Abstract
Introduction
Cost-of-illness (COI) studies provide policy-relevant information for cross-country, longitudinal, and other cost comparisons. Prior studies have called for standardization in COI methods. We investigated trends, identified factors associated with variation in COI estimation methods, and characterized reporting of heterogeneity in COI estimates.
Methods
The review of COI studies was implemented following (i) a structured search of PubMed, SCOPUS and EMBASE; (ii) a review of abstracts; (iii) a full-text review; and (iv) classification of articles according to six COI estimation methods: Sum_All Medical, Sum_Diagnosis Specific, Matched, Regression, Other_Total and Other_Incremental. Descriptive and multivariable regression analyses were conducted.
Results
Of the 993 studies included in the full-text review, 186 (18.7 %) were Sum_All Medical, 458 (46.1 %) were Sum_Diagnosis Specific, 96 (9.7 %) were Matched, 97 (9.8 %) were Regression, 70 (7.1 %) were Other_Incremental, and 68 (6.9 %) were Other_Total. Compared with the early period, publications in the middle and late period were associated with lower odds of using Sum_All Medical compared with Sum_Diagnosis Specific (adjusted odds ratio [AOR]middle 0.14; 95 % CI 0.07–0.28; AORlate 0.44; 95 % CI 0.29–0.67). Overall, 640 articles (64 %) reported COI estimates across patient groups defined by patient-level factors, while 247 articles (25 %) reported COI estimates across patient groups defined by non-patient-level factors.
Conclusion
The disease-specific total costing method (Sum_Diagnosis Specific) was most commonly used and its use increased over the time period covered by this review. The investigation of subgroup heterogeneity in COI estimates represents an area for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We investigated trends, identified factors associated with variation in cost-of-illness (COI) estimation methods, and characterized reporting of heterogeneity in COI estimates. |
The disease-specific total costing method (Sum_Diagnosis Specific) was most commonly used, and its use increased over the 10-year period covered by this review. |
Over half of the articles reported COI estimates across patient groups defined by patient-level factors (e.g. age, race), while one-quarter reported COI estimates across patient groups defined by non-patient-level factors (e.g. hospital, geography). |
1 Introduction
Cost-of-illness (COI) studies provide estimates of the burden of disease and were among the earliest type of economic costing methods applied to the healthcare sector [1]. COI is computationally different and serves a different purpose compared with other costing methods such as cost-effectiveness analysis or cost minimization. The COI method is descriptive (vs. analytic) and does not involve a testable hypothesis. It involves several decisions, including the choice of study perspective, data, costing approach (e.g. top-down vs. bottom-up, disease-specific vs. total costs), and cost components to include (e.g. direct medical, direct non-medical and indirect costs), among other choices. Differences across studies in terms of these methodological decisions can lead to variation in the COI estimates for a given disease state.
Prior work has noted the absence of a standard methodology for calculating COI [2–4]. This variation in methodologies can result in a wide range of cost estimates for one disease state [5, 6], leading to questions regarding the reliability of COI studies and their usefulness for health-related decision making [7–10]. The usefulness of the COI estimate also has been questioned given that there is no concurrent attention paid to effect or benefit [11] or disease prevention. It has been argued that cost-effectiveness and cost-benefit analyses provide more useful information than COI because a more costly disease should not necessarily be allocated more resources [11–13]. Focusing on the use of COI methods for injury studies, Currie et al. argued that while the cost of injury estimates raises awareness on the burden of injuries, these studies provide limited useful information to decision makers in priority setting as more attention should be given to health economic studies that explore the cost of prevention interventions [13]. Rice [14] dissented with the claim of Currie et al. that COI studies are not useful, providing historical examples of its usefulness. Rice pointed to the National Institutes of Health (NIH) reports (1995, 1997, 2000 [15]) to Congress that highlighted the societal burden of various illnesses and noted the level of NIH support in each area of research. Rice emphasized that one must clearly ascertain the methodologies, scope, and data sources used for a given COI study in order to clearly interpret the reliability of results. Despite issues with COI methods and results, COI studies are commonly conducted and reported in the US and abroad.
