Abstract
The portion of society aged ≥60 years is the fastest growing population in the Western hemisphere. Aging is associated with numerous changes to systemic physiology that affect physical function and performance. We present a narrative review of the literature aimed at discussing the age-related changes in various metrics of physical performance (exercise economy, anaerobic threshold, peak oxygen uptake, muscle strength, and power). It also explores aging exercise physiology as it relates to global physical performance. Finally, this review examines the vascular contributions to aging exercise physiology. Numerous studies have shown that older adults exhibit substantial reductions in physical performance. The process of decline in endurance capacity is particularly insidious over the age of 60 years and varies considerably as a function of sex, task specificity, and individual training status. Starting at the age of 50 years, aging also implicates an impressive deterioration of neuromuscular function, affecting muscle strength and power. Muscle atrophy, together with minor deficits in the structure and function of the nervous system and/or impairments in intrinsic muscle quality, plays an important role in the development of neuromotor senescence. Large artery stiffness increases as a function of age, thus triggering subsequent changes in pulsatile hemodynamics and systemic endothelial dysfunction. For this reason, we propose that vascular senescence has a negative impact on cerebral, cardiac, and neuromuscular structure and function, detrimentally affecting physical performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Above the age of 60 years, aging is associated with a substantial decline in global physical performance (endurance exercise capacity and neuromuscular strength and power). |
Vascular senescence may represent a common link between declines in cardiac function, neuromuscular function, brain function, and physical function with aging. |
1 Introduction
Aging is a natural and complex process influenced by many factors that can be classified as intrinsic (primary aging, e.g., genetic factors), extrinsic (secondary aging, e.g., psychosocial and environmental factors) and related to the effects of disease [1, 2]. The biological nature of this phenomenon and the physiological mechanisms underlying its progression remain largely unknown. Despite that the United Nations has indicated that age ≥60 years may be denoted as old age, the developing world often delimits old age not by years but by new roles, loss of previous roles, or the inability to make an active contribution to society [3]. Thus, the definition of old age continues to change, especially as life expectancy in developed countries has risen to beyond 80 years. Gerontologists distinguish between three subgroups: younger older adults (60–74 years), older adults (75–85 years), and very old adults (>85 years) [4]. Since a considerable decrease in physical and cognitive function typically occurs beyond the age of 80 years, clinicians often distinguish between two additional subgroups of older adults: those aged <85 years (old) and those aged >85 years (old-old) [4], with some classifications considering adults aged ≥100 years as oldest-old.
The number of individuals aged >60 years worldwide is expected to nearly triple from 760 million in 2010 to 2 billion in 2050 [5]. The oldest group (≥80 years, including centenarians) is the most rapidly expanding among Westerners and is expected to increase by 387 % until the year 2030 [6–8]. However, longevity comes at a price that includes a higher rate of morbidity, functional, and mental disability and the eventual loss of independence [9], all of which may play an important role in an increasing use of healthcare services and a reduction in quality of life in the elderly [10, 11]. Human aging involves a progressive decline in cognitive function, cardiovascular (CV) function, skeletal muscle mass (sarcopenia), and neuromotor performance (dynapenia) (Fig. 1). In the elderly, loss of independence is therefore associated with neurodegeneration, reduced muscle strength/power, and increased physiological fatigue [12]. For this reason, one purpose of this narrative review of the literature was to discuss the age-related physiologic changes in global physical performance operationally defined as encompassing exercise endurance, exercise economy, anaerobic threshold, peak oxygen uptake (VO2peak), and neuromuscular strength and power. In addition, we intended to characterize the influence of aging on cerebrovascular, cardiorespiratory, and systemic vascular function. Finally, we argued that vascular changes with aging are important effectors of CV, cerebrovascular, and neuromuscular functional decline, impacting physical performance.
Schematic representation of the impact of vascular senescence on physical performance. Aging interacts with human physiology at the vascular level and this has a negative impact on cerebral, cardiac and neuromuscular structure and function, detrimentally affecting exercise performance. WMH white matter hyperintensities, LV left ventricle, NO nitric oxide
2 Changes in Physical Performance with Aging
2.1 Exercise Endurance and Aging
Within the general population, exercise endurance is objectively measured as the amount of work done during a standardized exercise protocol using large muscle mass (e.g., Bruce treadmill test or 6-min walking test). While a great deal of variation exists, an average decline of 20 % in endurance capacity has been reported between the ages of 40 and 60 years due to a deterioration in both aerobic and musculoskeletal function [13]. From then on, up until the age of 94 years, a linear age-related decline can be observed in both men and women for total duration of exercise in response to the Bruce treadmill test (~0.3 min/year) [14].
Aged athletes represent a human niche for which exercise endurance is more closely linked to their personal bests achieved during a training season [15]. For this reason, in these individuals, exercise endurance is typically quantified as the time to complete long-distance events (e.g., 10-km running time or 1500-m swimming time) [16]. Its values decline modestly after ~35 to 40 years of age and are well maintained until ~60 to 70 years [16–18]. From a physiological point of view, this suggests that most trained adults should be able to sustain functional capacity until this age [16]. In contrast, after 60–70 years of age, endurance exercise performance declines exponentially [16–18]. Research also indicates that this decrease follows a sexually dimorphic pattern and depends on task specificity. This is supported by data demonstrating that the deterioration in running endurance is up to threefold greater in women than in men, with the largest differences occurring at an age >60 years [16]. It is additionally corroborated by findings indicating that the overall magnitude of reduction in swimming endurance with advancing age is ~30 % smaller than that observed in running endurance. Moreover, it is interesting to note that sex differences in age-related declines in swimming endurance are limited to short-duration events [18]. These differences are no longer observed in response to longer-duration events (i.e. 1-h swimming distance) [19].
Cardiopulmonary exercise testing is a methodology that assesses physical function by linking individual endurance capacity to physiological parameters and the underlying metabolic substratum. In humans, VO2peak, together with exercise economy and the anaerobic threshold (AT), is an important physiological factor related to exercise endurance [15, 20, 21]. There is considerable evidence to support the clinical significance of all these factors as they are commonly reduced in several pathological conditions (e.g., coronary artery disease, myopathic heart disease, valvular heart disease, congenital heart disease, peripheral arterial disease; diabetes, anemia, obesity, obstructive lung disease, and restrictive lung disease) [20, 22]. Interestingly, the decline in endurance capacity with advancing age can be attributed to collective reductions in most of these physiological determinants of physical functioning [15, 23].
2.1.1 Exercise Economy and Aging
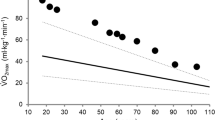
Exercise economy is an important factor that contributes to endurance capacity. Economy refers to how much speed or power can be generated for a given level of VO2 (ml kg−1 min−1) during activities such as walking and running. Exercise economy depends on the interaction of numerous factors, including muscle morphology, elastic elements, and joint mechanics in the efficient conversion of chemical energy to mechanical speed. Good exercise economy is manifested by lower fractional utilization of VO2peak at a given speed [24]. Even though VO2peak is an important determinant of health and physical function in the elderly, exercise endurance depends on a complex interplay between VO2peak and exercise economy (Fig. 2) [25].
Representative example of the aerobic profile of two older adults (age 75 years) obtained in response to a modified Bruce graded exercise protocol. The figure includes both submaximal and maximal data for VO2 (oxygen uptake) (circles) and heart rate (squares). Open symbols: lower exercise economy, but higher VO2peak; closed symbols: higher exercise economy, but lower VO2max. Protocol stages: 1st 2.7 km h−1, 0 % grade; 2nd 2.7 km h−1, 5 %; 3rd 2.7 km h−1, 10 %; 4th 4.0 km h−1, 12 %. Importantly, exercise is terminated at exactly the same stage of the Bruce protocol in both individuals. VO 2peak peak oxygen uptake, bpm beats per minute
The influence of exercise economy on age-related decreases in endurance capacity is poorly understood. However, it is evident that, while running economy remains relatively well preserved with aging, this is not sustained for walking economy. Walking has a 15–20 % greater metabolic cost in the elderly than in younger adults [21, 26]. Importantly, past research has shown that this is paralleled by deterioration in walking performance (a key predictor of morbidity among older adults) with advanced age [27, 28]. Over the past few years, there has been some interest in unraveling the factors underlying disturbed walking economy in this specific population. Data from these studies indicate that older adults exhibit a similar cost of balance and perform external mechanical work to a similar or lower level during walking than younger individuals [21, 29]. Yet, both a decrease in muscular efficiency and an increase in antagonist leg muscle co-activation have also been shown to contribute to the greater oxygen cost of walking in both sedentary and active older adults [21, 26, 29–31]. Nevertheless, to our knowledge, no study has found a single mechanical factor accounting for the 15–20 % greater metabolic cost of walking in this population.
