Abstract
Introduction
The role of macrolides for treatment of children with acute asthma or wheezing exacerbations is unclear.
Objective
The aim of this systematic review was to evaluate the effectiveness of macrolides in children with recurrent wheezing presenting with acute asthma or wheezing exacerbation.
Methods
We conducted an electronic search in MEDLINE, EMBASE, CINAHL, LILACS, CENTRAL, and ClinicalTrials.gov.
Study selection criteria
Randomized controlled trials of macrolides (any macrolide) compared with placebo or standard treatment in children up to 18 years with recurrent wheezing/asthma presenting with an acute exacerbation.
Outcomes
Primary outcomes were need for hospitalization and/or time of acute asthma/wheezing symptoms resolution; secondary outcomes were duration of stay in the emergency department (ED)/clinic, severity of symptoms of the index episode, use of additional systemic corticosteroids or short active β-2 agonists, changes in lung function measures, ED visit/hospitalization during first week after index episode, time to next exacerbation, or adverse effects (AEs).
Results
Only three studies met the inclusion criteria (n = 334 children, 410 treated episodes); two studies included recurrent wheezers and the third included asthmatic children. There was no difference in hospitalization between groups, but children treated with macrolides had a significantly lower time to symptoms resolution than controls, although the magnitude of benefit remains to be quantified due to no normal distribution data presented. There was no difference in time to next episode of exacerbation (HR 0.96; 95% CI 0.71–1.28; I2 = 0%; p = 0.77). In one study, children receiving macrolides had a significant decrease in the severity of symptoms, decrease use of salbutamol, and another study showed improved lung function. No study evaluated antibiotic resistance development.
Conclusions
Limited evidence support that a macrolide trial could be considered in children with acute asthma or recurrent wheezing exacerbation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Asthma in children is a heterogeneous disease and the best treatment for asthma exacerbations is still under debate, especially in younger children. |
Macrolides, due to their immunomodulatory and anti-inflammatory capacities, have been progressively studied and it has been hypothesized that macrolides may be helpful in asthma or wheezing exacerbations. |
We found limited evidence that macrolides reduce symptoms and duration of asthma exacerbation episodes and could be considered in children with recurrent wheeze. |
More studies need to be done to identify the group of children that can respond better to macrolides treatment and to measure the real risk of macrolide resistance development. |
1 Introduction
Asthma is one of the most common chronic, non-communicable diseases, and affects around 334 million people worldwide [1, 2]. Asthma affects people from all age groups and presents its peak incidence in childhood. Recent data from the general population showed that in children up to 5 years old, and for those aged between 12–17 years, the overall asthma incidence rates were 23/1000 and 4.4/1000 children per year, respectively [3]. Asthma affects quality of life, productivity at work and school, healthcare use, and can result in death. Asthma also places a significant economic burden on health systems; for example, in the US the total cost of asthma was estimated to be US$81.9 billion in 2013 [4]. Is important to mention that 48% of preschoolers with asthma had an exacerbation in the preceding year [5]. The annual rate of emergency department (ED) visits was 23–42 per 1000 for preschoolers and < 15 per 1000 for those aged 6–70 years, with the same pattern for hospitalizations [6, 7]. Furthermore, the age at first hospitalization for asthma has decreased over time [8]. Thus, the economic burden of poor asthma control in preschoolers is of paramount importance [9].
Two meta-analyses have confirmed the beneficial role of inhaled corticosteroids (ICS) among preschoolers with asthma or recurrent wheezing exacerbation, with daily medium-dose ICS reducing the risk of exacerbations by up to 40% [10, 11]. Even though international guidelines for treating acute asthma or recurrent wheezing exacerbations in preschoolers and school children recommend the use of systemic corticosteroids (SCS) for episodes that do not respond to short-acting β-2 agonists (SABA), recent studies have questioned the utility of this intervention in preschoolers [12, 13].
Therefore, additional approaches to prevent exacerbations have recently been investigated. Macrolides have beneficial anti-inflammatory effects in other inflammatory chronic lung diseases [14], and may also provide benefits in children with episodic wheeze based on their effect on the airway microbiome, as accumulating evidence suggests that bacteria are important determinants of asthma inception and progression [15]. Macrolides are already one of the most prescribed antibiotics in ED in the US; a recent study showed that macrolides were prescribed in 17% (1.1 million) of all visits to EDs in which antibiotics were prescribed, and the most frequently diagnoses where macrolides were used included acute otitis media (17%), non-viral pneumonia (14%), pharyngitis or tonsillitis (12%), bronchitis or bronchiolitis (11%;), and upper respiratory tract infection (10%) [16].
