Abstract
Background
Gastric emptying (GE) could influence exercise-induced changes in appetite and energy intake. GE also could contribute to changes in gastric symptoms and the availability of nutrients during exercise, which will subsequently affect performance.
Objective
The objective of this review was to determine the effects of acute exercise on GE using a systematic review and meta-analysis. The most common parameters to determine GE were selected, consisting of half-emptying time and volume emptied. Oral-caecal transit time (OCTT) was also examined.
Data Sources
Research databases (PubMed, Scopus, Google Scholar, EBSCOhost, SPORTDiscus) were searched through November 2013 for original studies, abstracts, theses and dissertations that examined the influence of acute exercise on GE.
Study Selection
Studies were included if they evaluated GE or OCTT during and/or after exercise and involved a resting control trial.
Study Appraisal and Synthesis
Initially, 195 studies were identified. After evaluation of study characteristics and quality and validity, data from 20 studies (35 trials) involving 221 participants (157 men; 52 women; 12 unknown) were extracted for meta-analysis. Random-effects meta-analyses were utilised for the three main outcome variables, and effect sizes (ES) are reported as Hedge’s g due to numerous small sample sizes.
Results
Random-effects modelling revealed non-significant and small/null main effect sizes for volume emptied (ES = 0.195; 95 % CI −0.25 to 0.64), half-time (ES = −0.109, 95 % CI −0.66 to 0.44) and OCTT (ES = 0.089; 95 % CI −0.64 to 0.82). All analyses exhibited significant heterogeneity and numerous variables moderated the results. There was a dose response of exercise intensity; at lower intensities GE was faster, and at high exercise intensities GE was slower. Walking was associated with faster GE and cycling with slower GE. Greater volume of meal/fluid ingested, higher osmolality of beverage and longer exercise duration were also associated with slower GE with exercise.
Limitations
The major limitation is that the majority of studies utilised a liquid bolus administered pre-exercise to determine GE; the relationship to post-exercise appetite and energy intake remains unknown. Study populations were also generally active or trained individuals. Furthermore, our review was limited to English language studies and studies that utilised resting control conditions.
Conclusions
These results suggest that exercise intensity, mode, duration and the nature of meal/fluid ingested all influence GE during and after acute exercise. The relationship of GE parameters with appetite regulation after exercise remains largely unexplored. Further integrative studies combining GE and alterations in gut hormones, as well as in populations such as overweight and obese individuals are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Exercise intensity, mode, duration and the nature of meal/fluid ingested all influence variation in gastric emptying (GE) results during and after acute exercise. |
GE could be one mechanism contributing to exercise-induced changes in appetite, but at present this linkage remains speculative. |
1 Introduction
The influence of exercise on the digestive function of the stomach has long been of interest, with reports of an experiment by Roman Emperor Frederick II dating back to the thirteenth century [1]. He was reported to have
“fed two men most excellently at dinner, one of whom he sent forthwith to sleep, and the other to hunt; and that same evening he caused them to be disembowelled in his presence, wishing to know which had digested the better: and it was judged by the physicians in favour of him who had slept.”—Coulton [1] p. 243.
Given the current levels of obesity and the need to better understand processes of appetite control and energy balance, research examining exercise-induced changes in appetite and energy intake has become increasingly pertinent. While it is intuitive that exercise energy expenditure drives the motivation to eat—a view that may deter some individuals from participating in physical activity [2]—this relationship is complex [3–5]. King et al. [6] observed a brief suppression of hunger and a delay in the onset of eating following high-intensity exercise (70 % maximal oxygen consumption [\( \dot{V}{\text{O}}_{2}{\rm max} \)]); an observation termed ‘exercise-induced anorexia’. Other studies have shown similar findings [7–9], and a recent meta-analysis demonstrated that energy intake during subsequent meals is not increased to match the energy expended during exercise [3]. This finding that individuals do not compensate with increased food intake in response to acute exercise [3] has since been confirmed through a systematic review of energy intake responses to acute, short- and long-term exercise [10]. However, various factors such as exercise mode [11], intensity [12] and individual characteristics including training status [3] or hedonic responses [13] to exercise may moderate the relationship between exercise and appetite.
To better understand changes in food intake with exercise, underlying mechanisms have been characterised [9, 13–16], and among them alterations in gut physiology. In particular, gastrointestinal (GI) peptides have been implicated [9, 15, 16]. A recent meta-analysis indicated that acute exercise suppresses acylated ghrelin levels by 16.5 % and increases levels of peptide YY, glucagon-like peptide 1 and pancreatic polypeptide (by 9, 13 and 15 %, respectively [16]. These peptides have a number of roles in appetite regulation, including regulation of gastric motility; regulation of glucose and insulin homeostasis; and control of pancreatic, gallbladder, stomach and intestinal secretions [17–23]. Although these hormonal responses to exercise may be short-lived [16], these data suggest that exercise can alter the release of gut peptides which could influence gastric responses and affect appetite and energy intake.
Given the integrative relationship between gut peptides and gastric emptying (GE, the rate at which solids and/or liquids empty from the stomach) in appetite control [24], GE is an additional mechanism which could mediate exercise-induced alterations in appetite and energy intake. Ghrelin, an orexigenic peptide, acts to accelerate GE [25] and a more rapid GE rate is in turn correlated with lower postprandial ghrelin concentrations [26, 27]. In contrast, peptide YY and glucagon-like peptide 1 act as satiety signals and to inhibit GE [28]. In addition to hormonal influences, mechanical or neural factors such as the relative dominance of parasympathetic or sympathetic tone may contribute to variable GE responses to exercise [29, 30].
GE, along with gastric accommodation (a vagally mediated reflex that results in reduction of tone, and provides a reservoir for the meal) [31], plays a key role in the regulation of appetite [32]. As the stomach fills and subsequently empties into the small intestine, a variety of factors including gastric distension and intestinal exposure of nutrients contribute to satiation and satiety [31, 32]. For a detailed review of GE and appetite control see Janssen et al. [32]. GE is also an important determinant of hydration status and carbohydrate stores during exercise [33, 34]. Furthermore, alterations in GE can contribute to GI upset during exercise [35]. Several comprehensive reviews on this subject have been published [36–39]. As a result, a number of studies have examined the acute effects of exercise on GE. The majority of evidence suggests that GE is delayed during strenuous exercise [40–46] and accelerated [29, 43, 47] or unchanged [48, 49] during mild to moderate exercise. However, to the best of our knowledge, the influence of variables which may moderate the relationship between exercise and GE have yet to be systematically examined. Furthermore, the majority of studies have focused on improving performance in athletes by manipulating the characteristics of the fluid and food. The implications of exercise-induced alterations in GE for appetite control and weight management have not been widely examined.
