Abstract
Introduction
Peripheral neuropathies (PNPs) encompass a large group of disorders of heterogeneous origin which can manifest themselves with sensory and/or motor deficits depending on the predominantly affected nerve fiber modality. It represents a highly prevalent disease group which can be associated with significant disability and poor recovery. Exercise has the potential to improve side effects of PNP.
Objective
Our objective in this systematic review was to analyze exercise interventions for neuropathic patients in order to evaluate the possible benefits of exercise.
Methods
Three independent reviewers used PubMed, MEDPILOT® (MEDLINE), Cochrane, and relevant reference lists to obtain the data. Relevant studies were graded according to the Oxford Levels of Evidence.
Results
Eighteen studies (ten randomized controlled trials and eight controlled clinical trials) met all inclusion criteria. Three (diabetic) studies were ranked very high quality [1b (A)], nine high quality (four diabetes, one cancer, four others) [2b (B)], while six (four diabetes, two others) showed low quality (4/C). Current data suggests that exercise is a feasible, safe, and promising supportive measure for neuropathic patients. This is best documented for patients with diabetic peripheral neuropathy (DPN), suggesting that endurance training has the potential to prevent the onset of and reduce the progression of DPN. In general, balance exercises showed the highest effect on the motor as well as sensory symptoms in all types of PNP.
Conclusion
Overall, balance training appears to be the most effective exercise intervention. Studies focusing exclusively on strength, or a combination of endurance and strength, appear to have a lower impact. For metabolically-induced neuropathies, endurance training also plays an important role. Further research with high methodological quality needs to be conducted in order to establish evidence-based clinical recommendations for neuropathic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Peripheral neuropathy (PNP) represents a group of diseases which affect motor, sensory and/or autonomous peripheral nerves. PNP can be subdivided by its etiology or by pathological features such as predominantly affected fiber modality. They can be further classified on the basis of primarily myelin or axonal damage resulting in demyelinating or axonal PNP. Furthermore, PNP is a highly prevalent disease; worldwide, approximately 168 million people are affected [1]. At the age of 55 years, around 5–8 % of all people suffer from symptomatic peripheral neuropathy, whereas in the age group above 65 years, almost one-third are estimated to have sensory symptoms attributed to peripheral neuropathy [1, 2]. Common symptoms include pain, altered sensation (numbness, burning, tingling, etc.), reduced or absent reflexes, muscle weakness, reduced balance control, insecure gait, and higher risk of falling [3, 4]. All of these symptoms can affect activities of daily living and subsequently reduce a patient’s quality of life [5].
PNP can develop genetically or be acquired. In approximately one-third of patients, PNP is caused by diabetes, another third results from a variety of factors such as medication (e.g. chemotherapeutic agents), genetics, autoimmune disorders, infections, nutritional deficiencies, and metabolic imbalance, whereas the remaining patients are termed idiopathic (cause unknown) [6]. PNP does not only have a severe impact on the activities of daily living, reducing patients’ quality of life, but for some patients it can even influence their survival [6, 7].
For patients with diabetic peripheral neuropathy (DPN), who represent the largest group (50 % of all diabetic patients; 110 million people) [8], small and large nerve fibers are damaged to different degrees, causing foot ulcers and non-traumatic foot amputation [9].
In cancer patients, PNP is the most common [10] neurological and clinically relevant side effect. Peripheral neuropathy can occur as a paraneoplastic manifestation, but, much more frequently, PNP is induced by neurotoxic chemotherapeutic agents (platinum derivatives, vinca alkaloids, and taxanes, as well as bortezomib, thalidomide, and epothilones) [6, 11–13]. Not only do patients have to deal with the debilitating side effects that these drugs induce, but chemotherapy-induced peripheral neuropathy (CIPN) has become a decisive limiting factor for therapy, causing treatment delays, dose reductions, or even discontinuation of therapy, which can affect the outcome and compromise survival [6]. Therefore, the occurrence of PNP presents a diagnostic dilemma because, up to now, approved and effective treatment options are lacking [6, 13].
Even though PNP causes so many symptoms that may even lead to life-threatening consequences, little research has been done to investigate the potentially beneficial effects of specific exercises to counteract the described symptoms. Research has focused on pharmacological therapies aimed at reducing PNP or treat selected side effects [10, 14, 15]. While this has been helpful for neuropathic pain, it does not address the many other sensory and motor side effects of PNP [12, 14, 16, 17]. To the contrary, many of these agents have been shown to have additional negative side effects [13].
Previous studies have shown that exercise can attenuate motor deficits induced by PNP. Apart from the obvious effect of strength training preventing muscle loss, it also improves inter- and intramuscular coordination as well as neural control, contributing to improved stability and gait [18, 19]. Endurance training improves cardiovascular fitness but also has an influence on metabolic factors such as glycemic control, insulin sensitivity, lipid abnormalities, and hypertension [20, 21], and therefore may also be able to improve related neuromuscular parameters [22].
Alternative interventions such as sensorimotor training (SMT), whole-body vibration (WBV) or Tai Chi for instance, have not received much attention so far but have considerable potential as they not only target motor components but simultaneously address small and large sensory nerve fibers [23–25].