In consideration of the controversy in the treatment of acute asthma exacerbations, especially in preschoolers with recurrent wheezing without persistent symptoms; the progressive evidence of the mechanisms of action of macrolides; and the widespread use of macrolides in children, we decided to perform this systematic review to evaluate the efficacy of macrolides in episodes of acute asthma or wheezing exacerbations in childhood.
2 Methods
2.1 Search and Selection Criteria
This study was registered with the International Prospective Register of Systematic Reviews (PROSPERO, http://www.crd.york.ac.uk/PROSPERO) as CRD42018115926. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to perform this review [17]. The authors identified studies published in MEDLINE—PubMed, EMBASE—Elsevier, LILACS—BIREME, the Cochrane Controlled Trials Register (CENTRAL) CINAHL—EBSCO databases, and ClinicalTrials.gov until September 2019, using the terms ‘asthma’, ‘acute asthma’, ‘asthma attack’, ‘asthma exacerbation’, ‘wheezing’, ‘wheeze’, ‘recurrent wheezer’, ‘recurrent wheeze’, ‘acute wheeze’, ‘wheezing episode’, ‘asthma episode’, ‘lung symptoms’, ‘macrolides’ ‘azithromycin’, ‘clarithromycin’, ‘erythromycin’ and ‘telithromycin’; we used free text, MeSH terms, Boolean operators and randomized controlled trial (RCT)/age limitations (filters) in different combinations (search details in supplement). Additionally, a search of relevant files from the drug manufacturer’s databases (published and unpublished) was performed. Language restrictions were not applied. To be included, studies had to meet all the following criteria: (i) children (preschoolers to adolescents aged up to 18 years) with recurrent wheezing/asthma presenting with an acute exacerbation; (ii) RCTs (parallel group or cross-over design) of any duration; (iii) comparison of macrolide (any type) with placebo or standard care, only when standard care did not include an antibiotic; and (iv) report at least one of the following primary outcomes: need for hospitalization and/or time to acute asthma/wheezing symptoms resolution; or the following secondary outcomes: duration of staying in the ED/clinic, severity of symptoms of the index episode, use of additional SCS or SABA, changes in lung function measures, ED visit/hospitalization during first week after index episode, time to next exacerbation, macrolides resistance or adverse effect (AEs) or severe adverse events (SAEs) of macrolides. An SAE was defined as any untoward medical occurrence that results in death, is life-threatening, requires inpatient hospitalization, or results in persistent or significant disability or incapacity [18].
We excluded studies that exclusively involved patients with their first wheezing episode or bronchiolitis episode and other chronic respiratory conditions (e.g., bronchopulmonary dysplasia, cystic fibrosis, primary ciliary dyskinesia, post-infection bronchiolitis obliterans) or congenital cardiopulmonary conditions. We also excluded studies that involved use of macrolides for asthma chronic treatment or long-term use.
2.2 Data Extraction and Assessment of Risk of Bias
Titles, abstracts, and citations were independently analyzed by two of the authors (JCR and MP). From the full text, all studies were independently assessed for inclusion. Both authors were independently involved in all stages of study selection, data extraction, and risk of bias assessment. The latter was assessed according to recommendations outlined in the Cochrane Handbook [19] for the following items: (i) adequacy of sequence generation; (ii) allocation concealment; (iii) blinding of participants and investigators; (iv) blinding of outcome assessment; (v) incomplete outcome data; (vi) selective outcome reporting and other bias. Disagreements were discussed and resolved by the third investigator (LBB).
2.3 Data Analysis
Analysis was performed by intention to treat and included all participants to minimize bias.
Outcomes were pooled using mean differences (MD) (inverse variance method) or Mantel–Haenszel risk ratios (RR). Estimate precision was quantified by 95% confidence intervals (CI). Statistical heterogeneity was measured by the I2 test (≤ 25% low heterogeneity; 26–39% unimportant; 40–60% moderate; and 60–100% substantial heterogeneity) [20]. A fixed-effects model was used when there was no evidence of significant heterogeneity in the analysis (I2 < 40%); if significant heterogeneity was found, a random-effects model was used [21, 22]. We presumed that the clinical heterogeneity between studies should be low, especially considering the narrow inclusion criteria. If clinical heterogeneity appeared to be high, we used a random-effects model. A priori subgroup analyses included type of macrolides, age (< 5 years versus > 5 years), severity of exacerbation (mild vs moderate to severe), atopic versus non-atopic children, and trials sponsored by pharmaceutical industry versus independent trials. The meta-analysis was performed with the Review Manager 5.3.5 software (Cochrane IMS, 2014).