2.1.2 Anaerobic Threshold and Aging
The ability to exercise for long periods at high fractions of the VO2peak is an important determinant of endurance capacity and physical performance. The self-selected fractional utilization of the VO2peak during prolonged submaximal exercise is linked to the AT [15]. It has long been determined that, when measured in absolute terms (i.e., absolute VO2 or speed), the AT declines with the aging process [32]. In line with previous reports, exercise endurance is closely associated with not only VO2peak but also velocity at the AT in both male and female runners aged 21–69 years [11, 33]. Thus, there is some evidence to support that the age-related reduction in endurance capacity may partially rely on a progressive decrease in the AT absolute values. This notwithstanding, it is generally believed that, while a reduction in the AT appears to play a primary role in limiting exercise endurance from young adulthood to middle age, this is not the case from early to later middle age. Under these circumstances, additional losses in endurance are mediated more strongly by the reduction in VO2peak [16].
When expressed as the percentage of VO2peak (relative terms), the AT values do not follow a linear decrease with aging. In fact, under these conditions, a U-shaped relationship has been described between the AT and VO2peak [34]. That is, it appears that the AT in individuals with low and high fitness levels is higher than that seen in those with normal fitness levels [34, 35]. Ultimately, this means that the physiological reserve for engaging in heavy exercise intensities is substantially reduced at both extremes of the physical fitness spectrum in the elderly.
2.1.3 Peak Oxygen Uptake and Aging
The upper limit of aerobic metabolism that can be achieved during exercise is termed VO2peak. This usually occurs during exercise using relatively large muscle mass (e.g., running, swimming, and cycling) and represents the integrative ability of the heart to generate a high cardiac output, total body hemoglobin, high muscle blood flow, and muscle O2 extraction, and, in some cases, the ability of the lungs to oxygenate the blood [36]. Despite being the most important determinant of age-related changes in large-muscle endurance in athletes, the fall in VO2peak with age is steeper than the reductions seen in long-distance exercise performance [16]. This is likely explained by a smaller decline in other determinants of exercise performance over the years (e.g., exercise economy and AT) [11, 35]. Conversely, in sedentary adults, the age-associated decline in VO2peak is similar to that seen in endurance work capacity from the age of 55 to 94 years [14].
In a longitudinal study (initial median age of 70 years), VO2peak was estimated to decline 6.9 and 3.9 ml kg−1 min−1 per decade in men and women, respectively [37]. In another report, the losses per decade in a group of participants aged 55–85 years were 4.3 and 1.9 ml kg−1 min−1 in men and women, respectively [38]. More recently, it has been reported that VO2peak remains constant to a large extent up to the age of 30–35 years and then declines 5–8 % per decade until the age of 60 years. From then on, individuals aged 60–69 years as well as those aged >70 years experience a more pronounced deterioration (20 % per decade) in the ability to transport oxygen from the air for consumption in the working muscles [39]. Thus, despite the differences in the existing literature, it seems that the decline in VO2peak is ≥4–5 ml kg−1 min−1 per decade and continues into later life [40].
2.2 Neuromuscular Performance: Muscular Strength and Power with Aging
In addition to changes in muscular endurance and VO2peak, aging may also be accompanied by decreases in muscle strength and power. These changes affect the ability of older people to perform functional tasks [41, 42]. Just as importantly, they also predispose to osteopenia, osteoporosis, reduced physical activity, decreased daily energy expenditure, and adverse changes in body composition (e.g., increased adiposity and visceral fat deposition) [43–45]. Low skeletal muscle strength has clinical consequences. For instance, strength is inversely and independently associated with death from all causes and from cancer from the age of 20 to 80 years, even after adjusting for VO2peak and other potential confounders [46, 47].
Muscle strength is usually measured by quantifying the maximal value of force that a muscle group produces during a single voluntary contraction. Strength levels usually peak between 25 and 35 years of age and then remain relatively stable until the age of 50 [12, 48–51]. Even though muscle strength decreases after the fifth decade of life, the higher rate of decline has been shown to occur after 60 years [52, 53]. Data from longitudinal studies indicate that, from then on, the age-related decline in muscle strength occurs at a rate of 2–5 % per year [54–57]. The magnitude of loss in muscle strength is more impressive in the lower than in the upper limb [58]. Strength loss is also more evident in concentric and isometric muscle actions than in those involving eccentric contractions [49, 59–62]. It is therefore evident that poor levels of muscle strength affect the ability of the elderly to perform motor actions involving the lower limbs (e.g., walking, stair ascent and/or descent), thus increasing the risk of falling in this specific population [63].
The vertical jump allows for determination of the instantaneous muscle power—an indirect index of the maximal rate of energy released from the hydrolysis of adenosine triphosphate stored in the muscles [64]. As described for muscle strength, instantaneous muscle power is substantially reduced at an older age [65, 66]. However, during the aging process, muscle power declines at an even faster rate than maximal strength [67–73]. The existing data suggest that, at 75 years of age, instantaneous muscle power decreases to about 50 % of the value measured at 20 years (both in absolute and relative terms—per unit of body mass) [65]. More recent research has shown that, in well-trained masters athletes, peak anaerobic power declines at a rate of ~8 % per decade from 35 to 64 years [66]. As described for muscle strength, the age-related reduction in muscle power is also more prominent in the lower limb [70, 74]. Because of its positive correlation with everyday task performance (i.e., rising from a chair, stair climbing, fast walking, dynamic balance, and postural sway), muscle power is believed to be more strongly associated with functional independence than maximal strength [10, 69, 75–77]. In fact, poor muscle power is associated with a threefold greater risk for mobility impairment than reduced muscle strength, and improving muscle power leads to improvements in physical function independent of changes in strength [76, 78]. Further corroborating this notion, previous findings also indicate that muscle power is a better predictor of falls among the community-dwelling elderly than is muscle strength [79]. Thus, its deterioration with aging can lead to a dramatic decrease in independence and quality of life.
3 Physiological Correlates of Age-Associated Changes in Physical Performance
3.1 Muscle Structure and Function
Physiological factors that contribute to age-related decrements in muscle strength, power, and endurance are multifactorial but may be partially mediated by changes in skeletal muscle structure and function. The gradual loss of muscle mass has been identified as a primary cause of age-related decrease in muscle strength (generally explaining >90 % of its variation) [56]. Loss of muscle mass, together with concomitant changes in metabolic machinery and vascular capillarization, have also been implicated in age-associated reductions in VO2peak via effects on peripheral oxygen delivery and extraction [80–83]. Thus, past studies support that muscle mass, strength, and power are all associated with exercise endurance and physical performance in older adults [80, 82, 84]. The reduction in muscle mass is particularly impressive after the age of 50 years. Evidence is compelling that total muscle mass declines by ~30 % from the fifth to the eighth decade of life [85]. Data from other reports suggest this process reaches a rate of decline of ~1.4 % per year after the age of 50 years [56]. The loss of muscle mass with aging is followed by an increase in the intramuscular content of non-muscle tissue (adipose and connective tissue), which may also independently affect muscle contractile properties and exercise endurance [86, 87].
Satellite cells are stem cells that have a primary role in the regulation of muscle growth and control of muscle mass [88]. Activation, proliferation, and differentiation of these cells represent important elements participating in the increase of myonuclei number, protein turnover, and muscle hypertrophy [89, 90]. Older adults exhibit a decreased number of satellite cells per muscle fiber than younger adults [91, 92] and, thus, a reduced myogenic potential. The activation of satellite cells, as well as the metabolic balance between muscle tissue anabolism and catabolism, is dependent on several chemical regulators. Insulin-like growth factor (IGF)-1 is one of the main factors that influences the activation of satellite cells positively. Conversely, myostatin has been demonstrated to exert a negative influence on muscle growth [90]. Interestingly, several studies have shown that serum myostatin levels are substantially increased in the elderly. This implicates greater inhibition of satellite cell activation (primarily in type II fibers), leading to age-related sarcopenia [93–95].
The progressive loss of muscle fibers is largely responsible for the decline in muscle mass with aging [85]. The vastus lateralis muscle undergoes extensive changes in the number of muscle fibers (loss of ~35 %) between the ages of 50 and 80 years [96]. Importantly, this affects all fiber types with similar magnitude [54, 85, 96–100]. Thus, in human aging, muscle deterioration is not characterized by substantial changes in muscle fiber type relative distribution. Further evidence has suggested that mitochondrial-mediated apoptosis may play an important role in the progression of muscle fiber loss in aging [101]. To date, the loss of muscle fibers past the age of 50 years has not been shown to be reversible by any form of training, which suggests that the progressive aging of skeletal muscle mass is not completely preventable [102].