Studies in healthy adults for instance, have revealed that SMT can induce supraspinal reorganization [26], regeneration of neuromuscular structures after injuries [27], reduction in reflex excitability [28], and diminish the prevalence of injuries [29], leading to improved proprioception [26], balance control, causing fewer falls [30], and increasing mobility. Similar effects have been shown with WBV. Kawanabe et al. [24] and Bogaerts et al. [31], for instance, showed that elderly people improve their gait after vibration exercises. Rittweger et al. [32] and Kirchner [33], found WBV to have a positive impact on pain reduction, while further studies showed an effect on deconditioned skeletal muscle [34], improved isometric strength [32, 35, 36], postural sway[37], and reduced fall frequency [31]. Tai Chi, a traditional Chinese martial art, improves balance [38], gait, reducing the risk of falling [39], inducing muscle strength [38], stabilization of the joints, flexibility [40], stamina, and coordination [41–45].
Nevertheless, the translation of those results to patients with neuropathic conditions is scarce. To date, treatment is predominantly symptom-orientated with little consensus regarding the benefits of the various exercises. Consequently, patients are uninformed as to how much exercise is advisable or if they should exercise at all during acute neuropathy.
Only in the last 3 years has the potential of exercise as a measure of supportive therapy gained more attention, for the first time enabling a systematic review based on sufficient evidence to derive preliminary recommendations.
This systematic review has the aim of analyzing all exercise interventions performed with neuropathic patients in order to critically review the exercises chosen and the influence they may have on the motor and sensory side effects of PNP. The intention is to improve future research and generate recommendations as to which exercises may be beneficial for which side effects of PNP, in order to better support neuropathic patients as well as the therapists guiding them, and improve their quality of life.
2 Methods
2.1 Literature Search
Three reviewers (FS, KM, and JR) independently searched the literature (April 2013–December 2013) using PubMed, MEDPILOT® (MEDLINE), and the Cochrane Database in order to find exercise intervention studies for patients with peripheral neuropathy. Additionally, relevant reference lists were hand-searched. We used the terms ‘peripheral neuropathy’, ‘PNP’, ‘CIPN’, ‘chemotherapy induced peripheral neuropathy’, and ‘diabetic neuropathy’, and combined these by using AND with the terms ‘physical activity’, ‘physical exercise’, ‘physical fitness’, ‘exercise’, ‘exercise program’, ‘exercise intervention’, ‘moving therapy’, ‘sports therapy’, ‘sport’, ‘endurance’, ‘aerobic training’, ‘resistance training’, ‘strength training’, ‘strength’, ‘balance’, ‘balance training’, ‘balance exercise’, ‘coordination’, ‘coordination exercises’, ‘gait’, ‘postural stability’, ‘postural control’, and ‘proprioception’. The German equivalents of all terms were also searched for.
To be included in the review, studies had to have examined the effect of an exercise intervention in patients with PNP, independent of the derivation. Animal studies, expert opinions without critical appraisal, or studies with less than ten patients, no control group, or combining exercise and nutrition, therapeutic footwear, medication for PNP, etc., therefore not enabling a clear interpretation of the results, were excluded. Reviews were excluded from analysis, yet analyzed for additional, possibly relevant, literature. Full-text articles of the studies meeting the inclusion criteria were then critically reviewed and graded according to the Oxford levels of evidence (see Table 1) by two authors (FS and FB) and, in case of doubt, by a third author (EZ), leading to grades of recommendation. This evaluation system by the Oxford Center for Evidence Based Medicine (OCEBM) was ranked most effective in a comparison by Atkins et al. in 2004 [46] and has been used for reviews in a similar context [47–50]. The evaluation is based on the study design, quality of the study, and its results, creating ten gradations of quality, which are then translated into four grades of recommendation (A = 1a, 1b; B = 2a, 2b, 3a, 3b; C = 4a, 4b; D = 5a, 5b) (see Table 1). Only high-quality studies [Level 1(A) and Level 2(B)] were considered to derive recommendations. Studies were abstracted for the tables to include the amount of study participants (N), type of exercise, duration, and frequency for which the exercise was performed, as well as the main outcome measures. Results given are based on intergroup comparison unless stated otherwise.
3 Results
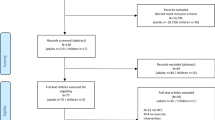
We screened 8,701 search results in PubMed, as well as 959 in MEDPILOT® (MEDLINE and Cochrane) and 177 in relevant reference lists. After careful reviewing, the total number of studies meeting the inclusion criteria for this review was 18 studies: ten randomized controlled trials (RCT) and eight controlled clinical trials (CCT) (see Fig. 1) evaluated the effects of an exercise intervention on the side effects of PNP, assessing a total of 841 patients.
Eleven studies assessed patients with diabetes-induced neuropathies, one study considered CIPN, while six studies dealt with PNP of other derivation such as liver-transplanted familial amyloid polyneuropathy (FAP), sensory neuron disease, hereditary sensorimotor neuropathy (HMSN) (Charcot Marie Tooth disease 1 + 2), chronic acquired PNP, toxic neuropathy, or antimyelin-associated glycoprotein. No studies were found for any other causes of PNP such as HIV or alcohol (see Table 2).
Critical grading of the 18 studies revealed 12 high-quality (Level 1 and 2) studies (seven diabetic PNP, 1 CIPN, four other) and six of poor quality (Level 4) (four diabetic PNP, two others) (see Table 3).
3.1 Diabetic Peripheral Neuropathy
Eleven studies (seven RCT and four CCT), assessing 576 diabetic adults, were evaluated regarding the side effects of PNP. Three studies were graded 1b (A), four 2b (B) and four 4 (C) (see Table 4).