In a prior review, we outlined four methodologies used to calculate COI: Sum_All Medical, Sum_Diagnosis Specific, Matched, and Regression [2]. The Sum_All Medical and Sum_Diagnosis Specific are total costing techniques, while Matched and Regression are incremental costing techniques. The Sum_All Medical cost method is the simplest design and involves adding all the medical costs of providing care to a patient, regardless of whether the cost was directly related to the disease or not. The Sum_Diagnosis Specific method involves adding only the costs associated with the disease of interest. Matched control group and regression analyses methods account for patient-specific factors using either matching or multivariable regression analysis for patients with and without the disease of interest. In the prior review, the regression method was the least-used method and the Sum_Diagnosis Specific method was the most commonly used method. A decade after the prior literature review, we sought to determine how these cost methods have been used in COI studies.
The primary goal of this paper was to update results from the 2006 publication [2] and describe trends over time in the methods used to calculate the COI among studies published in the last decade, i.e. 2005–2014. As a secondary objective, we conducted a descriptive analysis to identify factors (e.g. disease, country of origin, data source) that can explain variation in the COI method employed. Furthermore, we characterized the extent to which COI studies have investigated heterogeneity in the COI due either to patient-specific or contextual factors.
2 Methods
The study team implemented the review of COI articles in three stages: (i) we identified candidate abstracts in PubMed, SCOPUS, and EMBASE using prespecified search criteria; (ii) we identified COI articles based on the abstracts using prespecified criteria; and (iii) we extracted content based on a review of the full-text of articles that met prespecified inclusion criteria. Following data extraction, we conducted descriptive and multivariable statistical analyses to investigate variation in COI methods.
2.1 Literature Search Strategy
A literature review was completed in PubMed using the search term ‘cost of illness’ as a Medical Subject Heading (MeSH). Due to usage of the MeSH term, the search engine found articles that were also tagged to the term ‘cost of illness’, such as: cost of illness; illness cost; illness costs; sickness cost; cost, sickness; costs, sickness; burden of illness; illness burden; illness burdens; costs of disease; disease cost; cost, disease; costs, disease; disease costs; cost of sickness; sickness costs; cost of disease. The following search limits were applied: a custom date range of ‘01/01/2005–05/31/2014’, English language, abstract available, and humans. To further limit the search to articles specifically discussing cost of illness, articles that included ‘cost effectiveness’, ‘cost utility’, ‘cost benefit’, or ‘cost minim*’ within the title or abstract were excluded. Using ‘minim*’ allowed us to exclude variations of ‘cost minimization’, including cost minimal, cost minimization, cost minimizing, cost minimization, and cost minimizing.
We also completed a search within EMBASE to retrieve health/medical articles located within EMBASE that were not available in PubMed. We used the term ‘cost of illness’ as an EMTREE term and exact phrase within EMBASE, and extracted all articles tagged with the phrase ‘cost of illness’. The following limits were applied: date published 2005–2014, English, humans, abstract available, and article publication type. Records with the terms ‘cost effectiveness’, ‘cost utility’, ‘cost benefit’, ‘cost minimal’, ‘cost minimization’, ‘cost minimizing’, ‘cost minimization’, or ‘cost minimizing’ within the title or abstract were excluded. To minimize duplication, the source of records was limited to ‘EMBASE only’, which excluded articles available in PubMed.
Lastly, we completed a search via SCOPUS, utilizing the term ‘cost of illness’ as an exact phrase in the title, abstract, or as a keyword. The following limits were applied: date published after year 2005, human index term, English, and subject area limited to economics, econometrics, and finance. There was potential for overlap between SCOPUS and PubMed as well as between SCOPUS and EMBASE; thus, we searched the PubMed and EMBASE abstracts for duplicates from the SCOPUS abstracts. Search strings applied in PubMed, EMBASE and SCOPUS are provided in Appendix Table 5.
2.2 Abstract and Full-Text Review
The purpose of the abstract review was to identify likely COI studies based on the information available in the abstract. Articles with the phrase ‘cost minim*’, ‘cost benefit’, ‘cost effectiveness’, or ‘cost utility’ in the title or abstract were excluded during the abstract review. We defined a COI study as an article that reported an original, calculated cost about an applicable disease or illness, and defined an applicable disease as an illness that was identifiable using an International Classification of Diseases (ICD) diagnosis code. Articles that reported the cost of a specific treatment or other intervention were excluded because the focus of such articles was on the cost of the intervention rather than on the cost of illness. Seven reviewers involved in the abstract review decided whether articles were COI studies using the same decision algorithm and associated exclusion criteria. Abstracts were excluded from further consideration if they satisfied any of the following exclusion criteria: (i) disease cannot be identified using an ICD diagnosis code; (ii) COI was not original to the manuscript (e.g. the manuscript was a review article); (iii) cost was reported but not for an applicable illness (e.g. costs associated with a natural disaster or medical procedure); (iv) the illness was applicable but no cost analysis was reported; and (v) neither cost nor an illness was discussed.