The objectives of this review were to quantify the acute effects of exercise on GE using meta-analysis and to discuss the relevance of findings in relation to appetite control. A systematic review of the research findings will improve the current understanding of the acute effects of exercise on GE and characterise any moderating factors (including exercise characteristics and meal/fluid properties).
2 Methods
2.1 Study Selection and Assessment Criteria
Major research databases (PubMed, Scopus, Google Scholar, EBSCOhost, SPORTDiscus) were searched independently by two authors (KH and MS) utilising the same search strategy. Keyword searches were performed using ‘gastric emptying’, ‘orocecal transit time’, ‘gastric motility’, ‘mouth-to-cecum transit time’, ‘exercise’, ‘acute exercise’ and ‘physical activity’. All review articles and original papers had their references examined and cross-checked. Abstracts of relevant papers were examined to see if studies met the initial criteria of assessing GE (using one of the following parameters: volume retained, volume emptied, half-time and/or oral-caecal transit time [OCTT]).
Studies were then subjected to further scrutiny. Participants were required to be non-smoking adults, with no history of chronic disease or contraindications to exercise. Selection criteria were not limited by study duration, exercise intensity, exercise modality, or characteristics of the test fluid/meal (i.e., fluid, solid, energy content, carbonation). Studies without a control (resting) condition were excluded. Studies with differing environmental conditions or hydration status had data extracted for rest and thermo-neutral conditions only. Studies evaluating more than one parameter (e.g., simultaneous assessment of half-time and OCTT or half-time and volume emptied) were analysed as separate trials. Studies reporting volume retained were transformed into volume emptied by subtracting the volume retained from the initial bolus ingested.
2.2 Data Synthesis
Once a list of studies was identified and the full texts obtained, studies were assessed independently for quality and risk of bias using a previously developed 16-item checklist [50]. All studies had the following information extracted and recorded separately into a spreadsheet (Microsoft Excel 2010, Microsoft Office): citation information, parameter(s) used to assess GE and their associated values (mean ± standard deviation [SD], mean ± standard error of the mean [SEM], or median and range), exercise characteristics (duration, mode and intensity), beverage/meal characteristics (volume, energy content, time of ingestion, carbohydrate concentration, osmolality and form) and participant information. Studies reporting SEM had their values converted to standard deviation. For studies with non-parametric data reporting median and range, the equations of Hozo and colleagues [51] were used to estimate mean and SD. Aggregation and calculation of final results was conducted by one author (MS).
Exercise intensity was not consistently reported among the studies, so the following scheme was used to classify exercise bouts: low (<40 % \( \dot{V}{\text{O}}_{2} /{\text{maximal heart rate (HR}}_{ \hbox{max} } ) \)), moderate (40–70 % \( \dot{V}{\text{O}}_{2} /{\text{HR}}_{ \hbox{max} } \)) and high (>70 % \( \dot{V}{\text{O}}_{2} /{\text{HR}}_{ \hbox{max} } \)). Information on participant fitness was also not consistently reported, so it was decided to classify participants as untrained, recreationally active, or trained. If a study provided no information (qualitative assessment or \( \dot{V}{\text{O}}_{2} \) max values), the exercise intervention was examined and a subjective determination made by the authors. For intubation studies using the double-sampling technique that reported amount emptied at multiple time points, the final time point only was used in analyses. This was done to be consistent with the majority of studies that aspirated gastric contents at a single time point at the end of exercise. Meals were classified as liquid, solid, or mixed.
2.3 Meta-Analysis Procedures
All extracted data were entered into software designed specifically for meta-analyses (Comprehensive Meta-Analysis, version 2; Biostat, Englewood, NJ). The data entered included sample sizes, mean values for the control and exercise conditions with respective SDs, mean differences between control and exercise trials and inter-trial correlation coefficients. The inter-trial correlation coefficients between control and exercise conditions were calculated as previously described [52]. The median correlation coefficients were 0.63 for volume emptied, 0.511 for half-time and 0.463 for OCTT.
Separate analyses were conducted for the following parameters: volume emptied, OCTT and half-time. This approach was taken due to the varying metrics for each variable, as only variables with common metrics can be assessed in a meta-analysis. The software calculated the standardised difference in means to determine the effect size (ES) and we report it as Hedge’s g for each study to account for potential bias due to the small sample sizes in the reviewed studies. ES was defined as <0.2 as trivial, 0.2–0.3 as small, 0.4–0.8 as moderate and >0.8 as large, as per Cohen [53]. Random effects modelling was used because it accounts for both within and between study variability when estimating effects [54].
Heterogeneity was calculated as Cochrane’s Q and the I 2 index. Values of 25, 50 and 75 % were used for the I 2 analysis, and correspond to low, moderate and high heterogeneity, respectively [55]. For Cochrane’s Q, significant heterogeneity is considered to exist when the Q value exceeds the degrees of freedom (df) of the estimate [56]. Sensitivity analyses were conducted by excluding one study at a time to examine if results were driven by any one study.
Subgroups analyses were conducted using characteristics speculated or known to influence GE (determined a priori), including exercise intensity, liquid/solid meal, timing of energy intake and exercise mode. Meta-regressions were conducted to examine covariates statistically associated with estimated exercise effects on GE for exercise duration, energy content and volume, percent carbohydrate provided by the meal, and osmolality, where applicable.
Publication bias was assessed using funnel plots. Where there is no publication bias, studies are distributed evenly around the mean ES because of random sampling error. The trim-and-fill correction described by Duval and Tweedie [57] was used to assess bias. This technique allows for the computation and inclusion of potentially missing studies to create symmetry about the overall mean ES.
Statistical significance was assumed as p < 0.05 in a Z test analysis. The Z tests were utilised to examine if ES were significantly different from zero.
3 Results
3.1 Overview of Included Studies
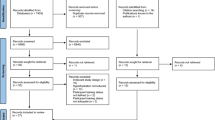
The initial search identified 195 studies. After removing duplicate studies and studies that failed to meet inclusion criteria, 20 publications were included in the analysis (Fig. 1). Two publications were conference proceedings (abstracts; [58, 59]) and the remainder had been published in peer-reviewed journals. The 20 publications (Table 1) involved a total of 221 participants (157 men; 52 women; 12 unknown). Eight publications (12 trials) reported half-time, eight publications (9 trials) reported OCTT and nine publications (19 trials) reported volume emptied (three of which were transformed from volume retained).