Five studies (Lee et al. [4], Akbari et al. [54], Song et al. [55], Allet et al. [56], and Richardson et al. [59]) assessed the influence of balance training on the side effects of PNP, showing a significant impact on balance control. Two studies (Song et al. [55] and Allet et al. [56]) also showed improved gait parameters, while Lee et al. [4] compared two interventions—WBV and a combination of WBV with balance exercises—with a control group. A further two studies (Ahn and Song [25] and Hung et al. [57]) chose Tai Chi as the intervention and showed improved motor, sensory, and metabolic symptoms of PNP. Kruse et al. [58] and Mueller et al. [53] chose a combination of endurance, balance, and strengthening exercises. Both groups performed progressive balance, flexibility, strengthening and aerobic exercises, although one group conducted the exercises standing or walking [weight-bearing group (WB; N = 15)], while the other group [non-WB group (NWB; N = 14)] was sitting or lying. Positive effects on motor performance could only be detected if exercises were performed standing or walking. Kruse et al. [58] instructed patients in leg strengthening and balance exercises as well as a graduated, self-monitored walking program for eight sessions, and then monitored patients while they continued home-based for 12 months. No significant intergroup differences were found.
The only existing preventive study was conducted by Balducci et al. [22], evaluating 78 diabetic patients over 4 years of endurance training (brisk walking on a treadmill at 50–85 % heart rate reserve). Intergroup comparison with the control group revealed significant sensory improvements.
No adverse events were reported by Dixit et al. [52], Ahn and Song [25], Kruse et al. [58], and Balducci et al. [22]. Mueller [53] reported that one patient sustained a calf strain during treadmill walking but was able to continue to exercise with lower intensity. Allet et al. [56] declared two patients had developed pain in their Achilles tendon, making it necessary to slow down the progression for ‘toe walking’ and ‘one leg stance’ exercises. The remaining five studies (Lee et al. [4], Akbari et al. [54], Song et al. [55], Hung et al. [57] and Richardson et al. [59]) did not indicate adverse events.
3.2 Chemotherapy-Induced Peripheral Neuropathy
So far, only one RCT, graded 2b (see Table 5), has assessed the effects of exercise intervention in patients suffering from CIPN. Streckmann et al. [60] was the first to show beneficial effects of exercise (sensorimotor, endurance, and resistance training) on motor as well as sensory side effects of CIPN in cancer patients (lymphoma). The number of patients with reduced deep sensitivity could be diminished significantly in the intervention group by 87.5 %, while no changes (0 %) were observed in the control group. Furthermore, patient’s quality of life as well as their level of activity were also improved significantly. No adverse events occurred.
3.3 Neuropathy of Other Derivation
Six studies (two RCT and four CCT) investigated 204 adults with neuropathies due to various causes [61–66]. Grading revealed four 2b (B) studies and two 4a (C) studies (see Table 6).
Apart from two studies that focused on liver-transplanted FAP (Tomás et al. [62]) and HMSN (Matjacic and Zupan [61]), all other studies evaluated a heterogeneous collective.
Only three studies were able to achieve improvements through the exercise regime chosen. Tomás et al. [62] and Nardone et al. [64] were able to improve balance and gait parameters, while Graham et al. [63] showed improved knee extensors and total work load. Tomás et al. [62] chose a combination of endurance, resistance, and balance exercises. Intergroup differences in favor of the exercising groups were shown for their walking capacity. Nardone et al. [64] compared neuropathic patients with patients with vestibular disorder. Both groups performed ten sessions of balance exercises on a powered platform, as well as Cawthorne–Cooksey and Frenkel exercises. Due to a crossover design, both groups received the same exercises, only in a different order. Regardless of the treatment order, both groups were able to improve their balance.
The other three studies (Matjacic and Zupan [61], Ruhland and Shields [65], and Lindeman et al. [66]) did not detect any significant intergroup results. The earliest study by Lindeman et al. [66] in 1995 investigated the effects of strength training. In 1997, Ruhland and Shields [65] also assessed the effects of strength training but combined it with endurance and stretching exercises. Patients were advised to train daily for 6 weeks (home-based). Matjacic and Zupan [61] combined strength training with passive stretching and dynamic balance exercises. Both groups performed the same exercises. They solely differed in dynamic balance training; the control group was managed by a physiotherapist, while the intervention group performed the exercises on a balance trainer.
Graham et al. [63] did not report any adverse events. All other authors (Tomás et al. [62], Nardone et al. [64], Matjacic and Zupan [61], Ruhland and Shields [65], and Lindeman et al. [66]) did not indicate adverse events.
4 Discussion
Although PNP is a highly prevalent and debilitating disease, affecting 168 million people worldwide [1], predominantly expert opinions and poor-quality studies have dominated the research field, hinting at the potential of exercise interventions for patients with PNP. It is only in the last 3 years that more and more high-quality studies have confirmed this presumption. Consequently, previous evidence has been insufficient to generate a systematic review until now. The only other existing review from 2010 [52] merely found one study that met the inclusion criteria.
Summarizing, one can say that the evidence for exercise interventions in neuropathic patients has improved, although study quality is diverse. Overall, the quality of the 18 included studies is 2b. Evidence is best in patients with diabetes and neuropathy, revealing most RCTs and therefore the highest quality in the field of neuropathic patients. With only one study on CIPN to date, results are promising but evidence is low. This also applies to the studies on the many other causes of PNP. Diseases such as HMSN or FAP, for instance, are also only represented in one study, while the many other causes of PNP are either merely represented with very few individuals in a heterogeneous patient group or not at all (see Table 2).