After completion of the abstract review, three co-authors responsible for the majority of the abstract reviews (>2000 each) randomly selected 20 abstracts from each set assigned to other reviewers, for a second review. If the discrepancy between decisions based on the initial review and on the second review was greater than 20 %, the full set of abstracts assigned during the initial review was re-reviewed by another co-author. Specifically, one set of 500 articles was reviewed again due to >20 % discrepancy based on the second review.
The primary focus of the full-text review was to summarize the COI calculation method that was used to determine the COI reported in the abstract. We characterized COI studies as employing one of six methods (Table 1), three of which were total cost methods and three of which were incremental cost methods. Articles that calculated the COI using only individuals with the disease of interest were categorized as using the Sum_All, Sum_Diagnosis Specific, or Other_Total method. Articles that calculated the COI by comparing the cost of disease in a sample of patients with and without the disease of interest were categorized as using the Matched_Control, Regression, or Other_Incremental method. We kept the same definitions used previously [2] and added two categories (i.e. Other_Total and Other_Incremental) to account for articles that utilized modeling methods, simulation, or other techniques not represented in the original categories. The ‘Other_Total’ method for calculation of COI identifies all patients with a diagnosis of the disease of interest and employs either a novel technique or mathematical modeling to calculate the costs. For example, Pisu et al. ran 10,000 Monte Carlo simulations to calculate the total costs of glucocorticoid-associated adverse events in rheumatoid arthritis [16]. The ‘Other_Incremental’ method for calculation of costs included studies that employed any novel technique or modeling approach to determine incremental costs associated with the disease of interest; for example, Brown et al. who calculated the incremental societal costs of pyelonephritis using a decision-tree model [17]. The Other_Incremental group also included articles that used a control group yet did not either explicitly state that patients were matched based on variables or specify that regression techniques were used to determine the COI.
Additional variables that were extracted during the full-text review included study perspective, disease state, funding source, follow-up period, COI reported, cost components, cost adjustment method across multiple years of data, source of patient-level data, modeling techniques, and continent of origin. Articles were characterized as North America, South America, Africa, Australia, Europe, Antarctica, or Asia. According to the article’s country of origin, articles were further classified by World Data Bank country lending group: low income, middle income, or high income. Articles were characterized according to the stated primary study perspective: societal, patient (out-of-pocket, household costs), caregiver (non-clinical), or clinical provider. The societal study perspective could include any combination or variation of cost components, including, but not limited to, direct medical (medication, supplies, hospitalizations, outpatient), direct non-medical (transportation, lodging, food), indirect (productivity loss), and intangible costs. In the absence of an explicit statement identifying the primary study perspective, we inferred that the study perspective reported in the abstract was the primary study perspective. In the case of multiple study perspectives reported in the study’s abstract, we defined the primary study perspective as the perspective that yielded the highest reported COI, providing a comprehensive estimate of the cost of illness. In the event that the author did not state the study’s perspective in the abstract, we reviewed the remaining article sections to determine the author’s stated study perspective. In the event that the author did not explicitly state the study’s perspective in the abstract or text, we categorized the study’s perspective as ‘NA’ for non-applicable or undetermined (see Table 2).
Diseases were listed using aggregated categories, e.g. occupational asthma was reported as asthma. An article was classified as reporting multiple diseases if separate COI estimates were reported for separate diseases. Diseases were classified using ICD Tenth Revision (ICD-10) code groupings (see Appendix Table 6 for list of grouping codes). Reviewers extracted information regarding the specific components of direct medical, direct non-medical, indirect, and intangible cost components. Direct medical costs were associated with the following health service utilization categories: inpatient, emergency department, laboratory/diagnostics, formal provider/ancillary personnel, formal facility-based services and prescription medications. Direct non-medical costs included informal caregiver care, child care, and transportation costs. Indirect costs included productivity loss while intangible costs included costs associated with pain, loss, and suffering. A complete list of all variables extracted is available from the authors upon request.