3 Results
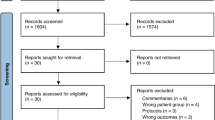
A total of 814 studies were found in a preliminary search; 787 were included from the databases search and 27 studies were included from the cross-reference search. Of these, seven studies were left after duplicates were removed and title and abstract were reviewed. We performed a full-text review of the seven articles; four of these studies were excluded because one did not have a comparison group [23], one had insufficient clinical data [24], one used macrolide intervention prior to lower respiratory tract symptoms [25], and one was an ongoing study (NCT02003911) (Fig. 1).
3.1 Included Studies
Three studies [26,27,28] published between 2012 and 2017 met our selection criteria and were included for quantitative analysis. These studies included 334 patients (aged 12 months to 14 years, 40–70% males; two studies [27, 28] recruited preschoolers and in the other study [26] the mean age was 9.09 ± 2.67 years) who presented a total of 410 episodes of acute asthma or lower respiratory symptoms. One study was performed in Canada [28], one in Greece [26], and one in Denmark [27]. We contacted the authors of two studies [26, 27] for additional data or clarifications. A summary of the characteristics of included studies is presented in Table 1.
As in our protocol, all included studies were randomized parallel-group trials. Two studies [27, 28] were blinded and one [26] was an open study. In two studies [26, 28], the randomization was done for each patient, and one study [27] randomized each episode of exacerbation (the number of episodes randomized per patient was 2.2 ± 1.5). One study [28] recruited patients presenting at the ED with wheezing, one study [26] recruited patients from a pediatric clinic, and one [27] study included patients from the COPSAC2010 birth cohort. This cohort is a single-center, population-based birth cohort of 700 children recruited from Danish population at 1 week of age and followed up prospectively at the Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) research unit.
All included patients met the criteria of acute asthma episodes, but with variation in the definition and severity. Mandhane et al. [28] included patients aged 12–60 months who presented in ED with a wheeze episode confirmed by physician or nurse; 62% were diagnosed with allergy or atopy, 35% were treated with ICS, 88% had previously had at least one episode of wheeze, and 35–40% had ever been diagnosed with asthma. This study included patients with a first episode of wheezing (29% of the sample) but presented data excluding this group of patients. Therefore, we considered only data from children with previous wheezing history in the outcomes and subgroup analysis whenever available. Koutsoubari et al. [26] included patients (6–14 years of age) diagnosed with intermittent or mild persistent asthma according to GINA (Global Initiative for Asthma) guidelines; 50% were atopic, 60% received ICS, and 4–22% were admitted for asthma exacerbation before the study. Stokholm et al. [27] included patients (1–3 years of age) diagnosed with recurrent asthma-like symptoms (troublesome symptoms consisting of cough, wheeze, or dyspnea, with a frequency of five episodes within 6 months or 4 weeks of continuous symptoms or a severe acute episode needing oral corticosteroids [OCS] or hospital admission) lasting at least 3 days. Almost 30% of the sample had atopic dermatitis, 11–15% had sensitization (skin prick test or specific IgE), and 28–44% had history of maternal asthma. Patients recieved concurrent ICS in 82% and montelukast in 60% of the randomized episodes.
Only one study [28] excluded patients who had previously received antibiotic therapy. Interestingly, in that study 69% of preliminary eligible children were excluded because they had received antibiotics within the prior 30 days. The presence of viral or bacterial infections was evaluated in two studies [26, 27]. Stokholm et al. [27] found a pathogenic bacteria in 90 of 135 tested episodes (67%), the most common bacteria identified was Moraxella catarrhalis, and any pathogenic virus was found in 58 of 135 tested episodes (43%) with similar distribution between rhinovirus, respiratory syncytial virus (RSV), and enteroviruses. Koutsoubari et al. [26] found that nasal washes were positive in 26/40 (65%) patients, (18 rhinovirus, 3 adenovirus, 2 Mycoplasma pneumoniae, 2 parainfluenza, 1 RSV, no Chlamydophila pneumoniae) and there were two patients with serologically confirmed mycoplasma infection, one in each group (p = 0.704). All studies used macrolides for intervention in acute presentation but with different starting criteria and settings. Mandhane et al. [28] started the intervention in patients presenting in ED with wheeze exacerbation, Koutsoubari et al. [26] started the intervention in patients with asthma exacerbation suspected by parents and confirmed by study physician, and in Stokholm et al. [28], the study intervention was started in patients who presented an episode defined as three consecutive days of troublesome lung symptoms (only 18% of the included patients had confirmed objective wheezing).