The current data suggests that exercise is feasible, safe, and beneficial (see Table 2) for patients with PNP. Overall, exercise-compliance was good and only two studies, both in diabetic patients, reported mild adverse events (Mueller et al. [53] and Allet et al. [56]), due to which patients had to modify their training schedule temporarily on account of pain in the Achilles tendon or the calf.
Currently, there is little evidence for a beneficial effect of supportive therapies such as vitamin E or high-dose vitamin B [67], electrolyte infusions (Ca/Mg), or electrotherapy in patients with PNP. Even neuroprotective treatments such as amifostine, nerve growth factors, or corticosteroids are not well evaluated or failed to demonstrate beneficial effects in clinical trials [13, 68, 69]. Specific treatment for nerve damage is currently not available [70] and the efficacy of available pharmaceutical interventions is limited. In DPN, for instance, 90 % of patients require two or more medications, and despite high prescription compliance, only 27 % respond to those pharmaceutical treatments [71–73]. There is no consensus regarding the treatment of PNP. To the contrary, most medication exerts additional side effects [10, 74]. Oncological patients with CIPN, for instance, were asked to report on the effect of supportive measures during rehabilitation. Patients reported that walking through granulated material as well as balance and gait exercises were most effective [75]. Therefore, exercise is currently a promising option in supportive therapy, which should be taken more seriously.
In general, the patient cohorts were quite heterogeneous with regard to symptoms and underlying cause. Therefore, future intervention studies should consider this shortcoming in study design. Groups should at least consist of patients with similar symptoms, not mixing diverse mechanisms or patients with symptoms only in the hands or face, for instance, with patients experiencing numbness in their feet, as most assessments performed are consequently biased.
Most studies reported on side effects caused by dysfunction of motor nerve fibers. All studies showing an additional impact on the sensory symptom balance control chose balance exercises as the intervention method, revealing improved parameters of balance control such as decreased sway paths, improved unipedal stance, less failed attempts and trunk repositioning errors, faster reaction time, better performance-orientated mobility, improved static and dynamic balance, and reduced concern regarding falling [4, 54–56, 60, 64]. Apart from specific gait training, balance exercises were also able to improve gait parameters such as gait speed, walking distance in the six- and ten-meter walk, and improved timed up-and-go. Lee et al. [4] showed additive effects of balance training when combined with WBV. Two studies by Ahn and Song [25] and Hung et al. [57] suggest that Tai Chi also targets balance control due to the high demand on balance control during the monopedal stances and weight-shifting movements.
A combination of strength and endurance training, not including any balance indices, was performed in two CCT studies (Graham et al. [63] and Tomás et al. [62]). They revealed improvements on the knee extensors and total work load as well as walking capacity. Lindeman et al. [66] detected significant improvements for knee torques in the HMSN group. These three studies only achieved improvements regarding muscle atrophy in general though not for specific PNP-related symptoms.
Interestingly, studies assessing either a combination of strength and endurance training, or strength training alone [Kruse et al. [58] (RCT), Matjacic and Zupan [61] (RCT), and Ruhland and Shields [65] (CCT)] did not detect any significant intergroup differences.
Only three RCTs (Dixit et al. [52], Streckmann et al. [60], and Balducci et al. [22]) demonstrated improvement on small and large sensory nerve fiber function. A combination of endurance, strength, and SMT revealed improved peripheral deep sensitivity in cancer patients (lymphoma) (Streckmann et al. [60]).
Balducci et al. [22] found that long-term, supervised endurance training was able to prevent the onset of PNP in diabetics, while Dixit et al. [52] achieved positive effects with moderate intensity (40–60 % heart rate reserve) aerobic exercise on the progression of DPN.
The underlying mechanisms for the beneficial effects of exercise on PNP have not yet been fully understood. Explanations may include positive modulation of regenerative mechanisms such as altered expression of growth factors, induction of remyelination, or accelerating axonal regeneration [76, 77]. Recently, it has been demonstrated that treadmill exercise has the potential to improve the regeneration of transected nerves by altered expression of neurotrophic growth factors such as nerve growth factor [78].
However, we will presumably have to address two different mechanisms of PNP in order to best target the symptoms. When analyzing the current data, it is noticeable that studies showing the effects of endurance exercises on sensory symptoms of PNP target DPN, which is metabolically induced, whereas the other types of PNP better respond to balance training. Exercise recommendations will probably have to differ, whether we desire to primarily target metabolically-induced PNP, such as DPN, or whether we have to target nerve cells damaged by toxins directly, as in CIPN.
In metabolically-induced PNP, exercise, especially endurance training, can induce glycemic control and reduce body weight. DPN, for instance, is attributed, amongst other mechanisms, to prolonged hyperglycemia, causing up to fourfold higher neuronal glucose levels [79] and additionally initiating an accumulation of sorbitol. Glucose and sorbitol in such concentrations disturb the homeostasis and cause neuronal damage [52]. Additionally, sorbitol requires a higher amount of antioxidants in order to detoxify, thereby contributing to enhanced oxidative stress, which leads to neuronal cell damage. Previous studies have shown that aerobic exercise has the potential to reduce the glucose level, therefore modulating the polyol-sorbitol pathway and increasing antioxidative capacity, consequently preventing and restoring neuronal damage [52, 80]. Recent studies have also revealed that neurons can alternatively use lactate as a substitute for glucose and therefore reduce the level of neuronal glucose and oxidative stress [81]. Endurance exercises, inducing a steady state of lactate and additionally removing surplus glucose, may therefore enhance the use of this alternative metabolic pathway and contribute to regulation of the glucose level. Consequently, the intensity and duration will also play a substantial role as a certain lactate level (presumably ≥2 mmol/l, in order to create the required gradient as the brain holds a lactate state of 1.9 mmol/l [82]) will have to be sustained. Therefore, the type of endurance exercise is probably secondary to the intensity necessary for each individual to obtain an effective lactate state. Furthermore, exercise also increases the blood flow through distal muscle groups, increasing oxygenation to the peripheral tissue.