3.2 Participant Characteristics and Exercise Intervention Information
Many studies did not adequately report participant characteristics. Twelve studies reported no information on height, weight, body mass index (BMI), or body composition. The remaining eight either reported BMI or provided data that allowed it to be calculated. The median among all studies was 22.2 kg•m−2 (range 22–25.3). Similarly, as discussed in the previous section (Sect. 2.2), 25 % of studies (n = 5) did not report on their participants’ fitness levels, generally using terms like ‘healthy’ or ‘active’. Seven studies provided either a direct statement that participants were trained or values from a \( \dot{V}{\text{O}}_{2} \) max test. Seven of the remaining studies grouped their participants into the ‘recreationally active’ category, and the final study utilised untrained, sedentary individuals.
Eight studies measured GE using intubation techniques, four using scintigraphy, three by 13C breath test and one using ultrasonography (Table 1). Of those that used intubation methods, two studies used a double sampling technique [45, 58] and in the other six studies the stomach was evacuated at a single time point [42, 43, 47, 49, 60, 61]. One study using a double sampling technique reported half-time only [58] and the other reported volume remaining every 10 minutes for 60 minutes [45]. Time of stomach evacuation in intubation studies ranged from 15 to 120 minutes (median = 60 min). Six studies that used intubation techniques also measured gastric secretions [42, 43, 47, 49, 60, 61]. One study did not state whether secretion was measured [58] and one study involving intermittent sprint exercise was unable to estimate secretions [45]. All eight studies that measured OCTT used the breath hydrogen method [29, 62–68].
Ten studies assigned exercise intensity as a percentage of maximal heart rate or oxygen consumption (Table 1). Two studies used percentage of maximal power output. Six used absolute speeds for treadmill walking (3.2–6.2 km/h) or running (13.3 km/h). One study used low-resistance/cadence cycling (33 rpm) and the final study used incremental exercise to 750 kpm/min (125 W). Duration ranged from 15 to 180 minutes (median = 75 min). Most studies utilised continuous exercise, although two utilised intermittent bouts [29, 45].
Ten studies utilised cycling as the mode of exercise [29, 42, 45, 49, 58–60, 65, 66, 68], two utilised running [47, 69], five utilised walking [62–64, 70, 71] and the remaining studies compared modes of exercise (one running/walking [43] and two running/cycling [61, 67]).
Thirteen studies provided participants with a liquid load, four used a solid meal and three involved a mixed meal. Energy content and volume ranged from 0–900 kcal and 40–1220 mL, respectively (medians = 248 kcal and 400 mL). Thirteen studies had participants ingest the meal pre-exercise, four during exercise, one post-exercise and two at all time points (liquid meals pre-/post-exercise and carbohydrate-electrolyte beverages (CHO-E) during exercise).
3.3 Meta-Analysis
Individual study statistics for each meta-analysis are available in the Electronic Supplementary Material [ESM], Tables S1–S3.
3.3.1 Volume Emptied
The meta-analysis revealed a trivial mean effect in favour of accelerated emptying of a fluid bolus ingested during or before exercise (Hedge’s g = 0.195, p = 0.392; 95 % CI −0.25 to 0.64, n = 19; Fig. 2) with a high degree of heterogeneity (Cochrane’s Q = 174.46, df = 18, p < 0.001; I 2 = 89.7 %). Sensitivity analyses showed that removal of individual studies had minimal influence on the mean effect.
Analysis of the moderator variables is displayed in Table 2. A number of grouping factors influenced the volume emptied during exercise. Intensity showed a dose–response effect, such that ES decreased as intensity increased (i.e., less volume emptied). The volume emptied in studies in which fluid was ingested during exercise (n = 5 trials) was significantly less than in studies in which the fluid bolus was ingested pre-exercise (n = 14). Whether the fluid bolus was water or a caloric beverage did not influence GE with exercise. Finally, less volume was emptied during cycling exercise than running and walking. Meta-regression analyses showed inverse relationships between volume ingested and volume emptied with exercise; duration of exercise and osmolality also showed inverse relationships with volume emptied with exercise (Fig. 3a–c).
Assessment of publication bias via inspection of the funnel plot and use of the trim-and-fill correction showed minimal publication bias and a symmetrical distribution about the mean (ESM, Figure S1).
3.3.2 Half-Time
The meta-analysis for half-time revealed a trivial mean effect for accelerated half-time (Hedge’s g = −0.109, p = 0.698; 95 % CI −0.66 to 0.442, n = 12; Fig. 4a). There was significant heterogeneity among these studies (Cochrane’s Q = 116.232, df = 11, p < 0.001; I 2 = 90.5 %). Sensitivity analyses showed that removal of individual studies had minimal influences on the mean effect.
Analysis of the moderator variables is shown in Table 2. There was a significant effect of intensity grouping, such that half-time increased with increased intensity. There was also a significant influence of exercise mode, with walking significantly accelerating half-time compared with running and cycling. Meta-regression of volume and ES (Fig. 5a) showed a positive relationship, such that the larger the ingested volume, the longer the half-time (i.e., slower GE) with exercise. The relationship was stronger when studies providing solid food were excluded (Fig. 5b).
Assessment of publication bias via inspection of the funnel plot and use of the trim-and-fill correction showed minimal publication bias and a symmetrical distribution about the mean (ESM, Figure S2).
3.3.3 OCTT
The final meta-analysis for OCTT revealed a trivial mean effect of increased OCTT (Hedge’s g = 0.089, p = 0.811; 95 % CI −0.638 to 0.816, n = 9; Fig. 4b). Considerable heterogeneity existed among these studies (Cochrane’s Q = 121.95, df = 8, p < 0.001; I 2 = 93.4 %). Sensitivity analysis revealed that a study by Keeling and Martin [62] unduly influenced the results. Its removal increased the ES to moderate values and indicated prolonged OCTT (g = 0.345).
Analysis of the moderator variables is shown in Table 2. As with the prior two analyses, intensity was a significant moderator with increasing intensity increasing OCTT (i.e., slower GE). OCTT during running was significantly slower than walking or cycling (p = 0.028). Finally, when energy was ingested before, during and after exercise, compared with only before exercise, OCTT was significantly slower. None of the meta-regression analyses were significant.
Publication bias was detected for this analysis, with the trim and fill correction suggesting one study was needed to create symmetry around the mean. However, this study would need a large negative ES (g = −1.3) (ESM, Figure S3).