Azithromycin was the most used intervention [27, 28]; only one study [26] used clarithromycin. Details of specific doses used and days of treatment are listed in Table 1. Two studies [27, 28] compared intervention with matching placebo. Koutsoubari et al. [26] compared intervention with standard care (oxygen, SABA, anticholinergics, and SCS according to clinical judgment). The three included studies [26,27,28] used SABA as needed. Montelukast and ICS were also used in some of the studies but not per protocol. Two [27, 28] reported funding information and all were funded by an independent sponsor. No pharmaceutical company funded any of the studies (Table 1).
3.1.1 Risk of Bias of Included Studies
We included three studies, all of which were properly randomized by computer, although one was limited by its open design. We judged unclear risk of bias in two studies because of the lack of a prospective register and apparently incomplete reported outcomes, although it appears not to be a source of high risk of bias. Risk of bias judgement of included studies is summarized in Fig. 2 and detailed in Table 2.
3.2 Primary Outcomes
3.2.1 Hospitalization
Two studies [26, 27] reported data for hospitalization outcome in established acute episodes confirmed by a physician. Stokholm et al. [27] presented mixed data on admission to hospital and the need for oral corticosteroids in the randomized episodes (three in the azithromycin group and two episodes in the placebo group). Koutsoubari et al. [26] reported that none of the included patients required hospitalization during the study period.
3.2.2 Time to Resolution of the Episodes
All studies [26,27,28] reported this outcome in a non-normal distribution of the data, so we were unable to perform a meta-analysis. Two studies [26, 27] showed a statistically significant reduction in the time for episode resolution, using macrolides versus placebo [27] or versus standard care [26]. Stokholm et al. [27] reported that the average number of symptom days was 3.4 in the azithromycin group versus 7.7 days in the placebo group, with a reduction in episode length in 63.3% (95% CI 56.0–69.3; p < 0.0001). Restricted to the first randomized episode, the mean duration was 4.0 days for the azithromycin group and 7.1 days for the placebo group with a reduction in symptom duration of 44% (95% CI 30.9–55.2; p < 0.0001). The reduction effect was better if the treatment was initiated before day 6 of the episode than if initiated ≥6 days after the episode (83% vs 36%; p < 0.0001). Concurrent treatment with ICS or montelukast did not significantly modify the treatment effect (p value for interaction = 0.57 for ICS, and p = 0.69 for montelukast). Similarly, Koutsoubari et al. [26] found a significantly shorter index episode median with clarithromycin compared with the control group, (5 [IQR 1] vs 7.5 [IQR 1], respectively; p < 0.00001); also, a significant reduction in the total duration of periods of loss of control was reported with clarithromycin versus controls (median 0.5 [IQR 3] vs 7 [IQR 5] for control group; p < 0.00001). The total duration of the loss of control episodes was 7.9 fewer days compared with control, independent of steroid administration. In the third study [28], a similar median time for resolution of symptoms was reported between azithromycin and placebo groups (4 days [IQR 3–7] and 4 days [IQR 3–6]; p = 0.49, respectively).
Only Stokholm et al. [27] reported data by atopic condition in this outcome and found that differences in episode duration were unrelated to allergic sensitization to inhalant or food allergens at 6 or 18 months of age (p = 0.173), or to atopic dermatitis presence (p = 0.323). No data was available to perform any planned subgroup analysis for this outcome.
As additional information, two studies [26, 27] reported time to resolution related to microbiological status. In one study [27], the presence of any pathogenic bacteria or virus during the episodes did not significantly modify the treatment effect, with the exception of Haemophilus influenzae, where azithromycin was more effective than placebo (2.7 vs 12.1 days, 77% reduction time [95% CI 58–87.4]; p ≤ 0.0001). In the other study [26], the presence of rhinovirus did not affect any of the outcomes in either groups; and since the presence of confirmed Mycoplasma pneumoniae was very low, no analysis could performed.
3.3 Secondary Outcomes
3.3.1 Time to Next Exacerbation
All studies [26,27,28] reported data for the next asthma or asthma-like exacerbation. We pooled the data from the two studies [27, 28] that reported hazard ratios (HRs) for the second episode of exacerbation or loss of control of symptoms and found no significant difference between preschoolers receiving macrolides versus placebo, HR 0.96 (95% CI 0.71–1.28; I2 = 0%, p = 0.77) (Fig. 3). However, Koutsoubari et al. [26] described that the time to first period of loss of control was significantly prolonged in the macrolide group compared with the control group (67.5 days [IQR 38] vs 26.5 days [IQR 33]; p = 0.003). This study found no difference between atopic and non-atopic patients. Not enough data were available to perform the sub-analysis by atopy or age group in this outcome.