Dixit et al. [52] even detected an influence of endurance exercise on the amount of oral hypoglycaemic agent (OHA) and insulin necessary. Further studies comparing this observation with exercise interventions would be highly desirable.
Whereas for non-metabolically-induced PNP, specific balance training such as SMT or whole-body vibration will probably play a more crucial role as they have the potential to induce neural adaptations [26]. The underlying mechanisms must also still be elucidated. Although one possibility could lie in the regenerative effect of SMT on nerve fibers [83]. A further possibility is attributed to the nervous system’s plasticity: (i) an increase in the density of receptors; (ii) activating deafferented neurons [83] by increasing metabolism; (iii) lowering the threshold for excitability [84]; or (iv) inducing supraspinal learning effects (Taube 2008).
Especially regarding the small and large sensory nerve fibers, the intensity, frequency, and choice of exercises seem to be crucial. Presumably, not every type of balance training will be able to induce sensory changes.
As previous studies on SMT in healthy adults have shown, neural stimulation is only achieved if exercises are performed within a range of 20–40 s, not exceeding five exercises, and allowing for sufficient regeneration time between exercises in order to prevent neural fatigue [23, 26].
The indication ‘balance training’ is very diverse and can include many different variations, targeting different effects. For this reason, studies should specify the balance training performed, and indicate frequency and duration in order to enable comparison and generate better recommendations in the future.
All studies applied the intervention at least twice a week (2–3×/week balance; 4–6×/week endurance) for at least 6 weeks (6–36 weeks balance; 8 weeks–4 years endurance).
This review also has limitations. Although the studies were ranked by three independent reviewers in order to minimize subjectivity, selection bias cannot be ruled out completely. It must also be considered that ranking according to the criteria of the Oxford Levels of Evidence-Based Medicine was hampered due to lack of access to the raw data in the papers. Many studies lacked confidence intervals and indications regarding adverse events. Consequently, studies were difficult to interpret and rank and may therefore be under- or over-rated. However, those limitations are well-known and also apply at different degrees for other evaluation strategies [86, 87].
To date, special recommendations regarding exercise interventions for neuropathic patients are scarce. Solely from a DPN point of view, the American Diabetes Association (ADA) and the American College of Sports Medicine (ACSM) have released a statement [70] recommending patients do 150 min/week of moderate-intensity exercise, or to refrain from non-weight-bearing activities such as swimming, bicycling, or arm exercises in case of foot injuries. Other than that, Internet sites, as well as ADA personnel, advise patients with diabetes and PNP to ‘be careful when exercising’ as ‘some physical activities are not safe for people with neuropathy’ [88]. Possible risks are mentioned, such as an increased risk of skin breakdown and infection as well as Charcot joint destruction, due to reduced sensitivity in the extremities [70]. However, current studies reveal that mild adverse events only occurred in 2 of 18 studies. Furthermore, patients exercising do not seem at higher risk for skin breakdown or foot ulceration, nor have weight-bearing exercises induced a higher risk than non-weight-bearing activities. Additionally, the efficacy of non-weight-bearing activities is low [53].
The large heterogeneity of the existing studies makes it difficult to define evidence-based recommendations, not only for peripheral neuropathy in general but also for the various subgroups. In order to give precise training guidelines, including duration, frequency, and intensity, more studies will be necessary. It is challenging to compare the various exercise programs of the individual studies as data is insufficient for the subgroups and a general comparison may be biased due to the potentially diverse underlying mechanisms of PNP that could alter the response to exercise. Furthermore, the studies differ in terms of the interventions, duration, frequency, heterogeneous inclusion- and exclusion criteria as well as outcome measures, which could also influence the effects. Since most neuropathies are characterized by a chronic disease course, exercise interventions at different timepoints during the disease course may impact their potential treatment benefit. Nevertheless, we will try to present some prevailing directions and therefore generate preliminary recommendations that will have to be confirmed by further studies.
According to the current evidence (see Table 7), balance exercises seem to have the highest effect on the crucial side effects of PNP, especially in primarily non-metabolic neuropathic disorders. Therefore, balance exercises should be included in exercise interventions and supportive care for PNP patients. Possible interventions to obtain this aim could be, for example, SMT, Tai Chi, and vibration exercises as these target the same mechanisms. Additionally, the type of exercises within the balance training will also have to be chosen carefully according to the symptom one wishes to address.
For patients with neuropathies of primarily metabolic origin, endurance exercises will presumably best target the onset as well as the progression of DPN. This is likely to also apply to other metabolically-induced neuropathies. Additional balance exercises or WBV [4] should be considered.
In accordance with other reviews on exercise interventions for various causes [47], better results were achieved if training was supervised rather than home-based or community-based [58, 68]. It also seems that exercises need to be repeated at least twice a week, preferably for 12 weeks or longer, as studies with very short interventions (12 days to 6 weeks) and less frequency (once a week) [58, 61, 65] fail to produce significant intergroup effects. Of course, it depends on the intervention and the aim. For instance, SMT is known to impact balance control in healthy older adults after just 4 weeks [30]. Further studies should evaluate the individual types of exercises and determine whether combinations of exercises, such as endurance and SMT, could have additive effects, as well as the intensity and duration necessary to achieve the highest effect for this specific patient cohort. Furthermore, the potential of exercise in various phases of the disease (preventive, acute, and rehabilitation) needs to be evaluated.