3.4 Study Quality
Utilising the scale developed by van Rosendal and colleagues [50], studies were evaluated for overall quality using a 16-item checklist, by allocating a percentage score calculated by dividing the total number of ‘yes’ items by the number of ‘non-applicable’ items and multiplying by 100. Of the 20 studies, ten were scored as ‘low quality’ (<40 %), six were of ‘moderate quality’ (<60 but ≥40 %) and four were of ‘high quality’ (≥60 %) (ESM, Table S4). When quality was analysed as a moderator variable (Table 2), it was found to be a significant moderator for all three meta-analyses. For volume emptied, low (g = 0.664; n = 5) and high (g = 0.336; n = 10) quality studies indicated increased volume emptied with exercise, while studies of moderate quality did not (g = −0.764; n = 4). As for half-time, low- (g = −0.438; n = 7) and moderate- (g = −0.632; n = 2) quality studies tended to indicate a faster half-time compared with high-quality studies (g = 0.977; n = 3). Finally, for OCTT, high- (g = 1.047; n = 2) quality studies indicated delayed OCTT compared with studies of moderate (g = −0.384; n = 4) and low quality (g = 0.045; n = 3).
4 Discussion
Changes in GE with exercise could have implications for appetite control, postprandial glycaemia, fluid/nutrient availability and GI symptoms. To the best of our knowledge, this is the first meta-analysis of the acute effects of exercise on GE. Collectively, it shows that there are a number of factors that influence GE with acute exercise. Although we observed small/null main effect sizes for GE and OCTT responses to exercise, numerous variables moderated the results in directions expected to attenuate gastric emptying with exercise. These included exercise intensity, mode and duration, and the volume and osmolality of the meal/fluid ingested.
4.1 Exercise Intensity
Exercise intensity significantly impacted on variation in findings. GE and OCTT were slower at higher intensities (>70 % \( \dot{V}{\text{O}}_{2}{\rm max} \)) compared with rest. In contrast, at lower intensities (<40 % \( \dot{V}{\text{O}}_{2} /{\text{HR}}_{ \hbox{max} } \)), a greater volume was emptied indicating a faster GE (Table 2). Although the small number of low-intensity trials included in the present analysis should be acknowledged, the findings are consistent with the majority of the literature to date. Two of the earliest documented studies suggested that mild to moderate exercise accelerates GE, while strenuous exercise inhibits emptying [40, 72]. Since then, a number of studies have examined the effects of exercise on GE at different intensities [29, 40–47]. Neufer and colleagues [43] compared the effects of exercise intensity on GE and reported a slower GE with exercise at >75 % \( \dot{V}{\text{O}}_{2}{\rm max} \) and an accelerated GE with both walking (at 28, 41 and 56 % of \( \dot{V}{\text{O}}_{2}{\rm max} \)) and running (at 57 and 65 % \( \dot{V}{\text{O}}_{2} { \hbox{max} } \)). Furthermore, GE is delayed with high-intensity intermittent exercise [45]. However, there is some disparity in findings regarding moderate-intensity exercise as others have shown GE to be unchanged [49, 68]. The results for the sub-group analysis for moderate exercise studies assessing volume emptied showed accelerated emptying but to a lesser degree than the magnitude observed for low-intensity exercise. Our findings support an inverse relationship between graded exercise intensity and GE [73], such that at lower intensities GE is faster, at moderate intensities the effect is less strong and consistent, and at high exercise intensities GE is slower.
4.2 Exercise Mode
Exercise mode also impacted on inconsistencies between studies. GE was faster with walking compared with other modes (cycling and running). Moore et al. [70] reported that compared with standing, light (3.2 km/h) and moderate (6.4 km/h) walking enhanced GE of a solid meal by 39 and 55 %, respectively. One explanation is that walking-induced increases in GE could be due to the inherently lower intensity associated with walking and a less dominant sympathetic drive. Two studies have directly compared running versus cycle exercise at the same intensity (at 70 % \( \dot{V}{\text{O}}_{2} { \hbox{max} } \) [67] and 75 % \( \dot{V}{\text{O}}_{2} { \hbox{max} } \) [61]) and found no difference in GE between cycling and running [61, 67]. Although for OCTT our findings demonstrate a slower transit time with running, this analysis is limited by the inclusion of only one study in the running subgroup. Slower GE or OCTT could contribute to GI distress, which is evident particularly in endurance running [39, 74, 75]. Overall, the present meta-analysis indicates GE is faster with walking. Further studies directly comparing cycling, running and walking exercise across a range of intensities are required to better understand the relative influence of exercise mode and exercise intensity on GE and OCTT. Furthermore, to the best of our knowledge, the effects of resistance exercise, swimming and other exercise modes on GE have yet to be reported.
4.3 Exercise Duration
The studies included in the present meta-analysis involved a broad range of exercise durations (15–180 min) and meta-regressions revealed an inverse relationship between exercise duration and volume emptied. This would suggest that as exercise duration increased, GE was slower. One potential mechanism could be a reduction in gut blood flow as exercise continues [36, 76]. However, as exercise duration did not influence variation in GE half-time or OCTT and the included studies did not cover a continuum of exercise durations, this finding should be interpreted with caution and the influence of exercise duration on GE requires further study.
4.4 Meal and Beverage Properties
Meal and beverage properties such as meal volume [77], osmolality [78], energy content [79] and carbohydrate concentration [78, 80] are factors known to influence GE. In the present review, meta-regressions indicated that volume ingested and osmolality were associated with variation in findings. As the volume ingested increased, exercise had a greater effect on delaying GE (volume emptied and half-time). This contrasts with findings from within the same experiments which have consistently demonstrated that increasing volume ingested is associated with faster GE during exercise and rest [48, 77, 81]. One possible explanation is the larger volumes (≥600 mL) ingested in five of the studies that were included in our meta-analysis reporting volume emptied [42, 45, 49, 60, 61]. Faster GE has been reported to occur with increasing volume ingested to a threshold level; at volumes ingested above 600 mL, GE begins to slow [48, 82]. In addition, meta-regressions explain variation in effect sizes of GE responses to exercise compared with rest, rather than variation within the same conditions. It could be speculated that a slower GE may only be detected with exercise compared with rest, when the meal/liquid ingested provides a sufficient functional challenge to the gut. Indeed, under resting conditions, variability in GE may go undetected if the test meal does not challenge motility [83]. Similar to volume ingested, meta-regression analysis revealed increased osmolality was associated with a slower GE during exercise. These findings are consistent with the previously well documented effects of slower GE with increased osmolality [48, 78]. Collectively, the present findings suggest that large volumes ingested and/or increased osmolality may increase the inhibitory effect of exercise on GE.