Despite not being the primary cause for muscle atrophy, decreased muscle fiber size also contributes to sarcopenia [54, 59, 85, 98, 99, 103]. The age-related muscle atrophy seems to be particularly impressive in type II fibers (~10 to 40 % smaller in muscle tissue collected from elderly than from young controls) [56]. In contrast, the size of type I fibers (i.e., slow twitch) does not change considerably with age [104–106]. Moreover, it has been shown that type IIB muscle fibers tend to be more affected than type IIA [54, 97]; this suggests the different subtypes of fast-twitch fibers are differently affected by senescence. This is relevant because this pattern of selective atrophy in type II muscle fibers likely underlies the etiology of reduced muscle strength and power in elderly. As importantly, since individuals who lack strength for upright locomotion may not reach the true limits of their VO2 reserve, muscle weakness may also contribute to decreases in aerobic capacity by limiting the ability of the elderly to reach higher levels of work [82, 84].
Muscle quality, also known as specific muscle tension, is defined as the maximum force normalized to muscle cross-sectional area [107]. The interaction between aging and specific muscle tension has generated some controversy in the literature. While some studies found that aging does not influence specific tension [107–110], others reported decreased muscle tension in the elderly [49, 74, 99, 111–114]. It has been argued that such contradictory results might reflect the following methodological differences between studies [115]: (1) muscles studied (upper or lower limb muscles) [49, 99], (2) participants’ sex [49, 114, 116], (3) contraction type [117], (4) velocity chosen for isokinetic testing [109], (5) experimental design (i.e., cross-sectional or longitudinal) [107], and (6) methods used to quantify specific tension (i.e., whole muscle or single fiber testing) [56]. For instance, Frontera et al. [56] found similar values for specific tension between younger and older individuals when testing the whole muscle cross-sectional area. In contrast, it has been shown that older skeletal muscle exhibits a 34 % reduction in intrinsic force-generating capacity [118]. Hypothetically, this might be caused by age-related alterations in cellular and molecular processes, such as changes in satellite cell population, excitation-contraction coupling, myofilament interaction, mitochondrial function, and adipocyte infiltration [119, 120]. However, another experimental design showed that differences in single muscle fiber peak force were virtually dissipated when normalized to cell size [103]. These findings do not support the notion that the intrinsic properties related to myofilament interaction are affected with age. Conversely, they indicate that the reduction in whole muscle strength and function with aging is more closely regulated by quantitative than by qualitative parameters of single muscle fiber contractile function [103].
Aging also influences other factors involved in the ability of individual muscle fibers to perform powerful contractions. Among these, the increase in the duration of twitch contraction together with prolonged half-relaxation time is particularly important [113]. Maximal muscle fiber shortening velocity has also been shown to be reduced with aging in some [121] but not all studies [103], and there is some evidence pointing towards changes in the excitation–contraction coupling process (i.e., decreased amount of Ca2+ release from the sarcoplasmic reticulum of fast-twitch fibers) [118, 122]. Finally, there is compelling evidence that glycation-related cross-linking of intramuscular connective tissue may contribute to altered muscle force transmission and function with healthy aging [123].
From a metabolic standpoint, while glycolytic enzymes seem to be slightly affected, aerobic enzymes have consistently been shown to decline throughout the aging process [97, 124, 125]. Cross-sectional experimental designs exploring the effects of aging on intramuscular capillarization have also reported some contradictory findings. Some indicate that the number of muscle capillaries tends to be reduced in older individuals [97, 126], whereas others were not able to replicate these findings [127, 128]. Nonetheless, with aging, there appears to be redistribution of muscle blood flow from highly oxidative to highly glycolytic muscle fibers [129]. Greater flow to type II muscle fibers may be a compensatory adaptation aimed at preserving contractile function concomitant with reduced fiber volume. Indeed, it has been shown that, despite age-associated changes in muscle mass, single fiber contractile properties of type II fibers are largely preserved [130].
3.2 Neural Structure and Function
The age-related decrements in strength, power, and muscular endurance may be partially explained by a decrease in voluntary neural drive [70, 115, 131]. Senescence leads to a decomposition of the α-motor neurons within the spinal cord [96, 132–136], with a consequent denervation and re-innervation of muscle fibers [134, 137]. This degeneration process occurs mostly after 60 years of age [100]. In fact, the number of motor neurons in the spinal ventral horn may be reduced by a margin of 50 % at the age of 60 years [132]. While some muscle fibers simply necrotize shortly after denervation, others are re-innervated by collateral branches arising from the remaining spinal motor neurons [134, 138]. The process of re-innervation typically means that the affected muscle fiber becomes innervated by a motor axon displaying a different pattern of synaptic discharge. This accounts for the phenotypic changes in muscle fibers and leads to a more homogeneous motor unit distribution within the muscle. Thus, in the aging muscle, each motor unit integrates a greater number of muscle fibers [115]. The reduction in the number of motor units, combined with the strengthening of the remaining units, affects motor unit recruitment and decreases the ability to discriminate force accurately. Thus, aged people experience decreased performance in most tasks involving fine motor control.
Aging also affects other mechanisms of intramuscular coordination, such as the rate coding (i.e., action potential firing rates at which motor neurons discharge), which has a fundamental role in power production and contraction speed [139]. The duration of twitch contraction of the tibialis anterior during maximal voluntary contraction is 23 % longer [140] and the maximal rate of torque during ballistic contractions is lower in older adults than in younger controls [141]. Both these studies also found lower motor neuron firing rates in the elderly. Importantly, this is similar to that described for voluntary isometric contractions of the first dorsal interosseous muscle performed at 20 and 50 % of maximal voluntary contraction [142]. The age-related prolongation of motor neuron after hyperpolarization [143] and the nervous fiber degeneration in the remaining motor neurons are possible explanations for the reduced maximal rate of motor unit discharge in the elderly. Electrophysiological studies within humans and animals found lower nerve conduction velocities in the peripheral nervous system of older than in younger adults [144]. These findings may well be secondary to alterations in myelinated fibers occurring with aging (i.e., size and intrinsic properties of myelin sheaths) [144].
An increase in agonist/antagonist co-activation during maximal voluntary contraction might further contribute to decreasing maximal strength in older individuals [70, 145, 146]; however, this is not a universal finding [147]. The agonist/antagonist co-contraction level is an important variable because part of daily life movement is ballistic in nature. Successful performance in ballistic movement depends on proper coordination between agonist and antagonist muscles, which relies on the integrity of the structures underlying the mechanism of reciprocal inhibition. During a visual step-tracking elbow movement, the elderly had higher levels of agonist/antagonist co-activation because they exhibited longer periods of forearm acceleration while performing slowly than young subjects [148, 149]. Despite slowing the expression of voluntary body movement, increased agonist/antagonist co-activation increases joint stiffness [150]. In the elderly, this may represent a strategy to minimize the age-related tendency for increased movement variability in ballistic movements [148, 151].
3.3 Brain Structure and Function
Aging is associated with profound structural changes to the brain, including many key regions necessary for motor performance, sensorimotor integration, perceived exertion, and volitional drive. Structural changes extend beyond the primary motor cortex and may also include the prefrontal cortex, cerebellum, and hippocampus [152]. These areas have been implicated in endurance exercise performance via their inter-connectivity to the motor cortex and central command (‘central governor’). They may also affect exercise performance via reception of afferent feedback regarding autonomic arousal, cardiac perfusion, thermoregulation, thirst/fluid loss, muscle damage, and perceived exertion [153–157].
Recent evidence indicates that age-related muscle weakness is not entirely explained by the classical concept of muscle atrophy. Overall, these findings suggest that the communication between the brain and skeletal muscle is impaired with advancing age, which raises the hypothesis that many neurologic changes associated with senescence are mechanistically linked to impaired skeletal muscle performance in the elderly [158]. Aging does not lead to a widespread loss of cortical neurons (i.e., pyramidal or stellate cells). In contrast, neuronal atrophy, together with a disruption in white matter integrity and an impressive reduction in myelinated nerve fiber length (~45 % from 18 to 93 years), is particularly incident among the elderly [159, 160]. Aging of the cerebral cortex may therefore be compatible with axon degeneration [161], thus affecting the connectivity of the cortex with itself and the spinal cord. Ultimately, from a functional standpoint, this might underlie impaired muscle strength in older adults [158].
As the structures of the brain age, the ability of the brain to transmit signals is progressively impaired. The loss of dopaminergic neurons in the striatum results in an age-associated inability to attenuate neural noise [162]. Neural noise disturbs the fine-tuning of central motor command, and this introduces a considerable level of unpredictability in motor unit discharge, likely affecting force production [158]. There is partial evidence that dopaminergic degeneration is secondary to the cytotoxic effects of high levels of extracellular glutamate around the striatal neurons [163, 164]. From a different perspective, it has also been reported that older adults exhibit more intra-cortical inhibition and less intra-cortical facilitation than younger controls [165]. Importantly, this is consistent with the notion of disuse-muscle weakness being associated with increases in intra-cortical inhibition [166, 167]. Taken together, these findings suggest that aging results in decreased motor cortical excitability and plasticity, which may affect voluntary activation capacity in older adults.