Scientists should preferably choose a control group that has the same disease but does not participate in the exercise intervention. If offering an intervention to the control group is desired, the intervention should not target the outcome measurements as intergroup results will be too weak and biased [61].
The recommendations generated are based on rather low evidence and very heterogeneous studies and can thus only present a preliminary direction. Therefore, many more studies will be necessary to develop comprehensive clinical exercise recommendations. Nevertheless, exercise is currently an effective supportive measure for neuropathic patients and a good alternative to pharmaceutical approaches. Therefore, the translation of the present knowledge into practice should be initiated. The various societies responsible ought to contribute to the education and instruction of therapists and physicians in order to guarantee the best possible support for patients. Interdisciplinary collaborations are essential in striving towards standardization of exercise in supportive therapy.
5 Conclusion
Exercise is feasible, safe, and effective for neuropathic patients. Balance training has the potential to improve sensory and motor symptoms in PNP, while in PNP of metabolic etiology, endurance training can prevent the onset and delay the progression of PNP. Exercise is therefore a supportive therapy for neuropathic patients that should be taken more seriously.
References
Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62(4):310–8.
Mold JW, Vesely SK, Keyl BA, et al. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17(5):309–18.
Richardson JK, Ashton-Miller JA. Peripheral neuropathy: an often-overlooked cause of falls in the elderly. Postgraduate Med. 1996;99(6):161–72.
Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp Med. 2013;231(4):305–14.
Liedberg GM, Vrethem M. Polyneuropathy, with and without neurogenic pain, and its impact on daily life activities: a descriptive study. Disabil Rehabil. 2009;31(17):1402–8. doi:10.1080/09638280802621382.
Stubblefield MD, Burstein HJ, Burton AW, et al. NCCN task force report: management of neuropathy in cancer. J Natl Compre Canc Netw. 2009;7 Suppl 5:S1–S26; quiz S7–8.
Poeck K, Hacke W. Neurologie. Springer; 2006.
Sartor CD, Watari R, Passaro AC, et al. Effects of a combined strengthening, stretching and functional training program versus usual-care on gait biomechanics and foot function for diabetic neuropathy: a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:36. doi:10.1186/1471-2474-13-36.
Boulton AJ. Lowering the risk of neuropathy, foot ulcers and amputations. Diabet Med. 1998;15(Suppl 4):S57–9. doi:10.1002/(SICI)1096-9136(1998120)15:4+<S57:AID-DIA741>3.0.CO;2-D.
Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17.
Antoine JC, Camdessanche JP. Peripheral nervous system involvement in patients with cancer. Lancet Neurol. 2007;6(1):75–86. doi:10.1016/S1474-4422(06)70679-2.
Kaley TJ, Deangelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol. 2009;145(1):3–14. doi:10.1111/j.1365-2141.2008.07558.x.
Wonders KY, Reigle BS, Drury DG. Treatment strategies for chemotherapy-induced peripheral neuropathy: potential role of exercise. Oncol Rev. 2010;4:117–25.
Tofthagen C, Visovsky C, Berry DL. Strength and balance training for adults with peripheral neuropathy and high risk of fall: current evidence and implications for future research. Oncol Nurs Forum. 2012;39(5):E416–24. doi:10.1188/12.ONF.E416-E424.
Uceyler N, Rogausch JP, Toyka KV, et al. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69(1):42–9. doi:10.1212/01.wnl.0000265062.92340.a5.
Smith EM, Cohen JA, Pett MA, et al. The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs. 2010;33(3):173–83. doi:10.1097/NCC.0b013e3181c989a3.
Smith BH, Torrance N, Bennett MI, et al. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain. 2007;23(2):143–9. doi:10.1097/01.ajp.0000210956.31997.89.
Granacher U, Muehlbauer T, Gollhofer A, et al. An intergenerational approach in the promotion of balance and strength for fall prevention: a mini-review. Gerontology. 2011;57(4):304–15. doi:10.1159/000320250.
Bruhn S, Kullmann N, Gollhofer A. The effects of a sensorimotor training and a strength training on postural stabilisation, maximum isometric contraction and jump performance. Int J Sports Med. 2004;25(1):56–60. doi:10.1055/s-2003-45228.
Balducci S, Leonetti F, Di Mario U, et al. Is a long-term aerobic plus resistance training program feasible for and effective on metabolic profiles in type 2 diabetic patients? Diabetes Care. 2004;27(3):841–2.
Goldhaber-Fiebert JD, Goldhaber-Fiebert SN, Tristan ML, et al. Randomized controlled community-based nutrition and exercise intervention improves glycemia and cardiovascular risk factors in type 2 diabetic patients in rural Costa Rica. Diabetes Care. 2003;26(1):24–9.
Balducci S, Iacobellis G, Parisic L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabet Complicat. 2006;20:216–23.
Granacher U, Mühlbauer T, Taube W, et al. Sensorimotor training. In: Cardinale M, editor. Strength and conditioning: biological principles and practical applications. San Francisco: Wiley; 2011: p. 399–409.
Kawanabe K, Kawashima A, Sashimoto I, et al. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J Med. 2007;56(1):28–33.
Ahn S, Song R. Effects of Tai Chi Exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. J Altern Complement Med. 2012;18(12):1172–8. doi:10.1089/acm.2011.0690.