3.3.2 Severity of Symptoms
Only one study [26] reported this outcome. The severity according to diary score was lower in the clarithromycin group than the control group during the index episode (14 [IQR 3] vs 18 [IQR 2]; p = 0.0002). There were no differences noted in this outcome between atopic and non-atopic children.
3.3.3 Short-Acting β-2 Agonists (SABA) Use
Two studies [27, 28] of preschoolers reported this outcome. However, these studies reported these data in different units, precluding the possibility of performing a meta-analysis. Mandhane et al. [28] described no significant difference in the number of days using SABA between azithromycin and placebo groups (5 days [IQR 1.3–7] vs 6 days [IQR 3–10]; p = 0.10, respectively). However, Stokholm et al. [27] reported that treatment with azithromycin reduced the duration of treatment with SABA after intervention: 8.9 days for azithromycin versus 10.1 days for placebo group, a 22.0% reduction (95% CI 7.0–34.6; p = 0.006). No data were available to perform the sub-analysis by atopy condition for this outcome.
3.3.4 Lung Function
Only one study [26] reported lung function data. There was a significant difference in minimum morning peak expiratory flow (PEF) during index asthma exacerbation between clarithromycin and control group (214.7 ± 49.1 L/min vs 275 ± 83.7 L/min, p = 0.032).
No significant difference between atopic and non-atopic subjects was found for this outcome. No data were available to perform the sub-analysis by age group in this study.
3.3.5 Use of Additional Systemic Corticosteroids (SCS)
Only one study include information for use of SCS as a measured outcome; Stokholm et al. [27] reported mixed data of admission to hospital and need for oral corticosteroids in the randomized episodes (three episodes in the azithromycin group and two episodes in the placebo group).
3.3.6 Adverse Events
Two studies [27, 28] reported this outcome; however, we excluded the Mandhane et al. [28] study because data for this outcome were not reported exclusively for recurrent wheezers. The most common AE described was gastrointestinal effects. Stokholm et al. [27] reported two SAEs, one hospitalization for gastroenteritis 4 days after RCT randomization in the azithromycin group and one hospitalization for pneumonia 20 days after randomization in the placebo group (p = 0.99) [29]. No other SAEs were reported in the rest of the studies.
3.3.7 Other Outcomes
We did not find any study that reported data for the following outcomes: antibiotics resistance, duration of staying in the ED/clinic, or ED visit during the first week after index episode.
4 Discussion
To our knowledge, this is the first systematic review of macrolides use exclusively in children with acute asthma or recurrent wheezing exacerbations. Two out of three [27, 28] studies included had an overall low risk of bias. We found that in two of the studies, children treated with macrolides had a significantly shorter time to symptom resolution than controls; and among secondary outcomes, children receiving macrolides had a significant decrease in the severity of symptoms, decreased use of SABA, and improved lung function (PEF) compared with controls. However, the magnitude of these benefits remains to be quantified because it was not possible to perform a meta-analysis. In contrast, no differences in reducing either hospitalization or time to the next exacerbation were found using macrolides or placebo.
Diagnosis of asthma in preschoolers is challenging. Further, the overlapping clinical presentation of viral infection-induced wheezing episodes, bronchiolitis, lower respiratory tract infection (LRTI), and asthma exacerbations is subtler among younger children. In the present review, two out of three studies [27, 28] included preschoolers with a minimum age limit of nearly 24 months; almost all of these included patients had experienced at least three episodes of acute exacerbation episodes (‘recurrent wheezers’), and 27–53% had history of parental asthma; one [28] of these studies also included data from children with bronchiolitis, but only data on preschoolers with recurrent wheeze were used in our analysis. The other study [26] included school children diagnosed with intermittent or mild persistent asthma according GINA guidelines.
A recent Cochrane review [30] evaluated the use of antibiotics in acute asthma exacerbation including three studies in children, only one of them used macrolides [26] and was included in our review. The authors found limited evidence that antibiotics given at the time of an asthma exacerbation may lead to more symptom-free days at follow-up and improved PEFR at 10 days compared with standard care or placebo, but with low confidence in the effect estimates [30]. Another systematic review [31] included RCTs in children with ‘reactive airway diseases’ (asthma, recurrent wheezing, and bronchiolitis) where macrolides were used for chronic and acute exacerbation treatment. They found that macrolides significantly improve lung function, lessen SABA use and recurrent wheezing, and limit growth of Moraxella catarrhalis from nasal swabs. Also, the same group of authors recently published a systematic review [32] of macrolides used exclusively during hospitalization of children with ‘reactive airway disease’ (asthma, recurrent wheezing and bronchiolitis) in which they found no effect on length of stay, oxygen duration, distress, and re-admission rates. However, the focus of these three systematic reviews [30,31,32] are completely different than in our review, where only RCTs of macrolides in children with recurrent wheezing presenting with acute asthma or wheezing exacerbation were included.