Voluntary activation is commonly assessed using the interpolated twitch method, which measures the electrical stimulation of the nerve or muscle itself during maximal voluntary contraction. It represents the proportion of maximal possible muscle strength produced during a voluntary contraction. Any increment in force evoked by the electrical stimulus indicates that voluntary activation is <100 % [168]. Despite the lack of consensus on this topic, several studies indicate that voluntary activation is substantially impaired in the elderly (by ~15 % or more) and that this is particularly evident in motor tasks involving knee extension and elbow flexion [169–171]. In fact, one previous report demonstrated that incomplete voluntary activation (ranging from 69 to 93 %) is found in virtually all people aged ≥87 years. Thus, deficits in voluntary activation likely contribute to a significant proportion of muscle weakness observed in the old and very old [169].
3.4 Cerebrovascular Function
With the aforementioned reductions in brain white/grey matter come commensurate reductions in cerebral vascularization. Aging is associated with reductions in regional and global cerebral blood flow [172]. Moreover, given limited energetic substrate storage in the brain, increases in metabolic demand upon neuronal activation during exercise necessitate increased delivery via increases in regional blood flow [173]. Reductions in cerebral oxygenation and disruptions in metabolic homeostasis during exercise have been directly linked to physical performance and fatigue [172]. It has been suggested that reduced oxygenation may impact on cortical activation, affecting skeletal muscle recruitment patterns. Indeed, older adults have reduced cerebral conductance during exercise, which may lead to impaired cerebral oxygen provision and central fatigue, although this is not an undisputed finding [174].
3.5 Cardiorespiratory Structure and Function
In aging individuals, respiratory function progressively deteriorates along with functional changes, and this might theoretically suggest that the respiratory system would become limiting to VO2peak. Increasing rigidity of the chest wall, decline in respiratory muscle strength and endurance, loss of elastic recoil, decrease in the alveolar surface area, and reduction in the number of capillaries perfusing the lung are among the prime candidates for affecting the VO2 response at peak exercise intensities [175]. However, it has been shown that the physiological response to peak exercise is characterized by a breathing reserve of 41 % independently of age and sex [176]. Moreover, in the elderly, maximal voluntary ventilation and lung diffusion capacity for carbon monoxide both decline at a slower rate than VO2peak (~5 to 6 % per decade). Finally, there is also evidence that exercise-induced arterial hypoxemia is very uncommon in this population [36, 175]. For all these reasons, the data from the existing literature do not support the hypothesis that VO2peak is limited by inadequate ventilation and/or pulmonary gas exchange in the healthy elderly. In contrast, the blood oxygen-carrying capacity decreases by ~10 % from 30 to 80 years of age, at least in men [36]. The oxygen-carrying capacity depends on blood hemoglobin levels, and reductions in the serum levels of this metalloprotein are well known to impact VO2peak (e.g., anemia) [177]. Thus, a progressive decrease in blood-carrying capacity along the aging process might hypothetically limit the VO2peak in the elderly to a small extent.
Even in the absence of disease and other risk factors, aging per se instigates pervasive changes in the CV system [178, 179]. The aging heart is marred by numerous structural and functional changes. While traditional views held that left ventricular (LV) mass increased with age, autopsy studies and findings from magnetic resonance imaging suggest that, in the absence of hypertension, cardiac mass does not change in women and may actually decrease in men [180, 181]. Structural changes to the heart are more complex than captured by conventional echocardiography, with newer 3D imaging modalities noting heterogeneous and asymmetric wall thickening to occur such that mass is redistributed from the free wall to the interventricular septum [181]. In addition, reduced autophagy, myocyte apoptosis, scarring, fibrosis, and collagen cross-linking ultimately reduces LV compliance, impacting cardiac function [182–185].
There is general agreement that reduced maximal cardiac output is the principal cause for the decrease in VO2peak and endurance exercise capacity in older people. It is believed that 70–85 % of the limitation in VO2peak is linked to cardiac output irrespective of age and sex [186]. Therefore, it is not surprising that any decline in cardiac output with aging will result in a secondary decrease in VO2peak. The main cause for such relevant reduction in cardiac output is the decline in the maximum achievable heart rate. Maximal heart rate decreases by ~3 % per decade, and the relative contribution of this drop to reduced maximal cardiac output with aging ranges from 40 to 100 % [187, 188]. Despite the etiological basis, an age-related decrease in maximal heart rate remains open to debate, and previous findings suggest it is largely caused by a progressive reduction in intrinsic heart rate and limited chronotropic responsiveness to β-adrenergic stimulation, with the reduction in intrinsic heart rate playing by far the greatest role [189].
LV systolic function remains largely unperturbed by age [190]. In aging, stroke volume is also mildly altered during intense aerobic exercise, while the ejection fraction is considerably influenced, as illustrated by the double increase in end-systolic volume compared with young adults [6]. During peak exercise, the ejection fraction normally decreases from 85 % in the third decade to 70 % in the ninth [191]. Therefore, in the elderly, the maintenance of stroke volume during exercise is primarily attained via the Frank–Starling mechanism (cardiac preload). Importantly, this is different from that seen in younger adults in whom stroke volume is adjusted to the metabolic demands of work rate by means of enhanced cardiac preload, positive inotropism, and decreased afterload. Aforementioned age-associated changes in LV cardiac structure and compliance most notably reduce diastolic function (e.g., diastolic filling and relaxation kinetics), an important determinant of exercise capacity. Interestingly, recent studies exploring cardiac twist mechanics note that cardiac twist may increase with age [192]. When viewed from the apex, the healthy LV maintains a ‘wringing’ systolic displacement, with an initial counterclockwise rotation followed by a clockwise rotation in the LV basal plane and a counterclockwise rotation in the LV apical plane [193]. In general, the apical and basal twist increases during the aging process, leading to an increased LV twist value at rest [193]. This accentuated twist may be an important compensatory adaptation with aging, as subsequent untwisting of the LV produces a suction effect that may enhance LV filling during diastole, particularly during exercise [194]. Loss of LV twist reserve with aging during exercise may thus affect stroke volume and be an important arbiter of reduced physical performance.
3.6 Systemic Vascular Structure and Function
Vascular senescence is a phenotypic expression of human aging. With advancing age, there is pervasive macro and microvascular dysfunction. Two key vascular changes that have recently received considerable attention are stiffening of central elastic arteries (i.e., aorta and carotid) and systemic endothelial dysfunction stemming largely from loss of nitric oxide (NO) bioavailability. Recent literature highlights increased large artery stiffness and endothelial dysfunction as novel correlates of reduced physical performance [195]. The aging arterial system may directly impair cardiac function with age by detrimentally altering ventricular–vascular coupling. Arterial compliance (the inverse of stiffness) reflects the ability of large central elastic arteries, such as the aorta and carotid, to expand and recoil during systole and diastole. Appropriate coupling between the LV and the compliant aorta results in an optimal transfer of blood from the LV to the periphery (optimal cardiometabolic efficiency) without excessive changes in hemodynamic pulsatility. Loss of arterial compliance, or an increase in stiffness, alters ventricular–vascular coupling such that arterial load is increased, as is pulsatility of pressure and flow [196]. With respect to cardiac function, increased aortic stiffness with age contributes to increased afterload, reduced coronary perfusion, altered LV twist mechanics, reduced LV synchronicity, increased myocardial work, myocardial deformation, and left atrium enlargement [197–199]. Increased arterial stiffness may thus detrimentally impact both systolic and diastolic function during exercise, contributing to reductions in exercise stroke volume. Increased stiffness of the aorta and carotid also reduces the sensitivity of baroreceptors housed within these vascular regions. Resultant autonomic dysregulation affects exercise heart rate, fostering chronotropic incompetence. Thus, arterial stiffness may be a key factor contributing to the aforementioned reductions in exercise cardiac output [200, 201].
It has been suggested that a decrease in the maximal difference between the arterial and venous oxygen content (a-vO2) with aging (~3 % per decade) contributes, in a small proportion, to the age reduction seen in the VO2peak [186, 202, 203]. There is a bi-directional association between arterial stiffness and endothelial dysfunction [204, 205]: loss of NO and subsequent endothelial dysfunction contributes to increases in arterial stiffness, and loss of the inherent buffering capacity of the central conduits owing to increased arterial stiffness begets further endothelial dysfunction via promulgation of pulsatile pressure and flow into the peripheral microcirculation [196]. Increases in arterial stiffness, with subsequent changes in blood flow pulsatility, affect blood flow delivery to working skeletal muscle [206–208]. Blood flow in the periphery is tri-phasic in nature with a large antegrade peak during systole owing to LV ejection, retrograde flow occurring during late systole/early diastole, and additional antegrade flow occurring during late diastole owing to the reservoir capacity of the elastic vessels [209]. Increased arterial stiffness results in more oscillatory flow (larger retrograde flow component), which increases with age [208] and may further damage the endothelium [210, 211] and impair oxygen extraction at the tissue level.
