life and lower body muscle strength. However, the previous analyses differed (differences in study inclusion and outcomes) limiting comparisons. Furthermore, only one analysis [39] considered strength outcomes, and this was limited to the lower body. Additionally, in the time since these previous analyses were conducted, several new studies have emerged. Given the current recommendations and guidelines for RT in HF, the aim of this review was to consider the evidence that underpins current recommendations and provide, where appropriate, an update to the evidence to further assist in clinical decision making. Clinical decisions are made on the basis of the best available evidence at the time, from various sources, all with careful consideration as to the quality of the evidence. It is therefore important to regularly update evidence available from research, particularly when the area or field of research does not have a large body of data to consider. Furthermore, as there is an increasing conversation around strength training in cardiac rehabilitation [40] as well skeletal muscle differences in the HF population [41], we therefore consider this work timely. Specifically, in this review and analysis, unlike the one previous analysis [39] to consider strength outcomes, we analysed both upper and lower body muscular strength, in addition to consideration of any potential adverse effects on left ventricular function, which has previously been the major concern for RT in this population.
Methods
This work was produced according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [42, 43].
Search strategy
Potential studies were identified by conducting systematic searches of PubMed, EMBASE, CINHAL and the Cochrane Library of Controlled Trials up until April 30, 2021. Search terms included the following ‘chronic heart failure’, ‘heart failure’, ‘heart failure preserved ejection fraction’, ‘heart failure reduced ejection fraction’, ‘resistance’, ‘resistance training’ and ‘strength training’. Additionally, systematic reviews, meta-analyses and reference lists of papers were hand-searched for additional studies. One reviewer (SF) conducted the search, and full-text articles were assessed for eligibility by two reviewers (SF and MJP). In the event of disagreement as to inclusion, a third reviewer (NAS) was consulted. A sample search strategy is presented in Supplementary Files (Table S1).
Study selection
Study type and participants
Randomised controlled trials (RCTs) and controlled trials (quasi and non-RCTs) of RT in HF patients 18 years or older were included. Any diagnosis of heart failure, preserved, moderately reduced and reduced ejection fraction was an inclusion criterion. Studies assessing intervention effect on acute or decompensated HF were excluded.
Intervention
To be included, studies must have included one of the following: (i) a RT intervention vs. usual care control or (ii) RT vs. AT. Resistance training is defined as an exercise where the individual exerts an effort against an external resistance or his or her body weight and includes dynamic (isotonic) and isometric (static) RT. For this review, only dynamic RT interventions were included. Resistance training interventions could be prescribed using a range of equipment (e.g. weight machines, free weights, resistance bands) or no equipment (i.e. body weight). Resistance training can be performed in various manners, and for this review, RT could be in the form of a traditional RT programme where the participant completes a predetermined number of sets and repetitions or RT could be performed in a circuit (e.g. circuit weight/resistance training). Both supervised and unsupervised facility and home-based interventions were included. Only interventions of a minimum of a 2-week duration were included.
Studies were excluded if (i) interventions included respiratory/inspiratory muscle training as part of the RT, (ii) RT was part of what was defined or described as a combined intervention of RT and AT, (iii) participants had participated within a formal exercise rehabilitation programme within the last 6 months and (iv) participants were recovering from an acute decompensation event that occurred within the previous 2 weeks. Additionally, abstracts were excluded.
Outcomes
Studies were eligible to be included in the review if they reported on one or more of the following outcomes: aerobic capacity: VO2peak or 6MWD; cardiovascular measures: resting heart rate (RHR), peak heart rate (PHR), resting systolic blood pressure (SBP) resting diastolic blood pressure (DBP); Echocardiographic parameters: left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), left ventricular end-diastolic diameter (LVEDD); and quality of life (QoL) and muscle strength and endurance. Muscle strength, endurance and QoL could be measured using any validated instrument.
Data extraction
One reviewer (SF) extracted the data, which was checked by a second reviewer (MJP), a third reviewer resolved disputes (NS). For each study, the following information was extracted: (1) author and year of publication, (2) demographic and clinical characteristics, (3) intervention characteristics, (4) outcome data (e.g. pre- and post-mean and SD or change mean and SD, p values and/or 95%CI, or range (IQRs) or range (min, max), and the main findings for the specified outcomes), and (5) compliance and adverse events.
Data synthesis
For participant and intervention characteristics, a narrative synthesis was conducted. For meta-analysis, aggregate data was used in the statistical analyses which were performed using Revman 5.4 (The Nordic Cochrane Centre, Copenhagen, Denmark). Individual meta-analyses were completed for continuous data by using the change in the mean and standard deviation. Variance data reported as standard error (SE) were converted to SD. Where the change in the mean and SD was not reported, the change in mean was calculated by subtracting the pre-intervention mean from the post-intervention mean, and Revman 5.4 (The Nordic Cochrane Centre, Copenhagen, Denmark) enabled calculations of SD using the number of participants in each group and within-group p values or 95% CI. Where exact p values or 95%CIs were not provided, the standard deviation of the mean difference was calculated using the formula: SD = square root [(SDpre-treatment)2 + (SDpost-treatment)2 – (2r x SDpre-treatment x SDpost-treatment)], assuming a correlation coefficient (r) = 0.75 [44]. We also conducted a sensitivity analysis using r = 0.5 which is considered a conservative estimate [45, 46] to determine whether the overall results of the analyses were robust to the use of imputed correlation coefficients. Where data was not presented in text or tables and authors could not be reached, data presented in figures were extracted where possible using the software ‘WebPlotDigitizer’ [Version 4.4].