Taube W, Gruber M, Beck S, et al. Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol (Oxf). 2007;189(4):347–58. doi:10.1111/j.1365-201X.2007.01665.x.
Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47(4):678–85.
Taube W, Kullmann N, Leukel C, et al. Differential reflex adaptations following sensorimotor and strength training in young elite athletes. Int J Sports Med. 2007;28(12):999–1005. doi:10.1055/s-2007-964996.
Verhagen E, van der Beek A, Twisk J, et al. The effect of a proprioceptive balance board training program for the prevention of ankle sprains: a prospective controlled trial. Am J Sports Med. 2004;32(6):1385–93. doi:10.1177/0363546503262177.
Granacher U, Gollhofer A, Strass D. Training induced adaptations in characteristics of postural reflexes in elderly men. Gait Posture. 2006;24(4):459–66. doi:10.1016/j.gaitpost.2005.12.007.
Bogaerts A, Delecluse C, Boonen S, et al. Changes in balance, functional performance and fall risk following whole body vibration training and vitamin D supplementation in institutionalized elderly women: a 6 month randomized controlled trial. Gait Posture. 2011;33(3):466–72. doi:10.1016/j.gaitpost.2010.12.027.
Rittweger J, Beller G, Armbrecht G, et al. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone. 2010;46(1):137–47. doi:10.1016/j.bone.2009.08.051.
Kirchner E. Pflegerische Interventionen und Möglichkeiten bei krebstherapiebedingter Polyneuropathie. DLH-INFO. 2008;37:19–21.
Blottner D, Salanova M, Puttmann B, et al. Human skeletal muscle structure and function preserved by vibration muscle exercise following 55 days of bed rest. Eur J Appl Physiol. 2006;97(3):261–71. doi:10.1007/s00421-006-0160-6.
Lau RW, Liao LR, Yu F, et al. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25(11):975–88. doi:10.1177/0269215511405078.
Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. 2011;32(2):75–99. doi:10.1055/s-0030-1268010.
Spiliopoulou SI, Amiridis IG, Tsigganos G, et al. Vibration effects on static balance and strength. Int J Sports Med. 2010;31(9):610–6. doi:10.1055/s-0030-1249618.
Jacobson B, Chen H, Cashel C. The effect of Tai Chi Chuan training on balance, kinesthetic sense and strength. Percept Mot Skills. 1997;84:27–33.
Harmer PA, Li F. Tai Chi and falls prevention in older people. Med Sport Sci. 2008;52:124–34. doi:10.1159/000134293.
Lan C, Lai JS, Chen SY, et al. 12-month Tai Chi training in the elderly: its effect on health fitness. Med Sci Sports Exerc. 1998;30(3):345–51.
Richerson S, Rosendale K. Does Tai Chi improve plantar sensory ability? A pilot study. Diabetes Technol Ther. 2007;9(3):276–86. doi:10.1089/dia.2006.0033.
Wolfson L, Whipple R, Derby C, et al. Balance and strength training in older adults: intervention gains and Tai Chi maintenance. J Am Geriatr Soc. 1996;44(5):498–506.
Wong AM, Lin YC, Chou SW, et al. Coordination exercise and postural stability in elderly people: Effect of Tai Chi Chuan. Arch Phys Med Rehabil. 2001;82(5):608–12. doi:10.1053/apmr.2001.22615.
Taggart HM. Effects of Tai Chi exercise on balance, functional mobility, and fear of falling among older women. Appl Nurs Res. 2002;15(4):235–42. doi:10.1053/apnr.2002.35975.
Li F, Harmer P, McAuley E, et al. An evaluation of the effects of Tai Chi exercise on physical function among older persons: a randomized controlled trial. Ann Behav Med. 2001;23(2):139–46.
Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations. I: critical appraisal of existing approaches. The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38. doi:10.1186/1472-6963-4-38.
Baumann FT, Zopf EM, Bloch W. Clinical exercise interventions in prostate cancer patients: a systematic review of randomized controlled trials. Support Care Cancer. 2012;20(2):221–33. doi:10.1007/s00520-011-1271-0.
Mac Dermid J, Law M. Evaluating the evidence. In: Law MC MacDermid J, editor. Evidence-based rehabilitation: a guide to practise. Thorofare: SLACK Incorporated; 2008. p. 122.
Bicego D, Brown K, Ruddick M, et al. Effects of exercise on quality of life in women living with breast cancer: a systematic review. Breast J. 2009;15(1):45–51. doi:10.1111/j.1524-4741.2008.00670.x.
Weis J, Domann U. Interventions in the rehabilitation of breast cancer patients: a critical literature review of the state of the art. Die Rehabil. 2006;45(3):129–45. doi:10.1055/s-2005-915459.
Philipps B, Ball C, Sackett D, et al. Oxford Centre for Evidence-based Medicine-Levels of evidence. March 2009. Available from: http://www.cebm.net/index.aspx?o=1025. 2014.
Dixit S, Maiya AG, Shastry BA. Effect of aerobic exercise on peripheral nerve functions of population with diabetic peripheral neuropathy in type 2 diabetes: a single blind, parallel group randomized controlled trial. J Diabet Complicat. 2013;28(3):332–9. doi:10.1016/j.jdiacomp.2013.12.006.
Mueller MJ, Tuttle LJ, Lemaster JW, et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94(5):829–38. doi:10.1016/j.apmr.2012.12.015.
Akbari M, Jafari H, Moshashaee A, et al. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49(2):333–8.