The multivariable analysis compared all COI methods against Sum_Diagnosis Specific using a multinomial logistic model. The dependent variable included five categories: Sum_All, Matched, Regression, Other_Incremental, Other_Total. We specified a parsimonious model given the sample size for each outcome group, and included a categorical fixed effect representing the time period, consistent with the primary study objective of investigating trends over time. We also included factors that could explain variation in the COI method either because they would affect the type of costs available for analysis (i.e. prevalence or incidence costs, cost components) or because they could affect the study time horizon and resources (i.e. funding source). Other measures (e.g. geographical setting and aggregate disease group) were available but, in the interest of parsimony, were not entered in the regression model.
The set of predictor variables included indicators for time period, inclusion of direct medical cost, inclusion of direct non-medical cost, incidence (vs. prevalence) design, funding source (modeled ‘government funding’, ‘unfunded’, and ‘other’, with ‘pharmaceutical company’ as the reference funding source). The ‘time period’ variable included three categories—early, middle and late. The ‘early’ time period (the reference category) included studies published between the years 2005 and 2007, the ‘middle’ period included studies published between 2008 and 2010, while the ‘late’ period included studies from 2011 to mid-2014 (i.e. the end of the study period). Type III tests for the overall effects and covariate-adjusted odds ratios (AORs) for the separate logits provided information regarding the statistically significant variables.
3 Results
3.1 Sample Size
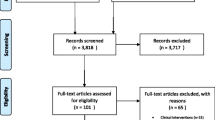
A total of 8299 records were identified as potential COI studies from PubMed, EMBASE, and SCOPUS. PubMed generated 93 % of the articles (n = 7686; see Appendix Fig. 4). The 8299 records were screened in the abstract review. A total of 6921 articles were eliminated in this process and 1378 articles were read in entirety in the full-text review (see Appendix Fig. 5). An additional 385 articles were excluded. Application of inclusion and exclusion criteria resulted in 993 articles that were eligible for analysis. Data from the remaining 993 articles were extracted and utilized in this review (see Fig. 1). Compared with the prior review (1996–2004), which included 365 eligible studies, the number of eligible studies in this review (2005–2014) almost tripled (2.7 times higher) to 993 studies.
3.2 Descriptive Results: Cost-of-Illness Methods
Of the 993 studies included in the review, 186 (19 %) were Sum_All Medical, 458 (46 %) were Sum_Diagnosis Specific, 96 (10 %) were Matched, 97 (10 %) were Regression, 70 (7 %) were Other_Incremental, and 68 (7 %) were Other_Total. The results from the prior review (1996–2004) indicated that the Matched method and the Regression method were not commonly used for COI studies. The use of the Matched approach remained steady over the 10-year period of the prior review. However, the Regression method saw an increasing trend from 0 % in 1996 to 10 % in 2004. One of the goals for this updated review was to determine whether the use of these two methods had increased over time. Figure 2 plots the proportion of studies employing each of the six methods for the early, middle, and late time periods for the updated review. We observed that a lower proportion of studies (10 %; N = 96) used the Matched method in this updated review compared with the prior review (19 %; N = 69). The proportion of studies using the Regression method was unchanged across the two review periods (10 %) but the overall number increased from 32 in the prior review to 97 in the updated review.
The continents of North America (37 %) and Europe (36 %) accounted for the most publications, followed by South America (23 %) and Asia (16 %). Studies conducted in Africa and Australia did not contribute significantly to the overall number of publications (3 and 4 %, respectively). The Matched method was employed in a higher proportion of studies in North America compared with other continents: 16 % for studies conducted in North America and 8 % for the next highest proportion, i.e. studies conducted in Australia. From Table 2, the Regression method was employed in a higher proportion of studies conducted in North America compared with other continents: 20 % for studies conducted in North America and 5 % for the next highest proportion, i.e. studies conducted in Europe.