Data were pooled for meta-analysis when two or more studies measured the same outcome and provided data in a format suitable for pooling [47]. Where a study included multiple intervention groups and a control group, if the intervention groups were included in the same meta-analysis, data was entered separately for each group and the sample size of the control group was divided by the number of intervention groups to eliminate over-inflation of the sample size. A random-effects inverse variance was used with the measure of the effects of mean difference (MD). We used a 5% level of significance and a 95% CI to report the change in outcome measures. For studies where the mean or SD of outcomes were not reported, but median, interquartile range (IQR) or median and range were reported, these were converted using the formula of Wan [48]. Where heterogeneity was apparent in a unit of measurements (e.g. combined QoL instruments or muscle strength) data was presented as a standardised mean difference (SMD). For SMD, effect sizes were categorised according to Cohen’s interpretation, < 0.20 trivial, ≥ 0.20 to < 0.50 small, ≥ 0.50 to < 0.80 moderated and ≥ 0.80 large [49].
Sensitivity analysis
To evaluate the influence of each study on the overall effect size, a sensitivity analysis using the leave-one-out approach was conducted. Additionally, where studies included more novel non-traditional RT protocols, sensitivity analyses were conducted.
Heterogeneity and publication bias
Heterogeneity was quantified using the Higgins I2 test [45, 50], which provides a measure of the degree of inconsistency with 0% < 25% low, 25% < 75% moderate and 75–100% high heterogeneity [50, 51]. Visual inspection of funnel plots [45, 52] assessed the risk of publication bias.
Study quality
Study quality was assessed using the Tool for the Assessment of Study Quality and Reporting in Exercise (TESTEX) [53] by two authors (NAS and SF). In the case of discrepancies, a third author (MJP) was consulted.
Results
Included studies
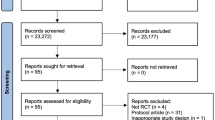
The initial search generated a total of 3472 articles. After removal of duplicates and exclusion of articles based on abstract and title, 1687 full-text articles remained for screening. Full screening resulted in 17 articles meeting the stated inclusion criteria (Fig. 1 PRISMA statement). Details of full-text articles reviewed but excluded are provided, with reasons, in Supplementary Table S2.
Participant characteristics
Table 1 shows the characteristics of all studies included in the review. Publication dates of included studies range from 1992 to 2020. Seventeen [31, 32, 54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] publications, corresponding to 15 separate studies were included in the review. Overall, 15 publications [31, 54,55,56,57, 59,60,61,62,63,64,65,66,67,68], relating to 13 studies compared RT to a control group, two [31, 55] of these studies also included an AT group for comparison. Two additional included studies [32, 58] only compared RT to AT with no comparison to a control group. Data related to one study [66] was retrieved from the associated thesis and confirmed to published information. The publications of Lan [60] and Maiorana [31] reported on the same trial, with additional outcomes reported by Lan and colleagues. With a greater number of strength outcomes reported by Lan, these were used in the strength analyses, as were the additional relevant echocardiographic data, and all other data for outcomes of interest were based on the Maiorana publication. A total of 347 participants were included in the comparison of RT to control; 193 participants were allocated to a RT intervention, with 154 control participants. Total participants included in the comparison of RT to AT interventions was 101; 51 RT and 50 participants allocated to an AT intervention. The mean age of participants from all included studies was 62.22 years for RT, 62.23 years for controls and 62.18 years for AT. Most participants had HFrEF, with only one study noting the inclusion of HFpEF participants based on mean LVEF% or inclusion criteria.
Intervention details
The length of the given RT programmes varied from 3 to 20 weeks. The frequency of RT varied from one per week to 5 times a week, ranging from 20 to 90 min per session. Specific AT programmes varied from 6 to 12 weeks. The frequency of AT varied from 2 to 3 times a week ranging from 40 to 60 min per session. Detailed intervention characteristics are included in Supplementary Table S3.
Outcomes
Tables 2 and 3 summarise the results of meta-analyses of all outcomes of interest. Outcomes for each of the selected studies in the review are presented in Supplementary Table S4.
Resistance training versus control
Lower extremity (LE) strength and endurance
Four studies assessed the LE strength using a 1RM Leg Press. A total of 39 participants in the intervention group and 32 participants in the control group were included in the analysis of 1RM of leg press. Resistance training significantly improved 1RM leg press [SMD: 0.76 (95% CI 0.26, 1.25), p = 0.003] compared with usual care (Fig. 2a). Knee extensor strength was analysed using RM Leg Extension in four studies significantly improved from RT when compared to control groups (SMD: 1.41 (95%CI 0.57, 2.25), p < 0.001, while no statistically significant improvement was found for knee flexor strength was analysed using RM Leg Curl in two studies (Supplementary Figures SF1-2). Strength measured via isokinetic peak torque of knee extensors at 60°/s Nm (three studies) and at 180°/s Nm (two studies) both demonstrated a trend towards improvement; however, neither result was statistically significant [SMD 0.42 (95%CI − 0.01, 0.85, p = 0.05; SMD 0.37 (95%CI − 0.17, 0.91), p = 0.18, respectively] (Supplementary Figures SF3-4). No statistically significant improvements were found for maximal isometric strength, isometric endurance or isokinetic endurance of knee extensors (Supplementary Figures SF5(a) – 7(b)).