With aging, limitations in oxygen and nutrient delivery limit contractile work, and age-associated impairment in endothelial-dependent vasodilation contributes to lower exercise hyperemia [212]. During exercise, vasodilation occurs via an increase in several vasoactive agents, including but not limited to NO. Resultant increases in blood flow ensure that oxygen delivery is closely matched to metabolic demand. Endothelium-dependent vasodilation increases circulatory time in the capillaries and allows for more effective oxygen extraction in the tissues [213]. Conversely, endothelial dysfunction will result in a state of heightened vasoconstriction, reducing blood flow into the capillary bed and preventing efficient oxygen extraction by muscle mitochondria (lower a-vO2), ultimately reducing exercise endurance [214]. A reduction in blood flow to working skeletal muscle (i.e., reduced perfusion) may limit the ability to perform functional tasks [206, 215]. With aging, the relative contribution of NO to exercise hyperemia is reduced by approximately 45 % [212]. Indeed, with aging there is a generalized reduction in limb blood flow and vascular conductance both at rest [216] and during exercise, contributing to reduced endurance capacity [217, 218].
Recent studies also support links between large artery stiffness, endothelial dysfunction (and loss of NO), and muscular strength/power in older adults [219]. NO is obligatory for optimal contractile function and has been shown to enhance velocity of muscle fiber shortening, reduce twitch time-to-peak contraction, and increase rate of force development [220–222]. Such effects are mediated by the NO-dependent activation of soluble guanylate cyclase and have been implicated in numerous aspects of excitation–contraction uncoupling with aging (e.g., reduced sarcoplasmic reticulum calcium–ATPase activity, reduced ryanodine receptor-mediated calcium release, and reduced calcium sensitivity) [221]. NO is also crucial for satellite cell activation and hypertrophy of fast-twitch muscle fibers [223]. Both arterial stiffness and endothelial dysfunction with aging have been causally linked to reduced muscle mass and sarcopenia via effects on reduced skeletal muscle perfusion [224–226]. Moreover, reduced muscle mass has been implicated in increased central hemodynamic load at rest and during exercise, which, as alluded to previously, impacts cardiac function [227–229]. Thus, vascular function may be an important effector of neuromuscular function in older adults.
Arterial stiffness has also been shown to be an important determinant of cerebral perfusion with advancing age. Similar to skeletal muscle during exercise, the brain is a high-flow target organ particularly susceptible to hemodynamic pulsatility [230]. Because the skull is a rigid structure, entry of excessive extracranial hemodynamic pulsatility into this non-expandable space may pervade the deep microcirculation of the brain [231, 232]. Indeed, hemodynamic pulsatility can be detected even in the venous efflux [232–235]. Excessive hemodynamic pulsatility may cause cerebral microbleeds, microvascular hypoperfusion, and focal lacunar infarcts, contributing to detrimental rarefaction and remodeling [236–238]. Pulsatility may also negatively affect the neurovascular unit by contributing to neuronal demyelination and parenchymal damage, ultimately manifesting as white matter hyperintensities [235, 239–243]. Thus, reduced oxygenation and cerebrovascular damage secondary to age-associated increases in arterial stiffness may detrimentally impact key regions of the brain necessary for regulating optimal physical performance. Indeed, older adults with increased arterial stiffness and blunted peripheral vasodilation report higher ratings of perceived exertion during exercise [206, 215].
4 Conclusions
Older adults aged 60–80 years exhibit substantial reductions in endurance capacity. The aging process is also characterized by an impressive deterioration of neuromuscular function affecting both muscle strength and power. Muscle atrophy has been viewed has one of the primary causes of dynapenia. However, more recent findings have clearly demonstrated that minor deficits in the structure and function of the nervous system and/or impairments in intrinsic muscle quality also play an important role in the development of dynapenia. Large artery stiffness increases as a function of age, thus triggering subsequent changes in pulsatile hemodynamics and systemic endothelial dysfunction. Ultimately, since target organs such as the brain, heart, and skeletal muscle are sensitive to hemodynamic pulsatility, this may represent a common link between declines in cardiac function, neuromuscular function, brain function, and physical function with aging. No single drug stands out as the optimal approach for improving and extending quality of life in the aging population. For this reason, the search for alternative strategies for management of human senescence must continue.
References
Glatt SJ, Chayavichitsilp P, Depp C, Schork NJ, Jeste DV. Successful aging: from phenotype to genotype. Biol Psychiatry. 2007;62:282–93.
Lupien SJ, Wan N. Successful ageing: from cell to self. Philos Trans R Soc Lond B Biol Sci. 2004;359:1413–26.
World Health Organization. Health statistics and information systems. Definition of an older or elderly person. Geneva: WHO; 2016. http://www.who.int/healthinfo/survey/ageingdefnolder/en/. Accessed 24 May 2016.
Schwartz JB, Zipes DP. Cardiovascular disease in the elderly. In: Braunwald E, Zipes DP, Libby P, editors. Braunwald’s Heart Disease. Philadelphia: WB Saunders; 2007. p. 1925–49.
United Nations. World population prospects: the 2010 revision. New York: United Nations; 2011.
Karavidas A, Lazaros G, Tsiachris D, Pyrgakis V. Aging and the cardiovascular system. Hellenic J Cardiol. 2010;51:421–7.
Robine JM, Paccaud F. Nonagenarians and centenarians in Switzerland, 1860–2001: a demographic analysis. J Epidemiol Community Health. 2005;59:31–7.
Waite LJ. The demographic faces of the elderly. Popul Dev Rev. 2004;30:3–16.
Christensen K, McGue M, Petersen I, Jeune B, Vaupel JW. Exceptional longevity does not result in excessive levels of disability. Proc Natl Acad Sci. 2008;105:13274–9.
Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond). 1992;82:321–7.
Evans SL, Davy KP, Stevenson ET, Seals DR. Physiological determinants of 10-km performance in highly trained female runners of different ages. J Appl Physiol. 1985;1995(78):1931–41.
Hurley BF. Age, gender, and muscular strength. J Gerontol A Biol Sci Med Sci. 1995;50:41–4.
Kenny GP, Yardley JE, Martineau L, Jay O. Physical work capacity in older adults: implications for the aging worker. Am J Ind Med. 2008;51:610–25.
Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill exercise testing in an epidemiologic study of elderly subjects. J Gerontol A Biol Sci Med Sci. 1998;53:B259–67.
Tanaka K, Takeshima N, Kato T, Niihata S, Ueda K. Critical determinants of endurance performance in middle-aged and elderly endurance runners with heterogeneous training habits. Eur J Appl Physiol Occup Physiol. 1990;59:443–9.
Tanaka H, Seals DR. Invited review: dynamic exercise performance in Masters athletes: insight into the effects of primary human aging on physiological functional capacity. J Appl Physiol. 1985;2003(95):2152–62.
Donato AJ, Tench K, Glueck DH, Seals DR, Eskurza I, Tanaka H. Declines in physiological functional capacity with age: a longitudinal study in peak swimming performance. J Appl Physiol. 1985;2003(94):764–9.
Tanaka H, Seals DR. Age and gender interactions in physiological functional capacity: insight from swimming performance. J Appl Physiol. 1985;1997(82):846–51.
Bongard V, McDermott AY, Dallal GE, Schaefer EJ. Effects of age and gender on physical performance. Age (Dordr). 2007;29:77–85.
Mezzani A, Agostoni P, Cohen-Solal A, Corra U, Jegier A, Kouidi E, Mazic S, Meurin P, Piepoli M, Simon A, Laethem CV, Vanhees L. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2009;16:249–67.
Ortega JD, Farley CT. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J Appl Physiol. 1985;2007(102):2266–73.
Wasserman K, Hansen J, Sue D, Stringer W, Whipp B. Principles of exercise testing and interpretation - including pathophysiology and clinical applications. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
Ortega JD, Beck ON, Roby JM, Turney AL, Kram R. Running for exercise mitigates age-related deterioration of walking economy. PLoS One. 2014;9:e113471.
Mian OS, Thom JM, Ardigo LP, Morse CI, Narici MV, Minetti AE. Effect of a 12-month physical conditioning programme on the metabolic cost of walking in healthy older adults. Eur J Appl Physiol. 2007;100:499–505.
Cadore EL, Pinto RS, Alberton CL, Pinto SS, Lhullier FL, Tartaruga MP, Correa CS, Almeida AP, Silva EM, Laitano O, Kruel LF. Neuromuscular economy, strength, and endurance in healthy elderly men. J Strength Cond Res. 2011;25:997–1003.
Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol. 1985;1992(73):200–6.
Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med Sci Sports Exerc. 1988;20:161–6.
Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–8.
Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf). 2006;186:127–39.
Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani F, Prefaut C, Caillaud C. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol. 1985;2003(95):2248–56.