4.5 Mechanisms
GE responses during exercise are likely a result of complex interactions of neural, hormonal and mechanical influences. Mechanisms proposed which could contribute to exercise-induced alterations in GE include changes in contraction frequencies, antral area [84] and gastric myoelectrical activity [85, 86]; hormonal [45, 47, 61] and neural factors (mainly vagal in origin) [29]; alterations in gut blood flow [43]; and the mechanical effects of ‘bouncing of the gut’ [43, 45, 47, 87] during exercise. Splanchnic blood flow is decreased by up to 80 % at an exercise intensity of 70 % of \( \dot{V}{\text{O}}_{2} { \hbox{max} } \) [88]. Limited GI blood flow may result in a diminished availability of oxygen and energy sources [89] and this local hypoxia of the GI tract could in turn result in an enhanced release of some gastroenteropancreatic hormones and subsequently delayed GE [90]. The relative dominance of sympathetic/parasympathetic activity is also influenced by exercise conditions [91]. During moderate intensity exercise, the vagal nerve appears to have a critical role in accelerating GE [30]. In addition, more powerful and stable gastric myoelectrical activity following moderate exercise could contribute to a faster GE [86, 92]. Different mechanisms may therefore have roles of varying relative importance depending on certain aspects of experimental methodology such as the relative exercise intensity.
4.6 Implications for Appetite Control
The role of gastric function in appetite control has been of interest for over a century [93]. GE plays an important role in the regulation of gastric distension and intestinal exposure of nutrients and hence control of satiation and satiety [32]. During resting conditions, GE is associated with appetite and energy intake [94–97]. However, despite early reports that gastric contractions associated with hunger were unaffected by walking but inhibited by running [98], few exercise studies have assessed appetite or energy intake in conjunction with GE or OCTT. Of the twenty studies in our analysis, only five included an assessment of hunger or fullness [58, 60, 61, 68, 71] and none reported energy intake. In one study, GE was accelerated by 12 % with postprandial walking but fullness and satiety were unchanged [71]; in another, fullness ratings were assessed at one time-point only [61], and in the other three of these studies GE was unchanged with exercise [58, 60, 68]. Evans et al. [58] assessed liquid GE and hunger ratings following either 30-min cycle exercise (at high intensity and low intensity) or rest. Although the authors observed no difference in GE between conditions, hunger in the hour after fluid ingestion was strongly and negatively correlated with total stomach volume after exercise. It was concluded that perceived hunger sensations and GI function appear closely linked. Mitchell and Voss [77] have shown similar findings, such that a greater volume retained in the stomach was associated with increased fullness during exercise. However, this study was not included in the present analysis due to a lack of a resting control condition. Therefore, while these studies suggest a link between GE and changes in appetite with exercise, overall, the specific relationship between exercise-induced changes in GE and appetite is inconclusive, mainly due to the lack of appetite and energy intake data reported in the majority of exercise GE studies.
The evidence suggests that exercise intensity moderates GE, leading to speculation that GE could contribute to exercise-induced changes in appetite. We observed a significant effect size towards delayed GE with high-intensity exercise (>70 % \( \dot{V}{\text{O}}_{2}{\rm max} \)) and it has previously been well documented that exercise ≥70 % \( \dot{V}{\text{O}}_{2}{\rm max} \) causes a transient suppression in hunger and does not automatically increase food intake for most individuals [3, 6–9, 99, 100]. As sensations of fullness and gastric distension are closely related [101, 102], delayed GE resulting in greater or prolonged gastric distension could be one mechanism contributing to exercise-induced anorexia with high-intensity exercise. Further study is required to examine whether exercise-induced changes in GE have a role in exercise-induced anorexia. The temporal patterns of changes in GE in relation to appetite, gut peptides and energy intake are also important to consider and are likely to depend on a number of factors such as the timing of measurement and meal ingestion. As only a few studies have examined GE rates after exercise, relationships to post-exercise appetite are speculative.
The potential implications of alterations in GE with exercise for health and weight loss are nevertheless worth considering. For example, it is possible that weight loss strategies could be enhanced by optimally timing exercise bouts relative to meals, such that higher intensity exercise performed in the postprandial period may extend the satiating effects of a meal [99]. On the other hand, evidence of faster GE with walking could have clinical applications for those with problems of postprandial fullness, gastric symptoms or other conditions such as delayed GE associated with diabetes. Such individuals could potentially benefit from low-intensity walking following food intake. Further study is required in this area.
4.7 Limitations, Methodological Considerations and Future Directions
As with all meta-analyses, the quality of the results is dependent on the existing literature and there are a number of limitations which should be considered. A large number of studies had to be excluded because they lacked a control condition or were conducted in extreme ambient conditions. In addition, over 50 % of the included studies were rated as ‘low quality’. While ideally we would have excluded these studies to reduce bias, we felt it was important to retain them due to the limited literature, and the use of subgroup analysis for study quality should have controlled for this. Furthermore, the majority of studies used a liquid bolus of water or a sports drink administered before or during exercise. While these studies have added considerable knowledge to how fluid and nutrient uptake is regulated during exercise, their relationship with GE from an appetite perspective must be considered with caution. Exclusion of conditions with varying temperature, although necessary for our analysis, also limits the generalisability of our results. For example, GE and gut blood flow have both been reported to be attenuated during and after exercise in the heat, likely due to a greater shift of blood flow to the skin to support the body’s thermoregulation [35, 76], and this could be related to attenuations in energy intake after exercise in warm conditions [103].
In interpreting findings, it is important to consider the measurement technique used. As GE is often exponential, the ideal method should reflect the kinetic nature of GE over time. However, six studies included in our analysis assessed gastric contents at a single time point [42, 43, 47, 49, 60, 61], meaning possible changes in the initial emptying phase and total emptying times are unknown [34]. A number of exercise studies have used modified versions of a double sampling technique [104], which allows serial measurements of gastric contents, but only two fit the inclusion criteria for the present analysis [45, 58]. Others were excluded due to insufficient information being reported to allow for meta-analyses (e.g., inconsistent outcome measures, results being presented graphically, no SD) [34, 35, 48, 105, 106]. These studies are important to acknowledge as they contribute greatly to knowledge on various aspects of GE responses to exercise (e.g., dehydration [35]). Although future meta-analyses of emptying curves would be ideal if sufficient information is available, the assessment of both volume emptied and half-time in our analyses should reduce some of the limitations of relying on one outcome measure.
