Abstract
Background
This study summarized the available randomized controlled trials (RCTs) to assess the efficacy and safety of macrolides on pathogens, lung function, laboratory parameters, and safety in children with bronchiectasis.
Methods
PubMed, EMBASE, and the Cochrane Library were searched for available papers published up to June 2021. The outcomes were the pathogens, adverse events (AEs), and the forced expiratory volume in one second (FEV1%) predicted.
Results
Seven RCTs (633 participants) were included. The long-term use of macrolides reduced the risk of the presence of Moraxella catarrhalis (RR = 0.67, 95% CI: 0.30–1.50, P = 0.001; I2 = 0.0%, Pheterogeneity = 0.433), but not Haemophilus influenza (RR = 0.19, 95% CI: 0.08–0.49, P = 0.333; I2 = 57.0%, Pheterogeneity = 0.040), Streptococcus pneumonia (RR = 0.91, 95% CI: 0.61–1.35, P = 0.635; I2 = 0.0%, Pheterogeneity = 0.515), Staphylococcus aureus (RR = 1.01, 95% CI: 0.36–2.84, P = 0.986; I2 = 61.9%, Pheterogeneity = 0.033), and any pathogens present (RR = 0.61, 95% CI: 0.29–1.29, P = 0.195; I2 = 80.3%, Pheterogeneity = 0.006). Long-term macrolides had no effect on FEV1% predicted (WMD = 2.61, 95% CI: –1.31, 6.53, P = 0.192; I2 = 0.0%, Pheterogeneity = 0.896). Long-term macrolides did not increase the risk of AEs or serious AEs.
Conclusion
Macrolides do not significantly reduce the risk of pathogens present (except for Moraxella catarrhalis) or increase FEV1% predicted among children with bronchiectasis. Moreover, macrolides were not associated with AEs. Considering the limitations of the meta-analysis, further larger-scale RCTs are needed to confirm the findings.
Impact
-
Macrolides do not significantly reduce the risk of pathogens present (except for Moraxella catarrhalis) among children with bronchiectasis.
-
Macrolides do not significantly increase FEV1% predicted among children with bronchiectasis.
-
This meta-analysis reports on the efficacy and safety of macrolides in the treatment of children with bronchiectasis, providing evidence for the management of children with bronchiectasis.
-
This meta-analysis does not support the use of macrolides in the management of children with bronchiectasis unless the presence of Moraxella catarrhalis is provenor suspected.
Similar content being viewed by others
Introduction
Bronchiectasis is a chronic, often progressive suppurative lung disease characterized by irreversibly dilated bronchi and chronic or recurrent bronchial infection and inflammation; it may be focal, where a single lobe or segment is involved, or diffuse with the involvement of both lungs.1,2,3,4 The exact epidemiology of bronchiectasis is unknown because many cases are misdiagnosed because of non-specific symptoms.3 Among children, the incidence may be higher in indigenous or socioeconomically disadvantaged groups.5 The incidence of bronchiectasis is estimated at 3.7 per 100,000 children in New Zealand6 but 202 per 100,000 children in an indigenous population in Canada.7 Exacerbations in children require treatments, cause parental anxiety and stress, and affect the quality of life of the whole family.8 When severe (i.e., requiring hospital admission), the exacerbations can negatively affect lung function in adolescence and adulthood.9,10 The complications of bronchiectasis include chronic respiratory failure, thoracic infection, cor pulmonale, hemoptysis, lung cancer, and vascular diseases.3,11
Macrolide antibiotics are antibacterial agents that possess anti-inflammatory and immunomodulatory properties.12 Macrolide mechanism of action is not strictly bacteriocidal and likely includes anti-inflammatory, immunomodulatory, and mucus-decreasing effects and inhibition of bacterial quorum sensing and toxin production.13,14 Because of these properties, a macrolide maintenance treatment might effectively prevent exacerbations inpatients with non-cystic fibrosis (CF) bronchiectasis. Indeed, macrolide antibiotics have been used to reduce the exacerbations of non-CF bronchiectasis.13 Macrolide antibiotics reach a high plasma concentration, have a long half-life, and display a broad antimicrobial spectrum.14 According to the guidelines, macrolides can be given to select patients with ≥3 exacerbations or ≥2 hospitalizations within the past year, with stratification based on Pseudomonas aeruginosa infection.15,16,17 On the other hand, the Thoracic Society of Australia and New Zealand does not support the use of long-term macrolides except in selected cases.18
A meta-analysis of three randomized controlled trials (RCTs) suggested that macrolides are effective in adult patients with bronchiectasis.19 A meta-analysis of 10 trials (including three in children) reached a similar conclusion.20 A Cochrane review that included 11 studies in adults and two studies in children supports the use of macrolides for bronchiectasis but highlights the low quality of the available evidence and the lack of data about adverse events (AEs).21 Still, the efficacy and safety of macrolides in the treatment of children with bronchiectasis remain inconsistent. In addition, much evidence is from observational studies. Therefore, this meta-analysis aimed to summarize the available RCTs to assess the efficacy and safety of macrolides on pathogens, lung function, laboratory parameters, and safety in children with bronchiectasis.