The study perspectives examined in this review included private payer, public payer, societal, patient, caregiver, and clinical provider perspectives. The majority of studies employed the societal perspective (44 %), followed by patient perspective (10 %). Interestingly, 26 % of the studies did not explicitly state a specific study perspective. A higher number of studies employed the Sum_Diagnosis Specific COI method, followed by Sum_All Medical and Matched for all study perspectives with the exception of the clinical provider perspective, where the distribution was equal among Sum_All Medical, Sum_Diagnosis Specific, and Regression (i.e. 29 % for each) followed by Matched (7.14 %). However, this difference in pattern from the other perspectives can be attributed to the small number of studies that employed the clinical provider perspective (n = 14). In addition, the caregiver perspective was employed by very few studies.
The various data sources employed by the studies in the review included healthcare claims (21 %), medical facilities (34 %), registries (10 %), surveys (10 %), randomized clinical trials [RCTs] (1 %), other (6 %) and ‘multiple sources’ (6 %). In addition, there were studies that employed non-patient-level data [e.g. modeling] (9 %) to generate the cost estimates. The use of the COI methods was comparable (20–25 %) among the studies that used healthcare claims as their data source. Studies using medical facility data mostly utilized the Sum_Diagnosis Specific COI method (66 %), as did studies with registry data (53 %), RCTs (54 %), ‘Other’ data source (58 %) and multiple data sources (55 %). Among studies utilizing non-patient-level data, studies identified as ‘Other’ for the COI method accounted for 67 % of the sample: Other_Incremental (28 %) and Other_Total (39 %).
Bottom-up (83 %) and top-down (11 %) methods were used for deriving the cost estimates in the COI studies. Together, Other_Incremental (6 %) and Other_Total (5 %) methods accounted for approximately one in ten studies using the bottom-up costing method. This can be compared with the top-down group, where Other_Incremental (14 %) and Other_Total (19 %) methods accounted for approximately three of ten studies using the top-down costing method. The Sum_Diagnosis Specific method accounted for 50 % of studies using the bottom-up approach and 30 % of studies using the top-down approach.
The Regression and Matched approaches were more commonly utilized for studies in the Central Nervous System, Mental and Behavioral disorder group compared with other disease groups (Fig. 3). The Sum_Diagnosis Specific was the dominant method for the Infectious/Parasitic and Skin Disease group, as well as for several other disease groups.
3.3 Descriptive Results: Heterogeneity
Of the 993 included articles, 640 articles (64 %) reported COI estimates across patient groups defined by patient-level factors, while 247 articles (25 %) reported COI estimates across patient groups defined by non-patient-level factors. The remaining articles either did not investigate heterogeneity in the COI estimate (N = 98) or it was unclear (N = 8) whether or not heterogeneity was investigated. From Table 3, the proportion of studies reporting heterogeneity in COI estimates based on patient-level factors was higher for studies using bottom-up costing methods compared with studies using top-down costing methods (66 vs. 60 %; p < 0.001). On the other hand, the proportion of studies reporting heterogeneity in COI estimates based on non-patient-level factors was lower for studies using bottom-up costing methods compared with studies using top-down costing methods (25 vs. 28 %; p < 0.001). An increasing trend was observed in the proportion of studies reporting COI estimates for patient subgroups when the subgroups were defined by patient-level factors: early: 52 %; middle: 68 %; late: 71 %; p < 0.001. No monotonic trend was observed for studies reporting COI estimates for patient subgroups when the subgroups were defined by non-patient-level factors: early: 16 %; middle: 38 %; late: 23 %; p < 0.001.
When articles reported COI estimates across patient-level subgroups, these subgroups were often defined using a clinical measure (n = 306; 48 % of 640). Many articles (n = 194; 30 % of 640) defined patient subgroups using more than one patient-level factor or using a demographic measure (n = 133; 21 % of 640). When articles reported COI estimates across patient subgroups defined using non-patient-level factors, these subgroups were often defined using a variety of different measures (n = 117; 47 % of 247). Forty-one articles (17 % of 247) defined the patient subgroups based on hospital/clinic, while 49 articles (20 % of 247) defined the patient subgroups based on geographic units.
3.4 Multivariable Regression
Results from the Type III tests indicated that the time period (p < 0.001), inclusion of direct non-medical cost (p < 0.001), inclusion of indirect cost (p = 0.02), and study funding source (p < 0.001) were important factors explaining variation in the COI method employed for a given study. The inclusion of direct medical cost and the choice of incident vs. prevalent cost were not statistically significantly associated with the COI method employed. Compared with the early period, a publication in the middle and late periods was associated with statistically significantly lower odds of using Sum_All Medical compared with Sum_Diagnosis Specific (AORmiddle 0.14, 95 % CI 0.07–0.28; AORlate 0.44, 95 % CI 0.29–0.67). Compared with a publication in the early period, a publication in the late period was associated with statistically significantly lower odds of using the Other_Total approach (AORlate 0.35, 95 % CI 0.17–0.72). Additional results from the regression model are available in Table 4.