In our review of three pediatric studies, we found that there were no significant differences in RR of hospitalization between macrolides and controls. However, for the other primary outcome, in two [26, 27] out of three studies, children on macrolides had significantly shorter times to resolution than those in the control group . Considering only data from preschoolers, one study [27] showed a significant reduction in time to symptom resolution of 44% with macrolides versus controls, but the other study [28] showed no difference between medians for this outcome. Among our secondary outcomes, only one [27] out of two [27, 28] studies showed a significant reduction in number of treated days with SABA. In relation to symptoms and progression to severe events, the use of macrolides significantly reduces the severity of symptoms of the index episode in the only study [26] that reported this outcome. Only one study [26] reported lung function, with significant improvement in PEF using macrolides compared with placebo. There was no difference in the outcomes between atopic or non-atopic patients, sensitized patients, or in patients with a family history of asthma [27].
Four studies were excluded in our review: Fonseca-Aten et al. [24], Volovitz et al. [23], Bacharier et al. [25], and an ongoing study (ClinicalTrials.gov identifier NCT02003911). Briefly, Fonseca-Aten et al. [24] conducted a randomized, placebo-controlled study enrolling 43 patients (4–17 years of age) that consulted in the ED with an acute exacerbation of wheezing. The intervention was clarithromycin 15 mg/kg twice daily for 5 days, and the primary outcome was total cytokine concentrations. Patients using clarithromycin had significantly and persistently lower nasopharyngeal concentrations of TNF-α, IL-1β, and IL-10 in children treated with clarithromycin compared with placebo, highlighting one of the proposed non-antibiotic mechanisms of action of macrolides. However, insufficient clinical data was reported to be included in our systematic review. Volovitz et al. [23], in a quasi-randomized crossover study including 100 children (5 months to 5 years old) with recurrent asthma exacerbations, included three protocols of treatment, one of which used azithromycin without any control group. Therefore, we decided not to include this study in our systematic review. Bacharier et al. [25] enrolled 607 participants and examined azitromycin intervention versus placebo prior to lower respiratory tract symptoms. This intervention was used at home by parents in respiratory tract infection (RTI) episodes as soon as included participants developed the symptoms or signs that parents defined as the child’s usual starting point before development of severe LRTI. These episodes may not have led to an exacerbation episode as we defined in our inclusion criteria, so severity of episodes, rescue therapy use, and time to next RTI episode may be significantly different by definition; therefore, we also decided to exclude this study.
Different mechanisms of action of macrolides have been proposed when considering their use in acute asthma or wheezing exacerbations: antibiotic, anti-inflammatory, and immunomodulator. Macrolides may inhibit the synthesis and/or secretion of proinflammatory cytokines and modulate the immune reponse. It is presumed than this effect occurs via suppression of NF-kB and activator protein 1, affecting the production of cytokines IL-1β, IL-6, IL-8, TNF-α, reducing predominantly neutrofilic inflamation, and reducing the migration and oxidative burst activity in phagocytes [24, 33,34,35,36,37,38,39]. Also, several studies show a role of macrolides in viral infections [38, 40,41,42]. It is well known that asthma exacerbations are triggered frequently by infections [43], predominantly viral infections, but also by atypical bacteria or coinfections [44, 45].
In our review, the presence of viral or bacterial infections was evaluated in two studies [26, 27]. Stokholm et al. [27] found a pathogenic bacteria in 67% (mostly Moraxella catarrhalis) and virus in 43% (rhinovirus, RSV, and enteroviruses). Koutsoubari et al. [26] found 65% of positive tests with a predominance of rhinovirus, and two patients had serologically confirmed mycoplasma infection (one in each group of treatment, p = 0.704). These studies excluded patients with suspected pneumonia or elevated C-reactive protein, suggesting that the action of macrolides in reducing the duration of index episode or severity of symptoms is not only explainable by a misdiagnosis of pneumonia or co-infection with atypical bacteria during the exacerbation. Perhaps the anti-inflammatory action of macrolides had a role.
We did not find any study that reported antibiotic resistance outcomes. However, there is still serious concern about the potential widespread use of macrolides in these patients in the context of a worldwide increase in antibiotic resistance [46, 47]. In our review, we found no differences in AEs in the macrolides group versus controls; the most reported AE was gastrointestinal. A recent Cochrane systematic review [48] evaluating AEs in both adults and children taking macrolide versus placebo for any indication, reported an OR of 2.16 (95% CI 1.56–3.00) for gastrointestinal disorders not otherwise specified, with a number needed to harm (NNTH) of 12.