Methods
Literature search
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline 2020.22,23 The study was elaborated based on the PICOS principle.24 PubMed, EMBASE, and the Cochrane Library were searched for available papers published up to June 2021 using the MeSH terms of “Bronchiectasis”, “Child”, “Macrolides”, “Azithromycin”, “Clarithromycin”, “Erythromycin”, “Roxithromycin”, “Spiramycin”, “telithromycin”, “Troleandomycin”, “Josamycin”, and “Oleandomycin”, as well as relevant keywords, followed by screening based on inclusion and exclusion criteria. The literature search process was performed independently by two investigators. This included the analysis of titles/abstracts followed by the full texts. Discrepancies were ruled by a third investigator.
Eligibility criteria
The inclusion criteria were (1) patients: children with bronchiectasis, (2) interventions: macrolides, (3) comparison: placebo or another drug, (4) study type: RCTs, and (5) outcome: compared the efficacy and safety of macrolides. The exclusion criteria were (1) conference abstract, case report, meta-analysis, review, animal study, or protocol, (2) full text not available in English, (3) full text cannot be obtained, (4) no data available, or (5) different reports for the same study (in which case only the most recent was included).
Data extraction
Data study characteristics (names of the first author, publication year, and country), characteristics of the patients (number of patients, age, and sex), treatment regimens, duration, and outcomes (pathogens, AEs, and FEV1% predicted) were extracted by two investigators using a standardized form. Discrepancies were solved by discussion until a consensus was reached.
Quality assessment
The RCTs were evaluated according to the Cochrane risk bias tool (ROB2).25
Statistical analysis
The incidences of pathogens and AEs were treated as dichotomous variables; they were expressed as risk ratio (RR) with 95% confidence intervals (CIs) and were presented using forest plots. The mean different changes in FEV1% predicted were treated as continuous variables and were expressed as weighted mean difference (WMD) with 95% CI for each study. Cochran’s Q statistic P < 0.10 indicated evidence of heterogeneity.26 When significant heterogeneity (P < 0.10) was observed, the random-effects model was used to combine the effect sizes of the included studies; otherwise, the fixed-effects model was adopted.27 In addition, sensitivity analyses were performed to identify the effects of individual studies on the pooled results and test the reliability of the results. Sensitivity analyses for result robustness were performed by sequentially excluding each study in turn. All analyses were performed using STATA SE 14.0 (StataCorp). P < 0.05 was considered statistically significant.
Results
Identification of the eligible RCTs
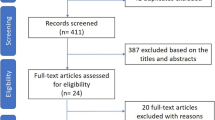
Figure 1 presents the RCT selection process. The initial search yielded 224 records, but 174 were removed before the screening. Then, 50 records were screened, and 39 were excluded. Among the 11 reports sought for retrieval, two could not be retrieved. Then, nine reports were assessed for eligibility; one was excluded because of inadequate comparison for the present meta-analysis, and one was excluded because of the lack of usable data. Therefore, seven RCTs were included in the present meta-analysis.