4 Discussion
The purpose of this review was to describe the methods used to calculate COI during the 10-year period from 1995 to 2014, and to investigate trends over time. We expected that COI methods progress towards more statistically complex methods over time and involve multivariable regression and mathematical modeling. We also expected to find a relatively small number of studies that investigated heterogeneity in the COI due to individual specific or contextual factors.
The Sum_Diagnosis specific method was the most commonly-used method and its use increased over time. While the use of the Regression, Matched and Other_Incremental approaches exhibited a positive trend over time, the absolute numbers indicated that these methods were not commonly used for estimating COI over the 10-year period covered by the current literature review. Compared with the Sum_Diagnosis Specific method, the Sum_All method was less likely to be utilized in more recent years, and in the most recent time period, the Other_Total was less likely to be utilized. Thus, among total cost methods, the Sum_Diagnosis Specific method appears to be the preferred method of choice. We did not find that the use of more complex statistical and mathematical models increased over time. Likewise, the odds of using an incremental costing method (i.e. Matched, Regression, Other_Incremental) were unchanged over the duration of the study period.
We investigated the reporting of subgroup variation in the COI estimate, or heterogeneity in the COI estimate. Interestingly, nearly nine of ten articles investigated heterogeneity in COI estimates. The number of studies that reported variation in patient-level sources of heterogeneity in the COI estimate increased monotonically over time; however, this trend did not carry over in studies reporting variation based on non-patient-level sources of heterogeneity. This result could be due to the fact that the ‘non-patient-level’ categorization included studies that defined subgroups using disparate units of analysis (e.g. geographic units, clinics, hospitals etc.) compared with the articles in the ‘patient-level’ categorization where subgroups were all based on measures that varied across patients (e.g. age, disease stage, comorbidity).
In addition to investigating trends over time as well as heterogeneity, we identified various factors that were associated with the choice of COI methods. The inclusion of direct non-medical costs and indirect costs were associated with lower odds of using the incremental cost COI methods. Our results indicated that studies including direct non-medical costs were less likely to list healthcare claims data as the primary data source, but were more likely to list healthcare facility data as the primary data source. Studies using survey or facility data may be less likely to include controls for incremental cost analysis due to the time and resource constraints associated with data collection. In addition, studies that included indirect costs were less likely to list healthcare claims data as the primary source and more likely to list survey data and registry data as the primary data source. The use of registry data precludes the use of an incremental cost approach due to the absence of disease controls.
Direct non-medical and indirect expenditures can contribute significantly to overall cost estimates and, depending on the disease of interest, will be important to consider. For example, these costs have been found to be significant when estimating the cost of asthma, cancer, hemophilia A, obesity, and congenital heart defects [7, 8, 18–20], but not for chronic low back pain [21]. Four of ten articles in the current review included direct non-medical costs, while six of ten articles included indirect costs. This stands in contrast to direct medical costs, which were included in nine of ten articles. Compared with direct medical costs, indirect costs and direct non-medical costs are harder to collect since they are not readily available through secondary data sources such as healthcare claims and disease registries. Studies conducted from a societal perspective may substantially underestimate the COI if they do not include the direct non-medical or indirect costs. On the other hand, these studies can potentially overestimate the societal COI if they are not utilizing an incremental cost approach. The net effect for the COI estimate will depend on whether the exclusion of cost components is more or less important than the use of total cost approaches for calculating the true COI.
The ideal approach for calculating an unbiased societal COI would combine design considerations for reducing bias (e.g. employ an incremental cost method) with practical considerations for enriching study measures (e.g. linking primary data from registries, surveys, or facilities to administrative data on health services utilization) to incorporate direct non-medical and indirect costs. Many studies will use existing data for practical considerations; however, these datasets are almost always created for purposes other than to calculate a COI estimate. Modeling studies offer an attractive alternative to existing data for use in calculating COI estimates. The use of mathematical models allows researchers to incorporate information from various data sources (e.g. healthcare claims, facility data, survey data, and registry data) in order to design COI studies.