Upper extremity (UE) strength and endurance
Four studies were pooled for analysis of upper body strength assessed using a 1RM for the pectoralis muscles. For analysis of pectoralis strength, three studies used a variation of a bench/chest press, while one study assessed 1RM of strength on a pectoral deck machine and an incline press machine. Analysis using the data from the peck deck for one study demonstrated that compared to controls RT significantly improved 1RM strength of the pectoralis muscles; SMD 0.85 (95% CI 0.35, 1.35), p = 0.0009 (Fig. 2b). Substitution of the pectoral deck data for the incline press data did not change the result to a large extent (Supplementary Figure SF8). Results from two studies indicated a statistically significant improvement in 1RM Lateral pulldown (back muscle strength) (Supplementary Fig. SF9).
Combined strength and endurance
Three studies provided data for combined muscle group strength. Analyses indicated a statistically significant improvement in isotonic and isokinetic strength [SMD 0.83 (95%CI 0.34, 1.31), p = 0.0008] (Supplementary Fig. SF10).
Aerobic/functional capacity
Eight studies, six RCTs and 2 quasi/non-RCTs assessed aerobic capacity using VO2peak. Resistance training significantly improved VO2peak compared to the control groups; MD 2.64 ml.kg − 1.min − 1(95% CI 1.67, 3.60) (p < 0.00001) (Fig. 3a). Removal of one study, with abnormal SDs did not impact the result [MD 2.64 (95%CI 1.63, 3.65,), p < 0.00001]. Removal of one study that included what could be considered a small amount of aerobic modalities of training in the circuit weight training (outside the warm-up and cool-down) resulted in a small reduction in the level of VO2peak improvement; however, it remained statistically significant [MD 2.49 ml/kg/min (95%CI 1.37, 3.60,), p < 0.0001]. Six studies assessed functional capacity with the 6MWT. Analysis revealed a statistically significant mean improvement in distance walked; MD 49.94 m (95%CI 34.59, 65.29) (p < 0.00001) (Fig. 3b). The result remained statistically significant with sensitivity analysis to remove Groennebaek [56] study that utilised the novel BFRRE protocol: MD 54.40 m (95%CI 36.17, 7.64) (p < 0.00001).
Quality of life
Six studies assessed QoL. Pooling of data available from five studies that used the Minnesota Living with Heart Failure Questionnaire (MLWHFQ); three RCTs and two quasi/non-RCTs demonstrated significant improvements in QoL in favour of RT; MD − 8.25 (95% CI − 11.51, − 4.99), p < 0. 00,001 (Fig. 4). The result remained statistically significant with sensitivity analysis to remove Groennebaek [56] study that utilised the novel BFRRE protocol: MD − 9.5 (95%CI − 14.60, − 4.39) (p = 0.003).
Echocardiographic parameters
LVEF% was assessed in seven trials; however, data was only suitable for pooling from six studies. Pooled data indicated a statistically significant improvement in LVEF% from RT compared to control; MD 2.75 (95%CI 0.90, 4.59), p = 0.004 (Supplementary Fig. SF11). Leave one out sensitivity analysis did not change the significance of the result. However, removal of the study by Sadek et al. 2018, did increase the p value, but the result remained statistically significant [MD 2.61 (95%CI 0.07, 5.15), p = 0.04]. No statistically significant improvement was noted as a result of the four studies pooled for analysis of LVEDV [MD − 5.28 ml (95%CI − 19.64, 9.08), p = 0.47] and LVESV [MD − 6.64 ml (95%CI − 15.88, 2.60), p = 0.16] or from the three studies pooled for analysis of LVEDD [MD 0.81 mm (95%CI − 2.20, 3.82), p = 0.60] and LVESD [MD − 1.31 mm (95%CI − 4.28, 1.67), p = 0.39] (Supplementary Figures SF11-15).
Cardiovascular parameters
Compared to the control groups, a statistically significant reduction in RHR was observed from the pooled data of five studies; MD − 4.03 bpm (95%CI − 7.52, 0.54), p = 0.02]. Analysis of five studies showed no significant change in PHR from RT [MD 2.62 bpm (95%CI − 3.86, 9.09), p = 0.43]. Neither resting SBP (four studies) [MD 3.21 mmHg (95%CI − 2.81, 9.23), p = 0.30] or resting DBP (two studies) [MD 2.63 mmHg (95%CI − 2.41, 7.67), p = 0.31] significantly improved in RT compared to controls (Supplementary Figures SF16-19).
Resistance training vs aerobic training
Only four studies (five publications) included in the review directly compared the RT group to the AT group.
Muscular strength and endurance
Only two of the four studies assessed the strength, and due to differences in strength testing and limited testing of the muscle groups in one study, data were not pooled for analysis. Feiereisen et al. (2007) [55] found isokinetic strength @ 60°/s Nm and @ 180°/s Nm improved with RT but not AT, while Lan et al. (2020) [60] reported a significant increase in 1RM strength for all muscle groups except the biceps from RT, with AT only improving strength in the left hip flexion and hamstring strength. Additionally, Feiereisen et al. (2007) [55] found knee extension endurance was improved with both RT (p < 0.0015) and AT (p = 0.0007).
Aerobic/functional capacity
Four studies assessed aerobic capacity using VO2peak. No statistically significant difference was observed between RT and AT; [MD 0.26 ml.kg − 1.min − 1 (95%CI − 0.90, 1.42), p = 0.66]. (Supplementary Figure SF20).
Quality of life
Two studies assessed QoL using the validated MLWHFQ, with no statistically significant difference between RT and AT; [MD 0.36 (95% CI − 4.72, 5.45), p = 0.89] (Supplementary Figures SF21).