Song CH, Petrofsky JS, Lee SW, et al. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabet Technol Ther. 2011;13(8):803–11.
Allet L, Armand S, de Bie RA, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53(3):458–66. doi:10.1007/s00125-009-1592-4.
Hung JW, Liou CW, Wang PW, et al. Effect of 12-week tai chi chuan exercise on peripheral nerve modulation in patients with type 2 diabetes mellitus. J Rehabil Med. 2009;41(11):924–9. doi:10.2340/16501977-0445.
Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568–79. doi:10.2522/ptj.20090362.
Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82(2):205–9.
Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25(2):493–9. doi:10.1093/annonc/mdt568.
Matjacic Z, Zupan A. Effects of dynamic balance training during standing and stepping in patients with hereditary sensory motor neuropathy. Disabil Rehabil. 2006;28(23):1455–9. doi:10.1080/09638280600646169.
Tomás MT, Santa-Clara H, Bruno PM, et al. The impact of exercise training on liver transplanted familial amyloidotic polyneuropathy (FAP) patients. Transplantation. 2013;95(2):372–7. doi:10.1097/TP.0b013e31827220e7.
Graham RC, Hughes RA, White CM. A prospective study of physiotherapist prescribed community based exercise in inflammatory peripheral neuropathy. J Neurol. 2007;254(2):228–35. doi:10.1007/s00415-006-0335-4.
Nardone A, Godi M, Artuso A, et al. Balance rehabilitation by moving platform and exercises in patients with neuropathy or vestibular deficit. Arch Phys Med Rehabil. 2010;91(12):1869–77. doi:10.1016/j.apmr.2010.09.011.
Ruhland JL, Shields RK. The effects of a home exercise program on impairment and health-related quality of life in persons with chronic peripheral neuropathies. Phys Ther. 1997;77(10):1026–39.
Lindeman E, Leffers P, Spaans F, et al. Strength training in patients with myotonic dystrophy and hereditary motor and sensory neuropathy: a randomized clinical trial. Arch Phys Med Rehabil. 1995;76(7):612–20.
Ang CD, Alviar MJ, Dans AL, et al. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;(3):CD004573. doi:10.1002/14651858.CD004573.pub3.
Albers JW, Chaudhry V, Cavaletti G, et al. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2014;(3):CD005228. doi:10.1002/14651858.CD005228.pub4.
Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413.
American Diabetes Assocation. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37 Suppl 1:S14–80. doi:10.2337/dc14-S014.
Tolle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabet Complicat. 2006;20(1):26–33. doi:10.1016/j.jdiacomp.2005.09.007.
Possidente CJ, Tandan R. A survey of treatment practices in diabetic peripheral neuropathy. Prim Care Diabet. 2009;3(4):253–7. doi:10.1016/j.pcd.2009.08.008.
Kessler NJ, Hong J. Whole body vibration therapy for painful diabetic peripheral neuropathy: a pilot study. J Bodyw Mov Ther. 2013;17(4):518–22. doi:10.1016/j.jbmt.2013.03.001.
Albers JW, Chaudhry V, Cavaletti G, et al. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2011;(2):CD005228. doi:10.1002/14651858.CD005228.pub3.
Steimann M, Kerschgens C, Barth J. Rehabilitation bei Chemotherapieinduzierter Polyneuropathie. Onkologe. 2011;17:940–7.
Sabatier MJ, Redmon N, Schwartz G, et al. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211(2):489–93. doi:10.1016/j.expneurol.2008.02.013.
Molteni R, Zheng JQ, Ying Z, et al. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101(22):8473–8. doi:10.1073/pnas.0401443101.
Park JS, Hoke A. Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PloS One. 2014;9(3):e90245. doi:10.1371/journal.pone.0090245.
Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9(1):36–45. doi:10.1038/nrn2294.
Kikkawa Y, Kuwabara S, Misawa S, et al. The acute effects of glycemic control on nerve conduction in human diabetics. Clin Neurophysiol. 2005;116(2):270–4. doi:10.1016/j.clinph.2004.08.011.
Lee Y, Morrison BM, Li Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–8. doi:10.1038/nature11314.
Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J. 2008;22(10):3443–9. doi:10.1096/fj.08-106104.
Taube W, Gruber M, Gollhofer A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiologica. 2008;193(2):101–16. doi:10.1111/j.1748-1716.2008.01850.x.
Gollhofer A. Proprioceptive training: considerations for strength and power production. In: P.V.K, editor. Strength and power in sport. 2nd ed. Oxford: Blackwell Publishing; 2003. p. 331–42.
Sjostrom PJ, Rancz EA, Roth A, Hausser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88(2):769–840. doi:10.1152/physrev.00016.2007.
Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi:10.1136/bmj.328.7454.1490.
Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–51. doi:10.1200/JCO.2007.15.4963.
ADA. 2014. www.diabetes.org. Accessed 3 Oct 2014.
Acknowledgments
Fiona Streckmann, Eva M. Zopf, Helmar C. Lehmann, Kathrin May, Julia Rizza, Philipp Zimmer, Albert Gollhofer, Wilhelm Bloch, and Freerk T. Baumann have no conflicts of interest to declare. No sources of funding were used to assist in the preparation of this manuscript. We acknowledge the advisory support of C. Brinkmann.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Streckmann, F., Zopf, E.M., Lehmann, H.C. et al. Exercise Intervention Studies in Patients with Peripheral Neuropathy: A Systematic Review. Sports Med 44, 1289–1304 (2014). https://doi.org/10.1007/s40279-014-0207-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-014-0207-5