As we look towards the next decade of COI research, it will be important that COI studies leverage current trends in health information technology, data availability and data linkage, as well as patient-centric concerns surrounding the burden of disease from a patient viewpoint. Current trends towards more comprehensive data linkage and the increased availability of big data present new opportunities for calculating COI estimates using enriched sets of measures. For example, the Optum Labs integrated electronic medical records and claims data provide a rich set of measures for estimating COI, including direct medical and indirect costs [22]. Moreover, the sample size and the availability of geographical and provider information facilitates the investigation of subgroups defined either at the patient (e.g. age, race, severity) or non-patient (e.g. geography, provider) level. With increasing evidence of cost hotspots and regional variations in costs of care [23–25], COI estimates that look beyond the average will be informative for public health decision makers. The current review indicated that many COI studies already report COI by patient-level subgroups. The increasing availability of larger, linked datasets facilitates the investigation of heterogeneity in the COI estimate not only at the patient or area level but also with respect to cross-level interaction effects. As the focus on patient centeredness increases, it will also be important to consider how patients view costs from both an out-of-pocket outlay of dollars as well as the physical and psychic burden of disease.
This review contributes new, updated information regarding the method used to calculate the COI, which has not been the focus of prior methodological reviews. Prior studies have investigated variation in methods relevant for COI studies. Some studies adopt a comprehensive view [8, 9, 14] while others focus on specific components such as indirect costs [5, 15] or the choice of quantitative/mathematical method [16]. The consensus finding among the studies is that there is a need for standardized methods for COI studies, including methods for calculating indirect costs, disease classification, use of top-down or bottom-up approaches, and the choice of study perspective.
There are a few limitations to note. We were not able to investigate all available predictors in multivariable regression analysis due to small samples overall and across the five modeled categories (e.g. indirect costs, intangible costs, disease groups). The results for the distribution of study perspective across COI methods may not be reliable given that one-quarter of the studies did not explicitly state the study perspective. Along with other recommendations that have been offered for improving standardization of COI methods, it will be important to highlight the importance of standardization in reporting characteristics of the COI study, particularly for measures such as the study perspective that determine the scope of the cost analysis.
5 Conclusions
In this review, we reported trends over time in the COI methods used in calculating the COI based on studies published during the past 10 years. We found that the disease-specific total costing method (Sum_Diagnosis Specific) was used most commonly, and that its use increased over the time period covered by this review and relative to the other total costing methods (i.e. Sum_All and Other_Total). There was no increase over time in the use of incremental costing approaches relative to the Sum_Diagnosis Specific approach. We also found that many studies report variation in the COI estimate, with the majority of studies reporting variation across patient-level factors. While some controversy exists regarding their ultimate use in decision making for healthcare resource allocation, COI studies provide useful information for policy makers and other decision makers. As long as there is demand for these COI estimates, the calls for standardization of methodologies will remain relevant and it will be important to consider the choice of the COI estimation method.
References
Tarricone R. Cost-of-illness analysis. What room in health economics? Health Policy. 2006;77(1):51–63.
Akobundu E, Ju J, Blatt L, Mullins CD. Cost-of-illness studies: a review of current methods. Pharmacoeconomics. 2006;24(9):869–90.
Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29(8):653–71.
Mauskopf J, Mucha L. A review of the methods used to estimate the cost of Alzheimer’s disease in the United States. Am J Alzheimers Dis Other Dement. 2011;26(4):298–309.
Dee A, Kearns K, O’Neill C, Sharp L, Staines A, O’Dwyer V, et al. The direct and indirect costs of both overweight and obesity: a systematic review. BMC Res Notes. 2014;7:242.
Ettaro L, Songer TJ, Zhang P, Engelgau MM. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics. 2004;22(3):149–64.
Molinier L, Bauvin E, Combescure C, Castelli C, Rebillard X, Soulie M, et al. Methodological considerations in cost of prostate cancer studies: a systematic review. Value Health. 2008;11(5):878–85.
Molinier L, Combescure C, Chouaid C, Daures JP, Housset B, Fabre D, et al. Cost of lung cancer: a methodological review. Pharmacoeconomics. 2006;24(7):651–9.
Rice DP. Cost-of-illness studies: fact or fiction? Lancet. 1994;344(8936):1519–20.