While meta-regressions showed associations between exercise duration, volume and osmolality of the meal with GE effect size, the magnitude of contribution of each remains to be determined. It is also important to note that the sub-group analyses and meta-regressions were conducted to examine the variance between studies and should not necessarily be viewed as predictors of GE. Further, it should be acknowledged that considering mean outcomes from trials is a limitation inherent in meta-analyses. Following both acute and long-term exercise, there is considerable inter-individual variability in biological and behavioural responses [14, 107] and there are a number of individual factors that could influence the effects of exercise on appetite including age, sex, training status, eating behaviour traits and body composition. These characteristics were not provided in sufficient detail to allow sub-group analyses in the present analysis; as such, future studies examining responses to exercise in different populations and characterising individual differences would be of interest.
The influence of different levels of exercise intensity when exercise volume and energy expenditure are matched also require further study. Although evidence from acute exercise studies suggests energy expenditure during exercise is not associated with alterations in energy intake [3], energy expenditure was reported inconsistently in GE studies in the present analysis and therefore the relative influence of energy expenditure, dose and intensity of exercise on GE could not be determined.
Macronutrient content of the meal/beverage could also influence exercise-induced changes in GE. Vist and Maughan [78] demonstrated a slower rate of GE with greater carbohydrate content using the double-sampling technique. Rehrer et al. [34] similarly demonstrated drink composition to be a strong inhibitor of GE during rest and cycling. However, carbohydrate content did not account for variation between study findings in the present analyses. One explanation may be the range of concentrations in the included studies (0–68 %) or that other moderating factors had a greater role. Costill and Saltin [48] demonstrated that when carbohydrate concentration exceeds 8 %, GE is slower. In contrast to carbohydrate, protein and fat content were either not reported or did not vary between the majority of studies in the current analysis. Under resting conditions, GE is slower following a high-fat compared with a low-fat meal [108]. The influence of macronutrient composition on GE, in particular dietary fat with exercise, requires further investigation.
Relationships between GE, gut hormones and appetite offer potential mechanisms contributing to alterations in energy intake with exercise. To date, no study has examined GE, gut blood flow, gut hormones, appetite perceptions and energy intake during or after exercise. Interactions between a reduced gut blood flow [88], alterations in appetite-related peptides [16] and increased gastric distension associated with a slowed GE may, for example, explain the transient suppression of appetite [6–9] that has been observed at higher exercise intensities. Integrative studies examining the temporal pattern of changes in these measures with different exercise conditions would be the capstone of the last three decades of studies on post-exercise appetite regulation, and results (combined with emerging neuroimaging study results) could perhaps support or refute the theories surrounding feeding behaviour post-exercise.
5 Conclusion
The current evidence base suggests that there are a number of factors which moderate exercise-induced changes in GE. GE appears to be accelerated with an acute bout of low-intensity exercise, unchanged or accelerated by moderate-intensity exercise and, at intensities above 70 % \( \dot{V}{\text{O}}_{2}{\rm max} \), GE is delayed. Walking was associated with faster GE compared with cycling and running, whereas increased exercise duration, large volumes ingested and increased osmolality were associated with a greater effect size towards delayed GE with exercise. Exercise-induced changes in GE may provide one mechanistic explanation for changes in appetite observed with exercise, and as such have implications for weight management and health. However, few studies have investigated exercise-induced changes in GE in relation to appetite and food intake and therefore at present the specific relationship is inconclusive. Future integrative studies combining GE, gut hormone, appetite and energy intake measurements are required.
References
Coulton GG. From St Francis to Dante: a translation of all that is of primary interest in the chronicle of the Franciscan Salimbene:1221–1288. London: David Nutt; 1906.
Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr. 1956;4(2):169–75.
Schubert MM, Desbrow B, Sabapathy S, et al. Acute exercise and subsequent energy intake: a meta-analysis. Appetite. 2012;63:92–104.
Hopkins M, King NA, Blundell JE. Acute and long-term effects of exercise on appetite control: Is there any benefit for weight control? Curr Opin Clin Nutr Metab Care. 2010;13(6):635–40.
King NA, Tremblay A, Blundell JE. Effects of exercise on appetite control: implications for energy balance. Med Sci Sports Exerc. 1997;29(8):1076–89.
King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. Eur J Clin Nutr. 1994;48:715–24.
King NA, Blundell JE. High-fat foods overcome the energy expenditure induced by high-intensity cycling or running. Eur J Clin Nutr. 1995;49(2):114–23.
Broom DR, Batterham RL, King JA, et al. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide yy in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R29–35.
Broom DR, Stensel DJ, Bishop NC, et al. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol. 2007;102(6):2165–71.
Donnelly JE, Herrmann SD, Lambourne K, et al. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS One. 2014;9(1):e83498.
Larson-Meyer DE, Palm S, Bansal A et al. Influence of running and walking on hormonal regulators of appetite in women. J Obes. 2012; 15 pages. doi:10.1155/2012/730409. Article ID 730409.
Thompson D, Wolfe L, Eikelboom R. Acute effects of exercise intensity on appetite in young men. Med Sci Sports Exerc. 1988;20(3):222.
Finlayson G, Bryant E, Blundell JE, et al. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol Behav. 2009;97(1):62–7.
Hopkins M, Blundell JE, King NA. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br J Sports Med. 2013. [Epub Ahead of Print]. doi:10.1136/bjsports-2012-091721.
Martins C, Morgan LM, Bloom SR, et al. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol. 2007;193:251–8.
Schubert MM, Sabapathy S, Leveritt M, et al. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014;44(3):387–403.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39.
Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85(2):495–522.
Kojima S, Ueno N, Asakawa A, et al. A role for pancreatic polypeptide in feeding and body weight regulation. Peptides. 2007;28(2):459–63.
Simpson K, Parker J, Plumer J, et al. CCK, PYY and PP: the control of energy balance. Handb Exp Pharmacol. 2012;209:209–30.
Stanley S, Wynne K, McGowan B, et al. Hormonal regulation of food intake. Physiol Rev. 2005;85(4):1131–58.
Wynne K, Stanley S, McGowan B, et al. Appetite control. J Endocrinol. 2005;184(2):291–318.
Delhanty PJ, Neggers SJ, van der Lely AJ. Mechanisms in endocrinology: ghrelin: the differences between acyl- and des-acyl ghrelin. Eur J Endocrinol. 2012;167(5):601–8.
Horner KM, Byrne NM, Cleghorn GJ, et al. The effects of weight loss strategies on gastric emptying and appetite control. Obes Rev. 2011;12(11):935–51.