Echocardiographic parameters
Three studies assessed LVEF%, with two of the three also assessing LVEDV and LVESV. No statistically significant difference was found between RT and AT [LVEF% MD − 2.10% (95%CI − 4.91, 0.72), p = 0.14; LVEDV [MD 2.15 ml (95%CI − 20.22, 24.52), p = 0.85] and LVESV [MD − 3.61 ml (95%CI − 25.40, 18.17), p = 0.75] (Supplementary Figures SF21-24).
Cardiovascular parameters
Analysis of three studies for PHR [MD 0.41 bpm (95%CI − 7.55, 8.37), p = 0.92], RHR [MD 0.43 bpm (95%CI − 3.41, 4.27), p = 0.83], SBP [MD − 1.58 mmHg (95%CI − 6.99, 3.83), p = 0.57], and DBP [MD − 2.29 mmHg (95%CI − 6.02, 1.44), p = 0.23] indicated no statistically significant difference between RT and AT (Supplementary Figures SF25-28).
Adverse events
Three of the included studies did not report any information on adverse events. Of the studies reporting on events, minimal adverse events occurred (Supplementary Table S5). There were no reported serious events related to exercise training, confirming the safety of RT exercise sessions in the HF population.
Study quality, heterogeneity and publication bias
A median TESTEX score of 11 out of 15 was obtained (range 8–12) (Supplementary Table S6). Notably one study, Feiereisen et al. (2007) [55] was a quasi-randomised controlled trial and the study of Levinger et al. (2005) [61, 62] was a controlled but nonrandomised trial. Notwithstanding the inclusion of these two trials, the areas that were frequently lacking in RCTs included allocation concealment, intention to treat analysis and activity monitoring of non-exercise control groups. Heterogeneity was assessed using I2, and all meta-analyses indicated a low level of heterogeneity except for muscle endurance of knee extensors which demonstrated a moderate level of heterogeneity (Table 2). Funnel plots demonstrated minimal evidence of publication bias (Supplementary File 2).
Discussion
The main finding of our analysis demonstrated that RT results in statistically significant improvements in measures of aerobic (peak VO2) and functional capacity (6MWD) in comparison to a control group, with no detriment to cardiac structure and function. Additionally, as one would intuitively expect from RT, pooled analyses demonstrated improved lower and upper body strength. Furthermore, from a more global perspective of wellbeing and function, health-related quality of life improved significantly after RT. Importantly, our analysis also indicated no major differences between RT and AT in aerobic/functional capacity and cardiac structure and function when these are directly compared in trials. Our analysis of aerobic capacity and QoL supports previous results for some outcomes [38, 39], notably; however, study inclusion across the previous reviews varies, and importantly, we have considered this closely when determining the inclusion criteria for RT studies.
Resistance training
While aerobic or endurance training has been the most utilised mode of exercise in HF patients, results of this current analysis demonstrate that RT produces improvements in aerobic and functional capacity. This is an important result given that cardiorespiratory fitness is linked to HF prognosis [38]. Our results support two previous meta-analyses for improvements in VO2peak and 6MWD [38, 39]. A prior concern for cardiac populations has been that RT may cause adverse cardiac remodelling due to the high afterload with lifting. Our results suggest a slightly improved LVEF%, hence no unfavourable remodelling from RT.
Importantly RT is the best method for improving lean muscle mass and the development of, or increase in muscular strength, endurance and power. Given that minimal levels of muscular strength, endurance and power are necessary for one to maintain functional independence throughout the lifespan, RT is the most effective mode for improving these parameters. Our analysis demonstrated improvements in both lower and upper body strength. The large improvement in 1RM leg press from our analysis is higher than that reported by a previous analysis [39]. Our analysis also addressed upper body strength, not reported in the previous review, demonstrating a significant improvement in 1RM strength of the pectoralis muscles. While lower body strength is undoubtedly important for mobility and function, upper body strength also affects a range of ADLs, e.g. lifting objects, including lifting, or pulling oneself up from the ground in the instance of a fall, not an uncommon event in older adults and individuals with chronic conditions and physical impairments. Strength is necessary for performance of ADLs, and HF patients have reduced ability in these functions [69]. Savage and colleagues (2011) [69] in a small trial found that the performance of ADLs was 30% lower in HF patients compared to age-matched non-HF controls, and this decrement was related to both reduced aerobic capacity and muscle strength. Recent evidence also indicates that in HFrEF knee extensor power is an independent predictor of rehospitalisation [70]. Furthermore, decreases in strength, endurance and power lead to reduced mobility and participation in life situations (e.g. recreation, community involvement) and therefore have an impact on an individual’s QoL. Importantly, our analysis also demonstrated a significant improvement in QoL, with a greater improvement in the MLWHFQ after RT, in line with previous reported reductions for exercise training in general (not mode specific) from the 2019 Cochrane Review [3] and from a 2019 IPD meta-analysis [71].