The present study has some limitations. First, only one [26] out of the three studies included school children with acute asthma exacerbation and this was the only study that performed lung function tests; therefore, we cannot directly apply these findings to schoolchildren with acute asthma exacerbation. The other two studies [27, 28] included preschoolers with a different definition of recurrent wheezing episodes, which represents frequent clinical presentation and is considered an important population of high asthma risk, even though the diagnosis of asthma could not be accurately done at this age. Second, there were differences in the basal treatment of patients included in the studies, suggesting also that we likely included patients with different severity of symptoms, although some studies [27, 28] reported no significant association between some outcomes and steroids (OCS or ICS) or montelukast. Third, the ‘time to next exacerbation’ outcome might be inaccurate because HRs may have been calculated with a different follow-up time. Fourth, microbiological assessment was different between studies, while some used culture for bacteria, others used PCR and serology. Fifth, we were unable to test our pre-established subgroup analysis because there were not enough reported data in specific age groups or severity of the exacerbation or sponsor (two studies [27, 28] were independently funded and the other did not mention funding), or atopic condition (only one study [27] reported data for this item).
5 Conclusion
We found limited evidence that macrolides used in children with acute asthma or wheeze exacerbation may reduce the duration and severity of the episode and may improve lung function. However, no difference in hospitalization, or reduction in the risk of a second episode was found. No study reported data for antibiotic resistance. Therefore, we suggest that a macrolide trial could be considered in children with acute asthma or recurrent wheezing exacerbations. The above cannot be extrapolated to patients with severe chronic symptoms. Future research should be oriented to properly identify and characterize the potential responder’s group, the risk of antibiotic resistance development, intervention with non-antibiotic macrolides, and to compare the use of intermittent ICS versus macrolides.
Change history
13 March 2020
An Online First version of this article was made available online at https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s40272-019-00371-5 on 14 January 2020. An error was subsequently identified in the article, and the following correction should be noted:
References
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2018. http://www.ginasthma.org. Accessed Nov 2019.
Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(17):783–800.
Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3(1):1.
Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018;15(3):348–56.
Garner R, Kohen D. Changes in the prevalence of asthma among Canadian children. Health Rep. 2008;19(2):45–50.
Lougheed MD, Garvey N, Chapman KR, Cicutto L, Dales R, Day AG, et al. The Ontario asthma regional variation study: emergency department visit rates and the relation to hospitalization rates. Chest. 2006;129(4):909–17.
Karaca-Mandic P, Jena AB, Joyce GF, Goldman DP. Out-of-pocket medication costs and use of medications and health care services among children with asthma. JAMA. 2012;307(12):1284–91.
Radhakrishnan DK, Dell SD, Guttmann A, Shariff SZ, Liu K, To T. Trends in the age of diagnosis of childhood asthma. J Allergy Clin Immunol. 2014;134(5):1057–62.
Szefler SJ, Zeiger RS, Haselkorn T, Mink DR, Kamath TV, Fish JE, et al. Economic burden of impairment in children with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2011;107(2):110–9.
Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics. 2009;123(3):e519–25.
Kaiser SV, Huynh T, Bacharier LB, Rosenthal JL, Bakel LA, Parkin PC, et al. Preventing exacerbations in preschoolers with recurrent wheeze: a meta-analysis. Pediatrics. 2016;137(6):e20154496.
Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51(8):868–76.
Beigelman A, Chipps BE, Bacharier LB. Update on the utility of corticosteroids in acute pediatric respiratory disorders. Allergy Asthma Proc. 2015;36(5):332–8.
da Silva Filho LV, Pinto LA, Stein RT. Uso de macrolídeos em doenças pulmonares: controvérsias da literatura recente. J Pediatr (Rio J). 2015;91(6 Suppl 1):S52–60.
Hernando-Sastre V. Macrolide antibiotics in the treatment of asthma. An update. Allergol Immunopathol (Madr). 2010;38(2):92–8.
Poole NM, Shapiro DJ, Fleming-Dutra KE, Hicks LA, Hersh AL, Kronman MP. Antibiotic prescribing for children in United States emergency departments: 2009–2014. Pediatrics. 2019;143(2):e20181056.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
Clinical safety data management: definitions and standards for expedited reporting E2A. ICH Harmonised Tripartite Guideline. 1994. https://database.ich.org/sites/default/files/E2A_Guideline.pdf. Accessed Nov 2019.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Deeks JJ, Altman DG, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey G, Altman D, editors. Systematic reviews in health care: meta-analysis in context. 2nd ed. London: BMJ Publishing Group; 2001. p. 285–312.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009.