Ortega JD, Fehlman LA, Farley CT. Effects of aging and arm swing on the metabolic cost of stability in human walking. J Biomech. 2008;41:3303–8.
Posner JD, Gorman KM, Klein HS, Cline CJ. Ventilatory threshold: measurement and variation with age. J Appl Physiol. 1985;1987(63):1519–25.
Iwaoka K, Fuchi T, Higuchi M, Kobayashi S. Blood lactate accumulation during exercise in older endurance runners. Int J Sports Med. 1988;9:253–6.
Thomas SG, Cunningham DA, Thompson J, Rechnitzer PA. Exercise training and “ventilation threshold” in elderly. J Appl Physiol. 1985;1985(59):1472–6.
Allen WK, Seals DR, Hurley BF, Ehsani AA, Hagberg JM. Lactate threshold and distance-running performance in young and older endurance athletes. J Appl Physiol. 1985;1985(58):1281–4.
Burtscher M. Exercise limitations by the oxygen delivery and utilization systems in aging and disease: coordinated adaptation and deadaptation of the lung-heart muscle axis—a mini-review. Gerontology. 2013;59:289–96.
Hollenberg M, Yang J, Haight TJ, Tager IB. Longitudinal changes in aerobic capacity: implications for concepts of aging. J Gerontol A Biol Sci Med Sci. 2006;61:851–8.
Stathokostas L, Jacob-Johnson S, Petrella RJ, Paterson DH. Longitudinal changes in aerobic power in older men and women. J Appl Physiol. 1985;2004(97):781–9.
Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–82.
Shephard RJ. Maximal oxygen intake and independence in old age. Br J Sports Med. 2009;43:342–6.
Aguirre LE, Villareal DT. Physical exercise as therapy for frailty. Nestle Nutr Inst Workshop Ser. 2015;83:83–92.
Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci. 1995;50:147–50.
Jilka RL, O’Brien CA. The role of osteocytes in age-related bone loss. Curr Osteoporos Rep. 2016;14:16–25.
Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–48.
St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–5.
Ruiz JR, Sui X, Lobelo F, Morrow JR Jr, Jackson AW, Sjostrom M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439.
Cooper R, Kuh D, Hardy R, Mortality Review G, Falcon, Teams HAS. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1985;1997(83):1581–7.
Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1985;1999(86):188–94.
Murray MP, Gardner GM, Mollinger LA, Sepic SB. Strength of isometric and isokinetic contractions: knee muscles of men aged 20 to 86. Phys Ther. 1980;60:412–9.
Viitasalo J, Era P, Leskinen A, Heikkinen E. Muscular strength profiles and anthropometry in random samples of men aged 31–35, 51–55 and 71–75 years. Ergonomics. 1985;28:1563–74.
Hakkinen K, Pastinen UM, Karsikas R, Linnamo V. Neuromuscular performance in voluntary bilateral and unilateral contraction and during electrical stimulation in men at different ages. Eur J Appl Physiol Occup Physiol. 1995;70:518–27.
Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:451–6.
Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve. 1986;9:585–91.
Bassey EJ, Harries UJ. Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clin Sci (Lond). 1993;84:331–7.
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 1985;2000(88):1321–6.
Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH, Health A. Body. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85.
Amaral JF, Alvim FC, Castro EA, Doimo LA, Silva MV, Novo Junior JM. Influence of aging on isometric muscle strength, fat-free mass and electromyographic signal power of the upper and lower limbs in women. Braz. J Phys Ther. 2014;18:183–90.
Hortobagyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci. 1995;50:B399–406.
Porter MM, Myint A, Kramer JF, Vandervoort AA. Concentric and eccentric knee extension strength in older and younger men and women. Can J Appl Physiol. 1995;20:429–39.
Porter MM, Vandervoort AA, Kramer JF. Eccentric peak torque of the plantar and dorsiflexors is maintained in older women. J Gerontol A Biol Sci Med Sci. 1997;52:B125–31.
Poulin MJ, Vandervoort AA, Paterson DH, Kramer JF, Cunningham DA. Eccentric and concentric torques of knee and elbow extension in young and older men. Can J Sport Sci. 1992;17:3–7.
Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–ii41.
Ferretti G, Gussoni M, Di Prampero PE, Cerretelli P. Effects of exercise on maximal instantaneous muscular power of humans. J Appl Physiol. 1985;1987(62):2288–94.
Grassi B, Cerretelli P, Narici MV, Marconi C. Peak anaerobic power in master athletes. Eur J Appl Physiol Occup Physiol. 1991;62:394–9.
Gent DN, Norton K. Aging has greater impact on anaerobic versus aerobic power in trained masters athletes. J Sports Sci. 2013;31:97–103.
De Vito G, Bernardi M, Forte R, Pulejo C, Macaluso A, Figura F. Determinants of maximal instantaneous muscle power in women aged 50–75 years. Eur J Appl Physiol Occup Physiol. 1998;78:59–64.
Hakkinen K, Hakkinen A. Muscle cross-sectional area, force production and relaxation characteristics in women at different ages. Eur J Appl Physiol Occup Physiol. 1991;62:410–4.
Izquierdo M, Aguado X, Gonzalez R, Lopez JL, Hakkinen K. Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol Occup Physiol. 1999;79:260–7.
Izquierdo M, Ibanez J, Gorostiaga E, Garrues M, Zuniga A, Anton A, Larrion JL, Hakkinen K. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle-aged and older men. Acta Physiol Scand. 1999;167:57–68.
Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–76.
Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing. 1994;23:371–7.
Young A, Skelton DA. Applied physiology of strength and power in old age. Int J Sports Med. 1994;15:149–51.
Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001;56:B443–8.
Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, Fielding RA. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–7.
Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:728–33.
Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, Singh MA. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–9.
Bean JF, Kiely DK, LaRose S, Goldstein R, Frontera WR, Leveille SG. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? J Am Geriatr Soc. 2010;58:2363–8.
Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31:119–25.
Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1985;1988(65):1147–51.
Spina RJ. Cardiovascular adaptations to endurance exercise training in older men and women. Exerc Sport Sci Rev. 1999;27:317–32.
Vincent KR, Braith RW, Feldman RA, Kallas HE, Lowenthal DT. Improved cardiorespiratory endurance following 6 months of resistance exercise in elderly men and women. Arch Intern Med. 2002;162:673–8.
Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Fujimoto N, Palmer D, Boyd K, Levine BD. The effect of age-related differences in body size and composition on cardiovascular determinants of VO2max. J Gerontol A Biol Sci Med Sci. 2013;68:608–16.
Frank P, Andersson E, Ponten M, Ekblom B, Ekblom M, Sahlin K. Strength training improves muscle aerobic capacity and glucose tolerance in elderly. Scand J Med Sci Sports. 2015;26:764–73.
Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–94.
Csapo R, Malis V, Sinha U, Du J, Sinha S. Age-associated differences in triceps surae muscle composition and strength—an MRI-based cross-sectional comparison of contractile, adipose and connective tissue. BMC Musculoskelet Disord. 2014;15:209.
Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–6.
Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5.
Pallafacchina G, Blaauw B, Schiaffino S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr Metab Cardiovasc Dis. 2013;23(Suppl 1):S12–8.
Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–314.
Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004;29:120–7.
Sacco A, Puri PL. Regulation of muscle satellite cell function in tissue homeostasis and aging. Cell Stem Cell. 2015;16:585–7.
Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86.
McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012;26:2509–21.
Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging. 2002;6:343–8.
Lexell J, Downham D, Sjostrom M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci. 1986;72:211–22.
Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–6.
Essen-Gustavsson B, Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand. 1986;126:107–14.
Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140:41–54.
Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6:588–95.
Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–27.
Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol. 1985;2011(111):1497–504.
Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58.
Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. 2011;4:192–9.
Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 1985;2000(89):81–8.
Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292:E151–7.
Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54:B207–18.
Alway SE, Coggan AR, Sproul MS, Abduljalil AM, Robitaille PM. Muscle torque in young and older untrained and endurance-trained men. J Gerontol A Biol Sci Med Sci. 1996;51:B195–201.
Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1985;1991(71):644–50.
Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1985;1999(87):22–9.
Bruce SA, Newton D, Woledge RC. Effect of age on voluntary force and cross-sectional area of human adductor pollicis muscle. Q J Exp Physiol. 1989;74:359–62.
Davies CT, Thomas DO, White MJ. Mechanical properties of young and elderly human muscle. Acta Med Scand Suppl. 1986;711:219–26.
Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol. 1985;1986(61):361–7.
Young A, Stokes M, Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985;5:145–54.
Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–24.
Young A, Stokes M, Crowe M. Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest. 1984;14:282–7.
Overend TJ, Cunningham DA, Kramer JF, Lefcoe MS, Paterson DH. Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol. 1992;47:M204–10.
Russ DW, Grandy JS, Toma K, Ward CW. Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol (Oxf). 2011;201:391–403.