Resistance training intervention characteristics
We only included studies that utilised dynamic RT. A range of dynamic RT protocols was utilised throughout the included studies. Intensity/load varied across studies, starting at 25%1RM moving through to 80–90%1RM at the end of one trial, which currently reflects the broad range of intensity prescriptions throughout RT training guidelines in cardiac and HF patients [35, 36, 72]. With one of the key aims of RT being to increase muscle mass and strength, intensity prescriptions need to reflect the load necessary for these gains, with higher intensity RT suggested as leading to greater improvements than lower loads (intensity), although low loads can improve strength [15]. Interestingly, the initial reason for caution for RT in cardiac and HF populations was due to the concern over the increased pressure-load and subsequently altered hemodynamics associated with lifting, which was speculated would be greater with higher loads/intensities. However, Hansen et al. [40] recently noted from a review of healthy and CVD studies that higher intensity RT resulted in fewer increments in intra-arterial blood pressure and cardiac output, likely due to the fact that with a higher intensity load, the duration of the session is shorter and may reduce the overall response compared to a longer lower intensity RT sessions [40]; however, this has not been tested in the HF population to date. Interestingly, one study, included in our review, by Groennebaek and colleagues (2019) [56] employed dynamic low load RT with the addition of blood flow restriction (BFRRE), a relatively new RT protocol utilised in both healthy and clinical populations to promote muscle hypertrophy and strength. The use of BFRRE in clinical populations may be beneficial to promote muscle function where an individual is unable to tolerate RT training loads at an intensity generally considered necessary to elicit desired improvements [56]. Similar to the improvements in muscular strength demonstrated by Groennebaek et al.,[56] in HF patients, improvements in muscle strength using low load BFRRE have been seen in CAD patients [73]. However, even though studies in clinical populations, including HF [56] and CAD [73], have shown no adverse cardiovascular effects to BFRRE, more longitudinal research is required to confirm the safety of BFRRE in clinical populations [74].
As only dynamic RT interventions were included in our analysis, we did not include another emerging mode of RT, isometric handgrip training (IHG). Recently isometric resistance training (IRT), particularly IHG, has emerged as a beneficial training modality in patients with chronic conditions. In HF patients, only one RCT [47] to date has employed IRT. Gao and colleagues [80] used IHG training five times a week for 12 weeks in 15 patients with HF with significant improvements in QoL and vascular endothelial growth factor (VEGF). In healthy and some clinical populations, IRT demonstrates improvements in blood pressure [75, 76] and is now recommended as an exercise training modality in the treatment of hypertension [22]. Given the paucity of BFRRE and IHG studies in HF patients, whether these types of RT are appropriate for incorporation into RT exercise guidelines for HF patients at this time requires more research. However, given the reasons as to why these RT protocols may be appropriate in this population, it is an important area for further consideration, e.g. ease of use for IHG and with BFRRE allowing individuals who cannot train with higher loads to elicit improvements using loads traditionally not considered appropriate for improvements.
Resistance training versus aerobic training
Our analysis of studies that directly compared AT to RT highlights no significant difference between RT and AT for outcomes of interest. This is an important finding, as, for numerous reasons, AT may not be a feasible or a preferred training modality for some patients. It must be acknowledged that only four RCTs to date have directly compared an AT intervention to a RT intervention. While our analysis demonstrated no significant differences between AT and RT for any of the selected outcomes, only a small number of studies could be pooled for analyses of outcomes, and sample sizes were small, which is a limitation. In our analysis of trials directly comparing AT to RT, we found not statistically significant difference between AT and RT in terms of CRF measured via VO2peak; however, only four studies have directly compared AT to RT. Similarly, a meta-analysis of progressive resistance training (PRT) in coronary heart disease patients found comparable improvements in VO2peak from PRT and AT [77]. Importantly though, the pooled data failed to indicate any major difference between both modalities in relation to cardiac function and left ventricular remodelling. It must be noted however that the study by Lan and colleagues (2020) [60] assessed many echocardiographic parameters, both left and right heart function, that were not considered in our meta-analysis, as to our knowledge, it is the only study to date to provide data on the majority of these parameters. However, in contrast to our pooled findings indicating no significant difference in LVEF% when comparing RT to AT, Lan et al. (2020) [60] found significant improvement in LVEF% from RT and not AT. Interestingly, Lan and colleagues found that while RT was not associated with adverse changes in cardiac structure in patients with HFrEF, AT was associated with a deterioration in diastolic function, specifically E/e’, and AT resulted in some adverse ventricular remodelling. This adverse remodelling from AT in their study is inconsistent with previous studies and analyses indicating AT reverses LV remodelling, with no benefit of combined AT and RT [78]. Lan’s work also conflicts with other findings that moderate intensity continuous AT attenuates LV remodelling, with no changes from RT alone or combined training in LVEF [8]. In their study Lan and colleagues, do note however, that the reduction in diastolic function and indicators of adverse remodelling they observed are likely to be multifactorial, including possible differences in baseline characteristics, e.g. HF severity, medications and RT intervention characteristics compared to other trials [60].
Strength and limitations
Our review included more trials than all previous reviews. Furthermore, only one other analysis of RT alone, to our knowledge, has considered muscular strength improvements from RT in HF patients [39], and only reported on changes in lower body strength. Our analysis reported on a wider range of strength outcomes including upper body strength in addition to considering the effect of RT on endurance. As previously noted, unfortunately, the number of RCTs that consider RT in isolation is limited when compared to AT or combined AT and RT in the HF population. Given the small number of RCTs, we included quasi-RCTs and controlled studies in this review. We did, however, conduct sensitivity analysis removing these trials. Most of the studies included in the review only included HFrEF patients, with only one study noting the inclusion of HFpEF participants (~ 45% of included participants); therefore, generalisability of these results across HF phenotypes is not possible. Additionally, most included participants were male. As noted in our methods, where change SDs were not provided, these were imputed; however, we ran sensitivity analyses to confirm robustness of our imputations.