Volovitz B, Bilavsky E, Nussinovitch M. Effectiveness of high repeated doses of inhaled budesonide or fluticasone in controlling acute asthma exacerbations in young children. J Asthma. 2008;45(7):561–7.
Fonseca-Aten M, Okada PJ, Bowlware KL, Chavez-Bueno S, Mejias A, Rios AM, et al. Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: a double-blind, randomized, placebo-controlled trial. Ann Allergy Asthma Immunol. 2006;97(4):457–63.
Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses a randomized clinical trial. JAMA. 2015;314(19):2034–44.
Koutsoubari I, Papaevangelou V, Konstantinou GN, Makrinioti H, Xepapadaki P, Kafetzis D, et al. Effect of clarithromycin on acute asthma exacerbations in children: an open randomized study. Pediatr Allergy Immunol. 2012;23(4):385–90.
Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(1):19–26.
Mandhane PJ, Paredes Zambrano de Silbernagel P, Aung YN, Williamson J, Lee BE, Spier S, et al. Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: a placebo-controlled randomized trial. PLoS One. 2017;12(8):e0182411.
Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Pedersen TM, Vinding RK, et al. Supplement to: azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(1):19–26.
Normansell R, Sayer B, Waterson S, Dennett EJ, Del Forno M, Dunleavy A. Antibiotics for exacerbations of asthma. Cochrane Database Syst Rev. 2018. https://doi.org/10.1002/14651858.CD002741.pub2.
Lei W, Tsai M, Liu S, Yeh T. The effects of macrolides in children with reactive airway disease: a systematic review and meta- analysis of randomized controlled trials. Drug Des Dev Ther. 2018;12:3825–45.
Lin C, Yeh T, Liu S, Lin H, Cheng Y, Hung H, et al. Effects of macrolide treatment during the hospitalization of children with childhood wheezing disease : a systematic review and meta-analysis. J Clin Med. 2018;7(11):432.
Steel HC, Theron AJ, Cockeran R, Anderson R, Feldman C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediat Inflamm. 2012;2012:17.
Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics—part 1: biological mechanisms. Respiration. 2011;81(1):67–74.
Beigelman A, Gunsten S, Mikols CL, Vidavsky I, Cannon CL, Brody SL, et al. Azithromycin attenuates airway inflammation in a noninfectious mouse model of allergic asthma. Chest. 2009;136(2):498–506.
Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The immunomodulatory effects of macrolides-A systematic review of the underlying mechanisms. Front Immunol. 2018;9:302.
Beigelman A, Isaacson-schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, et al. Randomized trial to evaluate azithromycin ’ s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135(5):1171–8.
Wong EHC, Porter JD, Edwards MR, Johnston SL. The role of macrolides in asthma: current evidence and future directions. Lancet Respir. 2014;2600(14):1–14.
Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590–615.
Asada M, Yoshida M, Suzuki T, Hatachi Y, Sasaki T, Yasuda H, et al. Macrolide antibiotics inhibit respiratory syncytial virus infection in human airway epithelial cells. Antiviral Res. 2009;83(2):191–200.
Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediat Inflamm. 2012;2012:9.
Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36(3):646–54.
Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, et al. Viruses and bacteria in acute asthma exacerbations—A GA2LEN-DARE systematic review. Allergy. 2011;66(4):458–68.
Hahn DL, Webley W. Chronic Chlamydia pneumoniae lung infection: a neglected explanation for macrolide effects in wheezing and asthma? Lancet Respir. 2016;4(3):e8.
Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10(1):67–73.
Cohen RT, Pelton SI. Individual benefit vs societal effect of antibiotic prescribing for preschool children with recurrent wheeze. JAMA. 2015;314(19):2027–9.
Fleming-Dutra KE, Friedman CR, Hicks LA. Early azithromycin treatment to prevent severe lower respiratory tract illnesses in children. JAMA. 2016;315(19):2121–2.
Hansen MP, Scott AM, McCullough A, Thorning S, Aronson JK, Beller EM, et al. Adverse events in people taking macrolide antibiotics versus placebo for any indication. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD011825.pub2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Dr. Castro-Rodriguez’s contribution was funded in part by CONICYT PIA/ANILLO (Grant no. 170925013) from the Chilean Comisión Nacional de Investigación Científica y Tecnológica (CONICYT).
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pincheira, M.A., Bacharier, L.B. & Castro-Rodriguez, J.A. Efficacy of Macrolides on Acute Asthma or Wheezing Exacerbations in Children with Recurrent Wheezing: A Systematic Review and Meta-analysis. Pediatr Drugs 22, 217–228 (2020). https://doi.org/10.1007/s40272-019-00371-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-019-00371-5