Levin F, Edholm T, Schmidt PT, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91(9):3296–302.
Heath R, Jones R, Frayn K, et al. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol. 2004;180(2):273–81.
Blom WAM, Lluch A, Vinoy S, et al. Effects of gastric emptying on the postprandial ghrelin response. Am J Physiol Endocrinol Metab. 2006;290(2):389–95.
Camilleri M. Peripheral mechanisms in the control of appetite and related experimental therapies in obesity. Regul Pept. 2009;156(1–3):24–7.
Cammack J, Read NW, Cann PA, et al. Effect of prolonged exercise on the passage of a solid meal through the stomach and small intestine. Gut. 1982;23:957–61.
Wang Y, Kondo T, Suzukamo Y, et al. Vagal nerve regulation is essential for the increase in gastric motility in response to mild exercise. Tohoku J Exp Med. 2010;222(2):155–63.
De Schepper HU, Cremonini F, Chitkara D, et al. Assessment of gastric accommodation: Overview and evaluation of current methods. Neurogastroenterol Motil. 2004;16(3):275–85.
Janssen P, Vanden Berghe P, Verschueren S, et al. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther. 2011;33(8):880–94.
Maughan RJ. Fluid and electrolyte loss and replacement in exercise. J Sports Sci. 1991;9:117–42.
Rehrer NJ, Beckers E, Brouns F, et al. Exercise and training effects on gastric emptying of carbohydrate beverages. Med Sci Sports Exerc. 1989;21:540–9.
Rehrer NJ, Beckers EJ, Brouns F, et al. Effects of dehydration on gastric emptying and gastrointestinal distress while running. Med Sci Sports Exerc. 1990;22:790–5.
Brouns F, Beckers E. Is the gut an athletic organ? Digestion, absorption and exercise. Sports Med. 1993;15:242–57.
Moses F, Ryan C, DeBolt J, et al. Oral cecal transit time during a two hour run with ingestion of water or glucose polymer. Am J Gastroenterol. 1988;83:1055.
Peters HP, De Vries WR. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48(3):435–9.
de Oliveira EP, Burini RC. The impact of physical exercise on the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2009;12(5):533–8.
Hellenbrandt FA, Tepper RH. Studies on the influence of exercise on the digestive work of the stomach. Its effect on emptying time. Am J Physiol. 1934;107:355–63.
Fordtran JS, Saltin B. Gastric emptying and intestinal absorption during prolonged severe exercise. J Appl Physiol Respir Environ Exerc Physiol. 1967;23(3):331–5.
Ramsbottom SJ, Hunt JN. Effect of exercise on gastric emptying and gastric secretion. Digestion. 1974;10:1–8.
Neufer PD, Young AJ, Sawka MN. Gastric emptying during walking and running: effects of varied exercise intensity. Eur J Appl Physiol Occup Physiol. 1989;58:440–5.
Marzio L, Formica P, Fabiani F, et al. Influence of physical activity on gastric emptying of liquids in normal human subjects. Am J Gastroenterol. 1991;86(10):1433–6.
Leiper JB, Broad NP, Maughan RJ. Effect of intermittent high-intensity exercise on gastric emptying in man. Med Sci Sports Exerc. 2001;33(8):1270–8.
Leiper JB, Nicholas CW, Ali A, et al. The effect of intermittent high-intensity running on gastric emptying of fluids in man. Med Sci Sports Exerc. 2005;37(2):240–7.
Neufer PD, Costill DL, Fink WJ, et al. Effects of exercise and carbohydrate composition on gastric emptying. Med Sci Sports Exerc. 1986;18(6):658–62.
Costill DL, Saltin B. Factors limiting gastric emptying during rest and exercise. J Appl Physiol. 1974;37:679–83.
Feldman M, Nixon JV. Effect of exercise on postprandial gastric secretion and emptying in humans. J Appl Physiol. 1982;53:851–4.
van Rosendal SP, Osborne MA, Fassett RG, et al. Guidelines for glycerol use in hyperhydration and rehydration associated with exercise. Sports Med. 2010;40(2):113–29.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Elbourne DR, Altman DG, Higgins JP, et al. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–9.
Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9.
Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Syn Meth. 2010;1(2):97–111.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or i2 index? Psychol Methods. 2006;11(2):193–206.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Evans GH, Shirreffs SM, Watson P, et al. Gastric emptying rate and perceived hunger after rest and exercise in man. Br J Sports Med. 2010;44(14):i20–1.
Laurens M-H, Thouvenot P, Antoine J-M, et al. The effects of physical effort on gastric emptying. Am J Clin Nutr. 1994;59(3):778S.
Mitchell J, Costill D, Houmard J, et al. Gastric emptying: Influence of prolonged exercise and carbohydrate concentration. Med Sci Sports Exerc. 1989;21(3):269–74.
Houmard JA, Egan PC, Johns RA, et al. Gastric emptying during 1 h of cycling and running at 75 % VO2max. Med Sci Sports Exerc. 1991;23(3):320–5.
Keeling WF, Martin BJ. Gastrointestinal transit during mild exercise. J Appl Physiol Respir Environ Exerc Physiol. 1987;63(3):978–81.
Meshkinpour H, Kemp C, Fairshter R. Effect of aerobic exercise on mouth-to-cecum transit time. Gastroenterology. 1989;96(3):938–41.
Keeling WF, Harris A, Martin BJ. Orocecal transit during mild exercise in women. J Appl Physiol. 1990;68:1350–3.
Soffer EE, Summers RW, Gisolfi C. Effect of exercise on intestinal motility and transit in trained athletes. Am J Physiol. 1991;260:698–702.
van Nieuwenhoven MA, Brouns F, Brummer RJ. The effect of physical exercise on parameters of gastrointestinal function. Neurogastroenterol Motil. 1999;11(6):431–9.
Van Nieuwenhoven MA, Brouns F, Brummer RJ. Gastrointestinal profile of symptomatic athletes at rest and during physical exercise. Eur J Appl Physiol. 2004;91(4):429–34.
Clegg M, McClean C, Davison GW, et al. Exercise and postprandial lipaemia: effects on peripheral vascular function, oxidative stress and gastrointestinal transit. Lipids Health Dis. 2007;6(30):1–8.
Carrio I, Estorch M, Serra-Grima R, et al. Gastric emptying in marathon runners. Gut. 1989;30:152–5.
Moore JG, Datz FL, Christian PE. Exercise increases solid meal gastric emptying rates in men. Dig Dis Sci. 1990;35:428–32.