Study characteristics and quality assessment
Table 1 presents the characteristics of the studies. The seven studies enrolled 633 participants. Four were from New Zealand/Australia,28,29,30,31 one from Korea,32 one from South Africa,33 and one from Turkey.34 Six were double-blind RCTs.28,29,30,31,32,33 Three RCTs had a low risk of bias for all ROB2 items,28,29,32 while the remaining four studies had some risk of bias for at least one item30,31,33,34 (Table 2).
Efficacy according to the pathogens
As shown in Fig. 2, long-term macrolides reduced the risk of the sputum presence of Moraxella catarrhalis (RR = 0.67, 95% CI: 0.30–1.50, P = 0.001; I2 = 0.0%, Pheterogeneity = 0.433), but not for Haemophilus influenza (RR = 0.19, 95% CI: 0.08–0.49, P = 0.333; I2 = 57.0%, Pheterogeneity = 0.040), Streptococcus pneumonia (RR = 0.91, 95% CI: 0.61–1.35, P = 0.635; I2 = 0.0%, Pheterogeneity = 0.515), Staphylococcus aureus (RR = 1.01, 95% CI: 0.36–2.84, P = 0.986; I2 = 61.9%, Pheterogeneity = 0.033), and any pathogens present (RR = 0.61, 95% CI: 0.29–1.29, P = 0.195; I2 = 80.3%, Pheterogeneity = 0.006).
The gray box indicates the point estimate of the study results. A horizontal line represents the 95% confidence interval of the study results, and each end represents the boundary of the confidence interval. The diamond represents the point estimate and confidence interval. The larger the study, the smaller the horizontal line and the larger the gray box.
FEV1% predicted
Figure 3 shows that long-term macrolides had no effect on the FEV1% predicted (WMD = 2.61, 95% CI: –1.31, 6.53, P = 0.192; I2 = 0.0%, Pheterogeneity = 0.896).
The gray box indicates the point estimate of the study results. A horizontal line represents the 95% confidence interval of the study results, and each end represents the boundary of the confidence interval. The diamond represents the point estimate and confidence interval. The larger the study, the smaller the horizontal line and the larger the gray box.
AEs
Figure 4a showed that long-term macrolides were not associated with AEs (all 95% CIs include 1). The same can be seen with serious AEs (Fig. 4b).
Other outcomes
The outcomes presented by only one study and that could not be summarized are presented in Fig. 5. Long-term macrolides decreased the sputum purulence score (WMD = –0.78, 95% CI: –1.32, –0.24)32 and increased the quality of life (WMD = 0.90, 95% CI: 0.29, 1.51).29 Other outcomes like the sputum leukocyte score, ΔFEV1max %, Bhalla score, exacerbations, FVC% predicted, and white blood cell count were not changed by long-term macrolides.
Sensitivity analysis
The sensitivity analysis of the effect of long-term macrolides on pathogens showed that the results were robust, and the exclusion of any study did not influence the results (Fig. 6).
Discussion
The reports about the efficacy and safety of macrolides in the treatment of children with bronchiectasis remain inconsistent. This study aimed to summarize the available RCTs to assess the efficacy and safety of macrolides on pathogens, lung function, laboratory parameters, and safety in children with bronchiectasis. The results suggest that macrolides do not significantly reduce the risk of pathogens present in sputum (except for Moraxella catarrhalis) or increase the FEV1% predicted among children with bronchiectasis. Moreover, macrolides were not associated with AEs.
The available guidelines broadly recommend long-term macrolides to any patient with ≥3 exacerbations or ≥2 hospitalizations within the past year, although some guidelines make additional stratification based on the pathogens.15,16,17,18 Still, the level of evidence in these guidelines is often moderate. Furthermore, studies specifically on children are rare. Indeed, although previous meta-analyses suggested that macrolides are effective in patients with bronchiectasis, only five RCTs in children were identified and included.19,20,21 Furthermore, a Cochrane review highlights the low quality of the available evidence and the lack of data about AEs.21 Previous meta-analyses did not examine the pathogens. In the present meta-analysis of seven RCTs, macrolides did not significantly reduce the risk of pathogens present (except for Moraxella catarrhalis). Therefore, long-term macrolides could be of limited efficacy in children with bronchiectasis. Still, no study presented data about Pseudomonas aeruginosa, which is associated with significant morbidity and mortality in patients with lung infections due to this pathogen.15,16,17 Therefore, the results of this meta-analysis must be taken with caution and highlight the lack of data about the efficacy of long-term macrolides in children with bronchiectasis.