Resistance training recommendations in heart failure and future research
Despite the fact RT is currently a recommended component of cardiac rehabilitation, the heterogeneity in published RT interventions and the low number of studies make it difficult to ascertain the optimal RT prescription. Current guidelines and position statements outline RT prescription in the HF population, but given the minimal number of RT studies compared to AT or combined training in HF patients, it would be prudent for researchers to now test these recommendations and validate the benefits, in a manner similar to what has occurred in other clinical populations [79]. Future research should also look to identify whether different RT prescriptions/protocols may be more effective in HFrEF, HFmrEF, HFpEF and HF subphenotypes, e.g. obese, sarcopenia and cachexia. Additionally, more long-terms trials are needed in this area to take into consideration that initial changes in the strength from RT are a result of neural adaptations [19]. Longer trials over extended time periods are needed to move past these initial neural adaptations to examine the muscular adaptations such as increases in muscle fibres and size that may lead to additional or greater changes in functional parameters. We believe the importance of RT in HF cannot be underestimated; given that skeletal muscle abnormalities in HF are related to reduced exercise capacity, a minimal amount of the muscle strength is required to perform ADLs, in addition to the fact that the level of dyspnoea, fatigue, physical impairments and comorbidities in some patients may make some modes and protocols of AT an unviable or unfavourable option. It is therefore essential for health clinicians, particularly exercise professionals, to clearly understand the safety, feasibility and benefits of RT in HF patients, to appropriately provide and increase confidence in the prescription of RT to patients.
Conclusion
First and foremost, with no evidence of adverse effects on left ventricular structure and function, RT appears to have been safely used in HF patients. RT is clearly the most effective mode of exercise to improve muscular strength. Given the functional consequences of HF, including skeletal myopathy and the cycle of associated sequelae, such as muscle weakness and frailty that can ensue in HF patients, inclusion of RT is necessary to address these deficiencies. Furthermore, given the ability of RT to improve exercise and functional parameters, the exercise clinician should be confident to prescribe RT where AT is deemed inappropriate or unviable, whether from an impairment or functional perspective or due to client preference. After all, the benefits of any exercise training will only occur if the client adopts and adheres to the programme; therefore, as clinicians, additional exercise options can only be advantageous.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O et al (2019) Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure: individual participant meta-analysis. J Am Coll Cardiol 73(12):1430–1443
Dieberg G, Ismail H, Giallauria F, Smart NA (2015) Clinical outcomes and cardiovascular responses to exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. J Appl Physiol 119(6):726–733
Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ et al (2019) Exercise‐based cardiac rehabilitation for adults with heart failure. Cochrane Database of Systematic Reviews (1)
Pearson M, Mungovan S, Smart N (2017) Effect of exercise on diastolic function in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 22(2):229–242
Pearson M, Smart N (2018) Exercise therapy and autonomic function in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 23(1):91–108
Pearson M, Smart N (2017) Effect of exercise training on endothelial function in heart failure patients: a systematic review meta-analysis. Int J Cardiol 231:234–243
Ismail H, McFarlane JR, Nojoumian AH, Dieberg G, Smart NA (2013) Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: a systematic review and meta-analysis. JACC: Heart Failure 1(6):514–22
Tucker WJ, Beaudry RI, Liang Y, Clark AM, Tomczak CR, Nelson MD et al (2019) Meta-analysis of exercise training on left ventricular ejection fraction in heart failure with reduced ejection fraction: a 10-year update. Prog Cardiovasc Dis 62(2):163–171
Tucker WJ, Haykowsky MJ, Seo Y, Stehling E, Forman DE (2018) Impaired exercise tolerance in heart failure: role of skeletal muscle morphology and function. Curr Heart Fail Rep 15(6):323–331
Carbone S, Billingsley HE, Rodriguez-Miguelez P, Kirkman DL, Garten R, Franco RL et al (2019) Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol 100417
Ventura HO, Carbone S, Lavie CJ (2018) Muscling up to improve heart failure prognosis. Wiley Online Library
Lavie CJ, Carbone S, Neeland IJ (2020) Prevention and treatment of heart failure: we want to pump you up. American College of Cardiology Foundation Washington DC
Brubaker PH, Tucker WJ, Haykowsky MJ (2020) Clinical Considerations and exercise responses of patients with heart failure and preserved ejection fraction: what have we learned in 20 years? Journal of Clinical Exercise Physiology 9(1):17–28
Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR et al (2021) Physical rehabilitation for older patients hospitalized for heart failure. New England Journal of Medicine
Schoenfeld BJ, Grgic J, Van Every DW, Plotkin DL (2021) Loading recommendations for muscle strength, hypertrophy, and local endurance: a re-examination of the repetition continuum. Sports 9(2):32
World Health Organization (2010) t. Global recommendations on physical activity for health: World Health Organization
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M et al (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and science in sports and exercise 43(7):1334–59
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA et al (2018) The physical activity guidelines for Americans. JAMA 320(19):2020–2028