Delbono O. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J Nutr Health Aging. 2000;4:162–4.
Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol. 1997;157:247–53.
Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80:447–55 (quiz 56–7).
Delbono O, O’Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol. 1995;148:211–22.
Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 1985;2007(103):2068–76.
Kirkendall DT, Garrett WE Jr. The effects of aging and training on skeletal muscle. Am J Sports Med. 1998;26:598–602.
Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, Hans D, Gremion G, Kreis R, Boesch C, Canto C, Amati F. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014;99:1852–61.
Parizkova J, Eiselt E, Sprynarova S, Wachtlova M. Body composition, aerobic capacity, and density of muscle capillaries in young and old men. J Appl Physiol. 1971;31:323–5.
Grimby G, Danneskiold-Samsoe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78–81 year old men and women. Acta Physiol Scand. 1982;115:125–34.
Jakobsson F, Borg K, Edstrom L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol. 1990;80:459–68.
Hirai DM, Copp SW, Hageman KS, Poole DC, Musch TI. Aging alters the contribution of nitric oxide to regional muscle hemodynamic control at rest and during exercise in rats. J Appl Physiol. 1985;2011(111):989–98.
Reid KF, Doros G, Clark DJ, Patten C, Carabello RJ, Cloutier GJ, Phillips EM, Krivickas LS, Frontera WR, Fielding RA. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol. 2012;112:2289–301.
Bilodeau M, Henderson TK, Nolta BE, Pursley PJ, Sandfort GL. Effect of aging on fatigue characteristics of elbow flexor muscles during sustained submaximal contraction. J Appl Physiol. 1985;2001(91):2654–64.
Brown WF, Strong MJ, Snow R. Methods for estimating numbers of motor units in biceps-brachialis muscles and losses of motor units with aging. Muscle Nerve. 1988;11:423–32.
Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36:174–82.
Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1985;1993(74):868–74.
Sica RE, McComas AJ, Upton AR, Longmire D. Motor unit estimations in small muscles of the hand. J Neurol Neurosurg Psychiatry. 1974;37:55–67.
Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–9.
D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511.
Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–9.
Moritani T. Neuromuscular adaptations during the acquisition of muscle strength, power and motor tasks. J Biomech. 1993;26(Suppl 1):95–107.
Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1985;1999(87):843–52.
Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol. 1985;2008(104):739–46.
Erim Z, Beg MF, Burke DT, de Luca CJ. Effects of aging on motor-unit control properties. J Neurophysiol. 1999;82:2081–91.
Piotrkiewicz M, Kudina L, Mierzejewska J, Jakubiec M, Hausmanowa-Petrusewicz I. Age-related change in duration of afterhyperpolarization of human motoneurones. J Physiol. 2007;585:483–90.
Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208.
Hakkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ. Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol. 2000;83:51–62.
Hakkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Malkia E, Kraemer WJ, Newton RU, Alen M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol. 1985;1998(84):1341–9.
Billot M, Duclay J, Simoneau-Buessinger EM, Ballay Y, Martin A. Is co-contraction responsible for the decline in maximal knee joint torque in older males? Age (Dordr). 2014;36:899–910.
Darling WG, Cooke JD, Brown SH. Control of simple arm movements in elderly humans. Neurobiol Aging. 1989;10:149–57.
Seidler-Dobrin RD, He J, Stelmach GE. Coactivation to reduce variability in the elderly. Mot Control. 1998;2:314–30.
Karst GM, Hasan Z. Antagonist muscle activity during human forearm movements under varying kinematic and loading conditions. Exp Brain Res. 1987;67:391–401.
Cooke JD, Brown SH, Cunningham DA. Kinematics of arm movements in elderly humans. Neurobiol Aging. 1989;10:159–65.
Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–8.
Marino FE, Lambert MI, Noakes TD. Superior performance of African runners in warm humid but not in cool environmental conditions. J Appl Physiol. 1985;2004(96):124–30.
Noakes TD, Calbet JA, Boushel R, Sondergaard H, Radegran G, Wagner PD, Saltin B. Central regulation of skeletal muscle recruitment explains the reduced maximal cardiac output during exercise in hypoxia. Am J Physiol Regul Integr Comp Physiol. 2004;287:R996–9 (author reply R9–1002).
Noakes TD. St Clair Gibson A. Logical limitations to the “catastrophe” models of fatigue during exercise in humans. Br J Sports Med. 2004;38:648–9.
Noakes TD, St Clair Gibson A, Lambert EV. From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans. Br J Sports Med. 2004;38:511–4.
Tucker R, Marle T, Lambert EV, Noakes TD. The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J Physiol. 2006;574:905–15.
Manini TM, Hong SL, Clark BC. Aging and muscle: a neuron’s perspective. Curr Opin Clin Nutr Metab Care. 2013;16:21–6.
Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–52.
Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5:239–54.
Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct. 2011;216:85–9.
Darbin O. The aging striatal dopamine function. Parkinsonism Relat Disord. 2012;18:426–32.
Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–31.
Hills TT. Animal foraging and the evolution of goal-directed cognition. Cogn Sci. 2006;30:3–41.
McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 2010;45:671–8.
Clark BC, Issac LC, Lane JL, Damron LA, Hoffman RL. Neuromuscular plasticity during and following 3 wk of human forearm cast immobilization. J Appl Physiol. 1985;2008(105):868–78.
Clark BC, Taylor JL, Hoffman RL, Dearth DJ, Thomas JS. Cast immobilization increases long-interval intracortical inhibition. Muscle Nerve. 2010;42:363–72.
Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28:495–503.
Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22:831–9.
Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 1985;2002(93):457–62.
Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve. 2003;27:99–101.
Fisher JP, Hartwich D, Seifert T, Olesen ND, McNulty CL, Nielsen HB, van Lieshout JJ, Secher NH. Cerebral perfusion, oxygenation and metabolism during exercise in young and elderly individuals. J Physiol. 2013;591:1859–70.
Fisher JP, Ogoh S, Young CN, Raven PB, Fadel PJ. Regulation of middle cerebral artery blood velocity during dynamic exercise in humans: influence of aging. J Appl Physiol. 1985;2008(105):266–73.
Rasmussen P, Nielsen J, Overgaard M, Krogh-Madsen R, Gjedde A, Secher NH, Petersen NC. Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol. 2010;588:1985–95.
Taylor BJ, Johnson BD. The pulmonary circulation and exercise responses in the elderly. Semin Respir Crit Care Med. 2010;31:528–38.
Habedank D, Reindl I, Vietzke G, Bauer U, Sperfeld A, Glaser S, Wernecke KD, Kleber FX. Ventilatory efficiency and exercise tolerance in 101 healthy volunteers. Eur J Appl Physiol Occup Physiol. 1998;77:421–6.
Gledhill N. The influence of altered blood volume and oxygen transport capacity on aerobic performance. Exerc Sport Sci Rev. 1985;13:75–93.
Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46.
Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–64.
Scholz DG, Kitzman DW, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part I (Growth): a quantitative anatomic study of 200 specimens from subjects from birth to 19 years old. Mayo Clin Proc. 1988;63:126–36.
Khouri MG, Maurer MS, El-Khoury Rumbarger L. Assessment of age-related changes in left ventricular structure and function by freehand three-dimensional echocardiography. Am J Geriatr Cardiol. 2005;14:118–25.
Linton PJ, Gurney M, Sengstock D, Mentzer RM Jr, Gottlieb RA. This old heart: cardiac aging and autophagy. J Mol Cell Cardiol. 2015;83:44–54.
Kwak HB. Effects of aging and exercise training on apoptosis in the heart. J Exerc Rehabil. 2013;9:212–9.
Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2:158–73.
Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–805.
Ogawa T, Spina RJ, Martin WH 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503.
Eskurza I, Donato AJ, Moreau KL, Seals DR, Tanaka H. Changes in maximal aerobic capacity with age in endurance-trained women: 7-yr follow-up. J Appl Physiol. 1985;2002(92):2303–8.
Hawkins SA, Marcell TJ, Victoria Jaque S, Wiswell RA. A longitudinal assessment of change in VO2max and maximal heart rate in master athletes. Med Sci Sports Exerc. 2001;33:1744–50.
Christou DD, Seals DR. Decreased maximal heart rate with aging is related to reduced {beta}-adrenergic responsiveness but is largely explained by a reduction in intrinsic heart rate. J Appl Physiol. 1985;2008(105):24–9.
Ruan Q, Nagueh SF. Effect of age on left ventricular systolic function in humans: a study of systolic isovolumic acceleration rate. Exp Physiol. 2005;90:527–34.
Nussbacher A, Gerstenblith G, O’Connor FC, Becker LC, Kass DA, Schulman SP, Fleg JL, Lakatta EG. Hemodynamic effects of unloading the old heart. Am J Physiol. 1999;277:H1863–71.