Decreased pulmonary function in adulthood is a major concern for children with bronchiectasis and exacerbations.3,11 The present meta-analysis observed no improvement in FEV1% predicted or FVC, but the results must be taken with caution since the pulmonary measurements in the various studies were performed during the treatment period, and no long-term data were available regarding the changes in pulmonary function from childhood to adulthood. Still, there are some reports of decreased FEV1% later in life in children who had bronchiectasis,35,36 but additional studies will be necessary to quantify the risk and whether macrolides can slow down the process.
AEs might be a concern with the long-term use of antibiotics. Still, this study indicated no significant increase in AEs and serious AEs using long-term macrolides. Still, the examined AEs varied among the RCTs, and reporting was not uniform. Antibiotic resistance is another major public health concern with long-term antibiotics, but no data could be summarized about that. Future studies should quantify this risk, especially in the global context of the rational use of antibiotics.
Bronchiectasis significantly affects the quality of life of the patients and their families.3 Only one of the included studies examined the quality of life and reported improvements with the long-term use of macrolides. This outcome should be included in future studies.
This meta-analysis has limitations. First, there was substantial heterogeneity among the included studies, especially in the studies reporting any pathogens present. There were important differences among the RCTs in terms of antibiotics, dosage, and comorbidities. Second, some of the included studies had a relatively small sample size, which would overestimate the treatment effect compared with larger trials. Third, the duration of follow-up varied greatly among the included studies, at 3–24 months. Fourth, the data available from the included studies did not allow analyses on some AEs, such as QT prolongation.
In conclusion, long-term macrolides do not significantly reduce the risk of pathogens present (except for Moraxella catarrhalis) or increase the FEV1% predicted among children with bronchiectasis. Moreover, macrolides were not associated with AEs. Considering the limitations of the meta-analysis, further larger-scale RCTs are needed to confirm the findings.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Goyal, V., Grimwood, K., Marchant, J., Masters, I. B. & Chang, A. B. Pediatric bronchiectasis: no longer an orphan disease. Pediatr. Pulmonol. 51, 450–469 (2016).
McCallum, G. B. & Binks, M. J. The epidemiology of chronic suppurative lung disease and bronchiectasis in children and adolescents. Front. Pediatr. 5, 27 (2017).
Pasteur, M. C., Bilton, D. & Hill, A. T. British Thoracic Society Non-CF Bronchiectasis Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 65(Suppl 1), i1–i58 (2010).
McShane, P. J., Naureckas, E. T., Tino, G. & Strek, M. E. Non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 188, 647–656 (2013).
El Boustany, P. et al. A review of non-cystic fibrosis bronchiectasis in children with a focus on the role of long-term treatment with macrolides. Pediatr. Pulmonol. 54, 487–496 (2019).
Twiss, J., Metcalfe, R., Edwards, E. & Byrnes, C. New Zealand national incidence of bronchiectasis “too high” for a developed country. Arch. Dis. Child 90, 737–740 (2005).
Das, L. & Kovesi, T. A. Bronchiectasis in children from Qikiqtani (Baffin) Region, Nunavut, Canada. Ann. Am. Thorac. Soc. 12, 96–100 (2015).
Kapur, N., Masters, I. B., Newcombe, P. & Chang, A. B. The burden of disease in pediatric non-cystic fibrosis bronchiectasis. Chest 141, 1018–1024 (2012).
Munro, K. A. et al. Do New Zealand children with non-cystic fibrosis bronchiectasis show disease progression? Pediatr. Pulmonol. 46, 131–138 (2011).
Kapur, N., Masters, I. B. & Chang, A. B. Longitudinal growth and lung function in pediatric non-cystic fibrosis bronchiectasis: what influences lung function stability? Chest 138, 158–164 (2010).