Fragala MS, Cadore EL, Dorgo S, Izquierdo M, Kraemer WJ, Peterson MD et al (2019) Resistance training for older adults: position statement from the national strength and conditioning association. J Strength Cond Res 33(8):2019–2052
Grgic J, Garofolini A, Orazem J, Sabol F, Schoenfeld BJ, Pedisic Z (2020) Effects of resistance training on muscle size and strength in very elderly adults: a systematic review and meta-analysis of randomized controlled trials. Sports Medicine 1–17
Sharman JE, Smart NA, Coombes JS, Stowasser M (2019) Exercise and sport science australia position stand update on exercise and hypertension. Journal of human hypertension 1–7
Hayes SC, Newton RU, Spence RR, Galvão DA (2019) The Exercise and Sports Science Australia position statement: exercise medicine in cancer management. J Sci Med Sport 22(11):1175–1199
Hordern MD, Dunstan DW, Prins JB, Baker MK, Singh MAF, Coombes JS (2012) Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. J Sci Med Sport 15(1):25–31
Carbone S, Kirkman DL, Garten RS, Rodriguez-Miguelez P, Artero EG, Lee D-c et al (2020) Muscular strength and cardiovascular disease: an updated state-of-the-art narrative review. Journal of Cardiopulmonary Rehabilitation and Prevention 40(5):302–9
Schoenfeld BJ, Ogborn D, Krieger JW (2017) Dose-response relationship between weekly resistance training volume and increases in muscle mass: a systematic review and meta-analysis. J Sports Sci 35(11):1073–1082
Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ (2018) Effectiveness of exercise interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI Evidence Synthesis 16(3):752–775
Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP (2018) Association of efficacy of resistance exercise training with depressive symptoms: meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiat 75(6):566–576
Saeidifard F, Medina-Inojosa JR, West CP, Olson TP, Somers VK, Bonikowske AR et al (2019) The association of resistance training with mortality: A systematic review and meta-analysis. Eur J Prev Cardiol 26(15):1647–1665
Price KJ, Gordon BA, Bird SR, Benson AC (2016) A review of guidelines for cardiac rehabilitation exercise programmes: is there an international consensus? Eur J Prev Cardiol 23(16):1715–1733
McKelvie RS, McCartney N, Tomlinson C, Bauer R, MacDougall JD (1995) Comparison of hemodynamic responses to cycling and resistance exercise in congestive heart failure secondary to ischemic cardiomyopathy. Am J Cardiol 76(12):977–979
Maiorana AJ, Naylor LH, Exterkate A, Swart A, Thijssen DH, Lam K et al (2011) The impact of exercise training on conduit artery wall thickness and remodeling in chronic heart failure patients. Hypertension 57(1):56–62
Munch GW, Rosenmeier JB, Petersen M, Rinnov AR, Iepsen UW, Pedersen BK et al (2018) Comparative effectiveness of low-volume time-efficient resistance training versus endurance training in patients with heart failure. J Cardiopulm Rehabil Prev 38(3):175–181
Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B et al (2000) Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription an advisory from the committee on exercise, rehabilitation, and prevention, council on clinical cardiology. American Heart Association Circulation 101(7):828–833
Selig SE, Levinger I, Williams AD, Smart N, Holland DJ, Maiorana A et al (2010) Exercise & Sports Science Australia Position Statement on exercise training and chronic heart failure. J Sci Med Sport 13(3):288–294
Shoemaker MJ, Dias KJ, Lefebvre KM, Heick JD, Collins SM (2020) Physical therapist clinical practice guideline for the management of individuals with heart failure. Phys Ther 100(1):14–43
Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I et al (2020) Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. European journal of preventive cardiology 2047487320913379
Liguori G, Medicine ACoS. ACSM’s guidelines for exercise testing and prescription: Lippincott Williams & Wilkins; 2020.
Jewiss D, Ostman C, Smart NA (2016) The effect of resistance training on clinical outcomes in heart failure: a systematic review and meta-analysis. Int J Cardiol 221:674–681
Giuliano C, Karahalios A, Neil C, Allen J, Levinger I (2017) The effects of resistance training on muscle strength, quality of life and aerobic capacity in patients with chronic heart failure - a meta-analysis. Int J Cardiol 227:413–423
Hansen D, Abreu A, Doherty P, Völler H (2019) Dynamic strength training intensity in cardiovascular rehabilitation: is it time to reconsider clinical practice? A systematic review. Eur J Prev Cardiol 26(14):1483–1492
Lavie CJ, Carbone S, Neeland IJ (2021) Prevention and treatment of heart failure: we want to pump you up. American College of Cardiology Foundation Washington DC
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med 6(7):e1000097
Weir CJ, Butcher I, Assi V, Lewis SC, Murray GD, Langhorne P et al (2018) Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol 18(1):1–14
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al (2019) Cochrane handbook for systematic reviews of interventions: John Wiley & Sons
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Valentine JC, Pigott TD, Rothstein HR (2010) How many studies do you need? A primer on statistical power for meta-analysis. Journal of Educational and Behavioral Statistics 35(2):215–247
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14(1):135
Thompson B (1998) Statistical significance and effect size reporting: portrait of a possible future. Res Sch 5(2):33–38
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Higgins J, Green S (2008) Cochrane handbook for systematic reviews. The Cochrane Collaboration 5(0)
Simmonds M (2015) Quantifying the risk of error when interpreting funnel plots. Syst Rev 4(1):24
Smart NA, Waldron M, Ismail H, Giallauria F, Vigorito C, Cornelissen V et al (2015) Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 13(1):9–18
Cider A, Tygesson H, Hedberg M, Seligman L, Wennerblom B, Sunnerhagen KS (1997) Peripheral muscle training in patients with clinical signs of heart failure. Scand J Rehabil Med 29(2):121–127