Shiell A, Gerard K, Donaldson C. Cost of illness studies: an aid to decision making. Health Policy. 1987;8(3):317–23.
Kymes S. “Can we declare victory and move on?” The case against funding burden-of-disease studies. Pharmacoeconomics. 2014;32(12):1153–5.
Byford S, Torgerson DJ, Raftery J. Economic note: cost of illness studies. BMJ. 2000;320(7245):1335.
Currie G, Kerfoot KD, Donaldson C, Macarthur C. Are cost of injury studies useful? Inj Prev. 2000;6(3):175–6.
Rice DP. Cost of illness studies: what is good about them? Inj Prev. 2000;6(3):177–9.
Varmus H. Disease-specific estimates of direct and indirect costs of illness and NIH support. Washington, DC: National Institutes of Health; 2000.
Pisu M, James N, Sampsel S, Saag KG. The cost of glucocorticoid-associated adverse events in rheumatoid arthritis. Rheumatology (Oxford). 2005;44(6):781–8.
Brown P, Ki M, Foxman B. Acute pyelonephritis among adults: cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics. 2005;23(11):1123–42.
Cisternas MG, Blanc PD, Yen IH, Katz PP, Earnest G, Eisner MD, et al. A comprehensive study of the direct and indirect costs of adult asthma. J Allergy Clin Immunol. 2003;111(6):1212–8.
Gerber AU, Torre AH, Buscher G, Stock SA, Graf C, Schickendantz S, et al. Direct non-medical and indirect costs for families with children with congenital cardiac defects in Germany: a survey from a university centre. Cardiol Young. 2010;20(2):178–85.
Zhou ZY, Koerper MA, Johnson KA, Riske B, Baker JR, Ullman M, et al. Burden of illness: direct and indirect costs among persons with hemophilia A in the United States. J Med Econ. 2015;18(6):457–65.
Depont F, Hunsche E, Abouelfath A, Diatta T, Addra I, Grelaud A, et al. Medical and non-medical direct costs of chronic low back pain in patients consulting primary care physicians in France. Fundam Clin Pharmacol. 2010;24(1):101–8.
Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood). 2014;33(7):1187–94.
Institute of Medicine. Variation in health care spending: target decision making, not geography. Washington, DC: Institute of Medicine; 2013.
Gawande A. The hot spotters. The New Yorker. New York: Conde Nast; 2011.
Gawande A. The cost conundrum. The New Yorker. New York: Conde Nast; 2009.
Acknowledgments
The authors wish to thank Sohani Patel, Amy Howard, Jacinda Tran, and Mercedes Wilkes for their assistance with the review of article abstracts and data cleaning. Any errors in the interpretation and reporting of the results are the sole responsibility of the authors.
Author contributions
The interpretation and reporting of these data are the sole responsibility of the authors. Eberechukwu Onukwugha contributed to the study design, data collection, conduct and interpretation of the statistical analysis, and drafted and revised the manuscript with input from all co-authors. Jacquelyn McRae contributed to the study design, data collection, interpretation of the analysis, and drafted, revised and commented on/edited all drafts of the manuscript. Alex Kravetz and Stefan Varga contributed to the data collection, interpretation of the analysis, and reviewed and commented on/edited all drafts of the manuscript. Rahul Khairnar contributed to the data collection, data cleaning, conduct and interpretation of the analysis, and reviewed and commented on/edited all drafts of the manuscript. Daniel Mullins contributed to the study design and interpretation of the analysis, and reviewed and commented on/edited all drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the conduct of this study.
Conflict of interest
Dr. Onukwugha has received consulting income from AstraZeneca and Janssen Analytics, and Dr. Mullins has received consulting income from Regeneron, Novartis, Daiichi-Sankyo, Bayer, Bristol-Myers Squibb, Mundi Pharma, and Pfizer. Jacquelyn McRae, Alex Kravetz, Stefan Varga, and Rahul Khairnar have no conflicts of interest to declare.
Appendix
Rights and permissions
About this article
Cite this article
Onukwugha, E., McRae, J., Kravetz, A. et al. Cost-of-Illness Studies: An Updated Review of Current Methods. PharmacoEconomics 34, 43–58 (2016). https://doi.org/10.1007/s40273-015-0325-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-015-0325-4