Hill, A. T. et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 74, 1–69 (2019).
Khoo, J. K., Venning, V., Wong, C. & Jayaram, L. Bronchiectasis in the last five years: new developments. J. Clin. Med. 5, 115 (2016).
Haworth, C. S., Bilton, D. & Elborn, J. S. Long-term macrolide maintenance therapy in non-CF bronchiectasis: evidence and questions. Respir. Med. 108, 1397–1408 (2014).
Steel, H. C., Theron, A. J., Cockeran, R., Anderson, R. & Feldman, C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediators Inflamm. 2012, 584262 (2012).
Smith, D. et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. Thorax 75, 370–404 (2020).
Polverino, E. et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 50, 1700629 (2017).
Chang, A. B. et al. European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur. Respir. J. 58, 2002990 (2021).
Chang, A. B. et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Med. J. Aust. 202, 21–23 (2015).
Chalmers, J. D. et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir. Med. 7, 845–854 (2019).
Wang, D., Fu, W. & Dai, J. Meta-analysis of macrolide maintenance therapy for prevention of disease exacerbations in patients with noncystic fibrosis bronchiectasis. Medicine (Baltimore) 98, e15285 (2019).
Kelly, C. et al. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 3, CD012406 (2018).
PRISMA 2020. J. Clin. Epidemiol. 134, A5–A6 (2021).
Swartz, M. K. PRISMA 2020: an Update. J. Pediatr. Health Care 35, 351 (2021).
Aslam, S. & Emmanuel, P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J. Sex. Transm. Dis. AIDS 31, 47–50 (2010).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Higgins, J. P. T. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (Cochrane Collaboration, London, 2020).
Goyal, V. et al. Amoxicillin-clavulanate versus azithromycin for respiratory exacerbations in children with bronchiectasis (BEST-2): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet 392, 1197–1206 (2018).
Goyal, V. et al. Efficacy of oral amoxicillin-clavulanate or azithromycin for non-severe respiratory exacerbations in children with bronchiectasis (BEST-1): a multicentre, three-arm, double-blind, randomised placebo-controlled trial. Lancet Respir. Med. 7, 791–801 (2019).
Hare, K. M. et al. Nasopharyngeal carriage and macrolide resistance in Indigenous children with bronchiectasis randomized to long-term azithromycin or placebo. Eur. J. Clin. Microbiol. Infect. Dis. 34, 2275–2285 (2015).
Valery, P. C. et al. Long-term azithromycin for Indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double-blind, randomised controlled trial. lancet Respir. Med. 1, 610–620 (2013).
Koh, Y. Y., Lee, M. H., Sun, Y. H., Sung, K. W. & Chae, J. H. Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double-blind, placebo-controlled study. Eur. Respir. J. 10, 994–999 (1997).
Masekela, R. et al. Lack of efficacy of an immunomodulatory macrolide in childhood HIV related bronchiectasis: a randomised, placebo-controlled trial. J. Antivir. Antiretrovir. 5, 044–049 (2013).
Yalçin, E. et al. Effects of claritromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. J. Clin. Pharm. Ther. 31, 49–55 (2006).
Ramsey, K. A. & Schultz, A. Monitoring disease progression in childhood bronchiectasis. Front. Pediatr. 10, 1010016 (2022).
McDonnell, M. J., Ward, C., Lordan, J. L. & Rutherford, R. M. Non-cystic fibrosis bronchiectasis. QJM 106, 709–715 (2013).
Author information
Authors and Affiliations
Contributions
Conceptualization: G.S. Data curation: M.P., W.L. Formal analysis: M.S., B.Z. Methodology: G.S., S.Y. Writing—original draft: G.S., Y.Z. Writing—review and editing: H.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, G., Zhang, Y., Yu, S. et al. Efficacy and safety of macrolides in the treatment of children with bronchiectasis: a meta-analysis. Pediatr Res 94, 1600–1608 (2023). https://doi.org/10.1038/s41390-023-02591-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02591-5
- Springer Nature America, Inc.