Feiereisen P, Delagardelle C, Vaillant M, Lasar Y, Beissel J (2007) Is strength training the more efficient training modality in chronic heart failure? Med Sci Sports Exerc 39(11):1910–1917
Groennebaek T, Sieljacks P, Nielsen R, Pryds K, Jespersen NR, Wang J et al (2019) Effect of blood flow restricted resistance exercise and remote ischemic conditioning on functional capacity and myocellular adaptations in patients with heart failure. Circ Heart Fail 12(12):e006427
Grosse T, Kreulich K, Nägele H, Reer R, Petersen B, Braumann K et al (2001) Peripheres Muskelkrafttraining bei schwerer Herzinsuffizienz. Deutsche Zeitschrift für Sportmedizin 52(1)
Jakovljevic DG, Donovan G, Nunan D, McDonagh S, Trenell MI, Grocott-Mason R et al (2010) The effect of aerobic versus resistance exercise training on peak cardiac power output and physical functional capacity in patients with chronic heart failure. Int J Cardiol 145(3):526–528
Koch M, Douard H, Broustet JP (1992) The benefit of graded physical exercise in chronic heart failure. Chest 101(5 Suppl):231s-s235
Lan NS, Lam K, Naylor LH, Green DJ, Minaee NS, Dias P et al (2020) The impact of distinct exercise training modalities on echocardiographic measurements in patients with heart failure with reduced ejection fraction. J Am Soc Echocardiogr 33(2):148–156
Levinger I, Bronks R, Cody DV, Linton I, Davie A (2005a) Resistance training for chronic heart failure patients on beta blocker medications. Int J Cardiol 102(3):493–499
Levinger I, Bronks R, Cody DV, Linton I, Davie A (2005b) The effect of resistance training on left ventricular function and structure of patients with chronic heart failure. Int J Cardiol 105(2):159–163
Palevo G, Keteyian SJ, Kang M, Caputo JL (2009) Resistance exercise training improves heart function and physical fitness in stable patients with heart failure. J Cardiopulm Rehabil Prev 29(5):294–298
Pu CT, Johnson MT, Forman DE, Hausdorff JM, Roubenoff R, Foldvari M et al (2001) Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol 90(6):2341–2350
Redwine LS, Wilson K, Pung MA, Chinh K, Rutledge T, Mills PJ et al (2019) A randomized study examining the effects of mild-to-moderate group exercises on cardiovascular, physical, and psychological well-being in patients with heart failure. J Cardiopulm Rehabil Prev 39(6):403–408
Sadek Z, Ahmaidi S, Youness M, Joumaa W, Awada C, Ramadan W (2018) editors. Impact of resistance training in patients with chronic heart failure. The Seventh International Conference on Global Health Challenges, Athens, Greece
Selig SE, Carey MF, Menzies DG, Patterson J, Geerling RH, Williams AD et al (2004) Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. J Cardiac Fail 10(1):21–30
Tyni-Lenné R, Dencker K, Gordon A, Jansson E, Sylvén C (2001) Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail 3(1):47–52
Savage P, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Ades PA et al (2011) Effect of resistance training on physical disability in chronic heart failure. Med Sci Sports Exerc 43(8):1379
Lee CJ, Ryu HY, Chun KH, Oh J, Park S, Lee SH et al (2021) Association of muscular fitness with rehospitalization for heart failure with reduced ejection fraction. Clin Cardiol 44(2):244–251
Taylor RS, Walker S, Ciani O, Warren F, Smart NA, Piepoli M et al (2019) Exercise-based cardiac rehabilitation for chronic heart failure: the EXTRAMATCH II individual participant data meta-analysis. Health Technol Assess 23(25):1–98
Fidalgo ASF, Farinatti P, Borges JP, de Paula T, Monteiro W (2019) Institutional guidelines for resistance exercise training in cardiovascular disease: a systematic review. Sports Med 49(3):463–475
Kambič T, Novaković M, Tomažin K, Strojnik V, Jug B (2019) Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: a pilot randomized controlled trial. Front Physiol 10:656
Cristina-Oliveira M, Meireles K, Spranger MD, O’Leary DS, Roschel H, Peçanha T (2020) Clinical safety of blood flow-restricted training? A comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. American Journal of Physiology-Heart and Circulatory Physiology 318(1):H90–H109
Smart NA, Way D, Carlson D, Millar P, McGowan C, Swaine I et al (2019) Effects of isometric resistance training on resting blood pressure: individual participant data meta-analysis. J Hypertens 37(10):1927
A. Correia M, Oliveira PL, Farah BQ, Vianna LC, Wolosker N, Puech‐Leao P et al (2020) Effects of isometric handgrip training in patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc 9(4):e013596
Hollings M, Mavros Y, Freeston J, Fiatarone SM (2017) The effect of progressive resistance training on aerobic fitness and strength in adults with coronary heart disease: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol 24(12):1242–1259
Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM (2007) A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol 49(24):2329–2336
Canning KL, Hicks AL (2020) Benefits of adhering to the Canadian physical activity guidelines for adults with multiple sclerosis beyond aerobic fitness and strength. Int J MS Care 22(1):15–21
Gao M, Lu X, Chen W, Xiao GH, Zhang Y, Yu R, Li J (2018) Randomized clinical trial of physiological ischemic training for patients with coronary heart disease complicated with heart failure: Safety of training, VEGF of peripheral blood and quality of life. Exp Ther Med 16(1):260–264
Funding
No funding has been received for the work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by S Fisher and M Pearson. Study quality was assessed by S Fisher and N Smart. The first draft of the manuscript was written by S Fisher. M Pearson and N Smart revised and completed the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. Meta-analysis of published data.
Consent for publications
All authors consent to publication of this article.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fisher, S., Smart, N.A. & Pearson, M.J. Resistance training in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 27, 1665–1682 (2022). https://doi.org/10.1007/s10741-021-10169-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10169-8