Sun JP, Lam YY, Wu CQ, Yang XS, Guo R, Kwong JS, Merlino JD, Yu CM. Effect of age and gender on left ventricular rotation and twist in a large group of normal adults–a multicenter study. Int J Cardiol. 2013;167:2215–21.
Tavakoli V, Sahba N. Assessment of age-related changes in left ventricular twist by 3-dimensional speckle-tracking echocardiography. J Ultrasound Med. 2013;32:1435–41.
Nelson MD, Haykowsky MJ, Petersen SR, DeLorey DS, Cheng-Baron J, Thompson RB. Increased left ventricular twist, untwisting rates, and suction maintain global diastolic function during passive heat stress in humans. Am J Physiol Heart Circ Physiol. 2010;298:H930–7.
Lizamore CA, Stoner L, Lucas SJ, Lucero A, Hamlin MJ. Does arterial health affect VO2peak and muscle oxygenation in a sedentary cohort? Med Sci Sports Exerc. 2015;47:272–9.
Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 1985;2008(105):1652–60.
Hwang JW, Kang SJ, Lim HS, Choi BJ, Choi SY, Hwang GS, Yoon MH, Shin JH, Tahk SJ. Impact of arterial stiffness on regional myocardial function assessed by speckle tracking echocardiography in patients with hypertension. J Cardiovasc Ultrasound. 2012;20:90–6.
Kiris A, Kiris G, Karaman K, Sahin M, Gedikli O, Kaplan S, Orem A, Kutlu M, Kazaz Z. Factors affecting left ventricular synchronicity in hypertensive patients: are arterial stiffness and central blood pressures influential? Turk Kardiyol Dern Ars. 2012;40:581–8.
Lantelme P, Laurent S, Besnard C, Bricca G, Vincent M, Legedz L, Milon H. Arterial stiffness is associated with left atrial size in hypertensive patients. Arch Cardiovasc Dis. 2008;101:35–40.
Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001;281:H284–9.
Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104.
Rivera AM, Pels AE 3rd, Sady SP, Sady MA, Cullinane EM, Thompson PD. Physiological factors associated with the lower maximal oxygen consumption of master runners. J Appl Physiol. 1985;1989(66):949–54.
Wiebe CG, Gledhill N, Jamnik VK, Ferguson S. Exercise cardiac function in young through elderly endurance trained women. Med Sci Sports Exerc. 1999;31:684–91.
McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–8.
Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–8.
Gonzales JU, Wiberg M, Defferari E, Proctor DN. Arterial stiffness is higher in older adults with increased perceived fatigue and fatigability during walking. Exp Gerontol. 2015;61:92–7.
Gonzales JU. Gait performance in relation to aortic pulse wave velocity, carotid artery elasticity and peripheral perfusion in healthy older adults. Clin Physiol Funct Imaging. 2013;33:245–51.
Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211:390–2.
Heffernan KS, Lefferts WK, Kasprowicz AG, Tarzia BJ, Thijssen DH, Brutsaert TD. Manipulation of arterial stiffness, wave reflections, and retrograde shear rate in the femoral artery using lower limb external compression. Physiol Rep. 2013;1:e00022.
Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986–92.
Schreuder TH, Green DJ, Hopman MT, Thijssen DH. Acute impact of retrograde shear rate on brachial and superficial femoral artery flow-mediated dilation in humans. Physiol Rep. 2014;2:e00193.
Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–36.
Boveris DL, Boveris A. Oxygen delivery to the tissues and mitochondrial respiration. Front Biosci. 2007;12:1014–23.
Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13:315–27.
Gonzales JU, Defferari E, Fisher A, Shephard J, Proctor DN. Calf exercise-induced vasodilation is blunted in healthy older adults with increased walking performance fatigue. Exp Gerontol. 2014;57:1–5.
Dinenno FA, Seals DR, DeSouza CA, Tanaka H. Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol. 2001;531:573–9.
Ridout SJ, Parker BA, Smithmyer SL, Gonzales JU, Beck KC, Proctor DN. Age and sex influence the balance between maximal cardiac output and peripheral vascular reserve. J Appl Physiol. 2010;108:483–9.
Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–74.
Heffernan KS, Chale A, Hau C, Cloutier GJ, Phillips EM, Warner P, Nickerson H, Reid KF, Kuvin JT, Fielding RA. Systemic vascular function is associated with muscular power in older adults. J Aging Res. 2012;2012:1–10.
Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol. 2011;589:2119–27.
Marechal G, Gailly P. Effects of nitric oxide on the contraction of skeletal muscle. Cell Mol Life Sci. 1999;55:1088–102.
Morrison RJ, Miller CC 3rd, Reid MB. Nitric oxide effects on force-velocity characteristics of the rat diaphragm. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:203–9.
Martins KJ, MacLean I, Murdoch GK, Dixon WT, Putman CT. Nitric oxide synthase inhibition delays low-frequency stimulation-induced satellite cell activation in rat fast-twitch muscle. Appl Physiol Nutr Metab. 2011;36:996–1000.
Ochi M, Kohara K, Tabara Y, Kido T, Uetani E, Ochi N, Igase M, Miki T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212:327–32.
Sampaio RA, Sewo Sampaio PY, Yamada M, Yukutake T, Uchida MC, Tsuboyama T, Arai H. Arterial stiffness is associated with low skeletal muscle mass in Japanese community-dwelling older adults. Geriatr Gerontol Int. 2014;14(Suppl 1):109–14.
Prior SJ, Ryan AS, Blumenthal JB, Watson JM, Katzel LI, Goldberg AP. Sarcopenia is associated with lower skeletal muscle capillarization and exercise capacity in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:1096–101.
Abbatecola AM, Chiodini P, Gallo C, Lakatta E, Sutton-Tyrrell K, Tylavsky FA, Goodpaster B, de Rekeneire N, Schwartz AV, Paolisso G, Harris T, Health ABCs. Pulse wave velocity is associated with muscle mass decline: Health ABC study. Age (Dordr). 2012;34:469–78.
Anoop S, Misra A, Bhardwaj S, Gulati S. High body fat and low muscle mass are associated with increased arterial stiffness in Asian Indians in North India. J Diabetes Complicat. 2015;29:38–43.
Kim TN, Park MS, Lim KI, Yang SJ, Yoo HJ, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Res Clin Pract. 2011;93:285–91.
Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–60.
Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56:1746–8.
Bateman GA, Levi CR, Schofield P, Wang Y, Lovett EC. The venous manifestations of pulse wave encephalopathy: windkessel dysfunction in normal aging and senile dementia. Neuroradiology. 2008;50:491–7.
Bateman GA. Pulse wave encephalopathy: a spectrum hypothesis incorporating Alzheimer’s disease, vascular dementia and normal pressure hydrocephalus. Med Hypotheses. 2004;62:182–7.
Bateman GA. Pulse-wave encephalopathy: a comparative study of the hydrodynamics of leukoaraiosis and normal-pressure hydrocephalus. Neuroradiology. 2002;44:740–8.
Jolly TA, Bateman GA, Levi CR, Parsons MW, Michie PT, Karayanidis F. Early detection of microstructural white matter changes associated with arterial pulsatility. Front Hum Neurosci. 2013;7:782.
Brisset M, Boutouyrie P, Pico F, Zhu Y, Zureik M, Schilling S, Dufouil C, Mazoyer B, Laurent S, Tzourio C, Debette S. Large-vessel correlates of cerebral small-vessel disease. Neurology. 2013;80:662–9.
Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, Witteman JC, Breteler MM, Mattace-Raso FU, Ikram MA. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke. 2012;43:2637–42.
Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–91.
Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43:2631–6.
Wahlin A, Ambarki K, Birgander R, Malm J, Eklund A. Intracranial pulsatility is associated with regional brain volume in elderly individuals. Neurobiol Aging. 2014;35:365–72.
Xiong YY, Mok V, Wong A, Leung T, Chen XY, Chu WC, Soo Y, Fu JH, Ding D, Hong Z, Wong KS. Evaluation of age-related white matter changes using transcranial Doppler ultrasonography. J Neuroimaging. 2013;23:53–7.
Hughes TM, Kuller LH, Barinas-Mitchell EJ, McDade EM, Klunk WE, Cohen AD, Mathis CA, Dekosky ST, Price JC, Lopez OL. Arterial Stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71:562–8.
Rosano C, Watson N, Chang Y, Newman AB, Aizenstein HJ, Du Y, Venkatraman V, Harris TB, Barinas-Mitchell E, Sutton-Tyrrell K. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension. 2013;61:160–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Goncalo V. Mendonca, Pedro Pezarat-Correia, João R. Vaz, Luís Silva, and Kevin S. Heffernan have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Mendonca, G.V., Pezarat-Correia, P., Vaz, J.R. et al. Impact of Aging on Endurance and Neuromuscular Physical Performance: The Role of Vascular Senescence. Sports Med 47, 583–598 (2017). https://doi.org/10.1007/s40279-016-0596-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-016-0596-8