Franke A, Harder H, Orth AK, et al. Postprandial walking but not consumption of alcoholic digestifs or espresso accelerates gastric emptying in healthy volunteers. J Gastrointest Liver Dis. 2008;17(1):27–31.
Campbell JMH, Mitchell MB, Powell ATW. The influence of exercise on digestion. Guys Hosp Rep. 1928;78:279–93.
Rehrer NJ, McLaughlin J, Wasse LK. Importance of gastrointestinal function to athletic performance and health. In: Maughan RJ, editor. The encyclopaedia of sports medicine: an IOC medical commission publication, volume xix, sports nutrition. Chichester: Wiley; 2014. p. 526–38.
Jeukendrup AE, Vet-Joop K, Sturk A, et al. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin Sci. 2000;98(1):47–55.
Riddoch C, Trinick T. Gastrointestinal disturbances in marathon runners. Br J Sports Med. 1988;22(2):71–4.
Rowell LB, Blackmon JR, Bruce RA. Indocyanine green clearance and estimated hepatic blood flow during mild to maximal exercise in upright man. J Clin Invest. 1964;43(8):1677–90.
Mitchell JB, Voss KW. The influence of volume on gastric emptying and fluid balance during prolonged exercise. Med Sci Sports Exerc. 1991;23(3):314–9.
Vist GE, Maughan RJ. The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J Physiol. 1995;486(Pt 2):523–31.
Velchik MG, Reynolds JC, Alavi A. The effect of meal energy content on gastric emptying. J Nucl Med. 1989;30(6):1106–10.
Vist GE, Maughan RJ. Gastric emptying of ingested solutions in man: effect of beverage glucose concentration. Med Sci Sports Exerc. 1994;26(10):1269–73.
Noakes TD, Rehrer NJ, Maughan RJ. The importance of volume in regulating gastric emptying. Med Sci Sports Exerc. 1991;23(3):307–13.
Brouns F, Saris WHM, Rehrer NJ. Abdominal complaints and gastrointestinal function during long-lasting exercise. Int J Sports Med. 1987;8:175–89.
Chey WD, Shapiro B, Zawadski A, et al. Gastric emptying characteristics of a novel c-13-octanoate-labeled muffin meal. J Clin Gastroenterol. 2001;32(5):394–9.
Brown BP, Ketelaar MA, Schulze-Delrieu K, et al. Strenous exercise decrease motility and cross-sectional area of human gastric antrum. Dig Dis Sci. 1994;39(5):940–5.
Lu CL, Shidler N, Chen JD. Enhanced postprandial gastric myoelectrical activity after moderate-intensity exercise. Am J Gastroenterol. 2000;95(2):425–31.
Kato M, Sakai T, Yabe K, et al. Gastric myoelectrical activity increases after moderate-intensity exercise with no meals under suppressed vagal nerve activity. Jpn J Physiol. 2004;54(3):221–8.
Rehrer NJ, Meijer GA. Biomechanical vibration of the abdominal region during running and bicycling. J Sports Med Phys Fitn. 1991;31:231–4.
Qamar M, Read A. Effects of exercise on mesenteric blood flow in man. Gut. 1987;28(5):583–7.
Moses FM. The effects of exercise on the gastrointestinal tract. Sports Med. 1990;9:159–72.
Brouns F. Etiology of gastrointestinal disturbances during endurance events. Scand J Med Sci Sports. 1991;1:66–77.
Robinson BF, Epstein SE, Beiser GD, et al. Control of heart rate by the autonomic nervous system: studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res. 1966;19(2):400–11.
Lu C-L, Shidler N, Chen JDZ. Enhanced postprandial gastric myoelectrical activity after moderate-intensity exercise. Am J Gastroenterol. 2000;95(2):425–31.
Park M-I, Camilleri M. Gastric motor and sensory functions in obesity. Obesity. 2005;13(3):491–500.
Sepple CP, Read NW. Gastrointestinal correlates of the development of hunger in man. Appetite. 1989;13(3):183–91.
Bergmann JF, Chassany O, Petit A, et al. Correlation between echographic gastric emptying and appetite: influence of psyllium. Gut. 1992;33(8):1042–3.
Nair NS, Brennan IM, Little TJ, et al. Reproducibility of energy intake, gastric emptying, blood glucose, plasma insulin and cholecystokinin responses in healthy young males. Br J Nutr. 2009;101(7):1094–102.
Delgado-Aros S, Camilleri M, Cremonini F, et al. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127(6):1685–94.
Carlson AJ. The control of hunger in health and disease. Chicago University Press; 1916.
Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab. 2010;57(2):36–42.
Martins C, Morgan L, Truby H. A review of the effects of exercise on appetite regulation: an obesity perspective. Int J Obes. 2008;32(9):1337–47.
Jones KL, Doran SM, Hveem K, et al. Relation between postprandial satiation and antral area in normal subjects. Am J Clin Nutr. 1997;66(1):127–32.
Santangelo A, Peracchi M, Conte D, et al. Physical state of meal affects gastric emptying, cholecystokinin release and satiety. Br J Nutr. 1998;80(6):521–7.
Shorten AL, Wallman KE, Guelfi KJ. Acute effect of environmental temperature during exercise on subsequent energy intake in active men. Am J Clin Nutr. 2009;90(5):1215–21.
George JD. New clinical method for measuring the rate of gastric emptying: the double sampling test meal. Gut. 1968;9(2):237–42.
Rehrer N, Brouns F, Beckers E, et al. Gastric emptying with repeated drinking during running and bicycling. Int J Sports Med. 1990;11(03):238–43.
Sole CC, Noakes TD. Faster gastric emptying for glucose-polymer and fructose solutions than for glucose in humans. Eur J Appl Physiol Occup Physiol. 1989;58(6):605–12.
King NA, Hopkins M, Caudwell P, et al. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes. 2008;32(1):177–84.
Stacher G, Granser GV, Bergmann H, et al. Slow gastric emptying induced by high fat content of meal accelerated by cisapride administered rectally. Dig Dis Sci. 1991;36(9):1259–65.
Acknowledgments
No sources of funding were used in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this review. KH and MS conceived the study, conducted the literature review, extracted and reviewed the data, prepared the tables and wrote the initial manuscript. MS performed the meta-analysis and prepared the figures. All authors reviewed and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. M. Horner and M. M. Schubert contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horner, K.M., Schubert, M.M., Desbrow, B. et al. Acute Exercise and Gastric Emptying: A Meta-Analysis and Implications for Appetite Control. Sports Med 45, 659–678 (2015). https://doi.org/10.1007/s40279-014-0285-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-014-0285-4