Abstract
Oral tafamidis (Vyndaqel®) is the first disease-modifying drug approved to delay the progression of peripheral neurological impairment in adults with early-stage transthyretin (TTR) amyloidosis (i.e. stage 1 symptomatic polyneuropathy), with recent treatment guidelines recommending it as first-line treatment. Tafamidis acts by kinetically stabilizing the TTR tetramer, thereby inhibiting amyloidogenesis. In adults with early-stage TTR familial amyloid polyneuropathy, tafamidis 20 mg once daily was superior to placebo in the efficacy evaluable population with regard to neurological deterioration and health-related quality-of-life. Long-term extension studies have demonstrated that the beneficial effects of tafamidis on these endpoints are maintained for up to 5.5 years, and studies in the clinical practice setting found that a marked proportion of tafamidis-treated patients achieved neurological stability. Tafamidis is generally well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adis evaluation of tafamidis in transthyretin amyloidosis
First disease-modifying drug to be approved |
New standard of first-line care in stage 1 disease |
Acts by stabilizing the transthyretin tetramers, thereby inhibiting monomer formation, misfolding and amyloidogenesis |
Slows deterioration of neurological function |
Maintains health-related quality-of-life |
Generally well tolerated |
What is the rationale for developing tafamidis?
Transthyretin (TTR)-related amyloidosis is a rare, but increasingly recognized, progressive, protein-misfolding disorder. It is characterized by endoneurial amyloid deposits leading to sensory, motor and autonomic neuropathy [1–4]. The most common form of TTR amyloidosis is inherited [familial amyloid polyneuropathy (FAP)] and is caused by point mutations in the TTR gene [3, 5, 6]. Approximately 120 different single or double point mutations have been identified, the most common of which is the V30M mutation [3, 5, 6]. TTR amyloidosis may also result from the deposition of wild-type TTR amyloid, which typically occurs in older individuals [3, 5, 6]. TTR-FAP is generally described based on whether the predominant clinical manifestation is neuropathy or cardiomyopathy; however, in clinical practice, a wide range of phenotypes are observed [3, 5, 6]. The condition is associated with substantial morbidity and mortality and the estimate of median survival from diagnosis in patients presenting predominantly with neuropathy is 5–15 years, but is considerably shorter if cardiomyopathy is dominant (2.5–4 years) [6].
Estimates of the prevalence of TTR-FAP vary, but TTR amyloidosis with polyneuropathy is estimated to affect ≈10,000 patients worldwide, or about one per million individuals [6]. The age at onset varies between different populations, but may occur at any time from early adulthood [5, 6]. It is likely that the condition is underdiagnosed because of the phenotypic variability and the need for biopsy testing to confirm the diagnosis [1, 4–6].
Until recently, the only treatment available to halt the progression of TTR-FAP was liver transplantation, which replaces the hepatic source of mutant TTR with less amyloidogenic wild-type TTR from the donor organ [1, 3, 4]. However, liver transplantation rarely leads to improved nerve function, needs to be performed early in the course of the disease, and the need for chronic immunosuppression may adversely affect clinical outcomes [2, 4–7]. Furthermore, liver transplantation does not prevent the development of the cardiac arrhythmia, ocular complications and CNS symptoms associated with amyloidosis [2, 4–7]. Liver transplantation is also limited by its high associated costs and the scarcity of available organs. The prognosis of patients following transplant are influenced by many factors, with long-term survival after liver transplantation being better in patients with early-onset disease (i.e. at <50 years of age), and good nutritional status and relatively short duration of disease at the time of intervention [8]. The TTR variant also influences the prognosis, with 10-year survival rates ranging from excellent to poor depending on the mutation, with large differences in survival also being shown between mutations with similar phenotypes [9].
Due to a greater understanding of the pathogenic processes and molecular biology associated with TTR-related amyloidosis, recent research has focused on developing molecules that can stabilize the TTR tetramer, thereby slowing tetramer dissociation and suppressing the disease [3]. Of these, tafamidis (Vyndaqel®) was selected for clinical development based on its favourable bioavailability and tolerability profile. Unlike certain NSAIDs (e.g. diflunisal) that act as non-selective TTR kinetic stabilizers, tafamidis is selective TTR stabilizer and, therefore, is not associated with the typical gastrointestinal, renal or cardiac adverse effects associated with chronic NSAID use [3]. Tafamidis is the first disease-modifying drug to be approved in the EU (and other countries, including Japan and Argentina) for the treatment of early-stage TTR amyloidosis in adults [3].
How does tafamidis work?
Tafamidis is an orally available benzoxazole derivative that binds to the two thyroxine binding sites on the TTR tetramer with high affinity and selectivity, thereby kinetically stabilizing the complex, which inhibits monomer formation, misfolding and amyloidogenesis [3, 10]. In clinical trials in patients with TTR-FAP, tafamidis stabilized TTR tetramers and slowed peripheral neurological impairment [11–13].
For whom is tafamidis indicated?
Tafamidis is indicated to delay the progression of peripheral neurological impairment in adults with early stage TTR (i.e. stage 1 symptomatic polyneuropathy) [14]. Table 1 provides a summary of the prescribing information for tafamidis in the EU [14].
What is the clinical efficacy of tafamidis?
Double-blind trial in patients with V30M mutation
The efficacy of tafamidis in adults with early-stage V30M TTR-FAP was demonstrated in the double-blind Fx-005 trial [11], in which patients were randomized to receive tafamidis 20 mg once daily (n = 64) or placebo (n = 61) for 18 months [11]. The co-primary endpoints were the between-group differences (BGDs) in the proportion of patients with a Neuropathy Impairment Score-Lower Limbs (NIS-LL) response (defined as a <2 point increase from baseline in NIS-LL score) and the least squares mean (LSM) change from baseline in Norfolk Quality-of-Life Diabetic-Neuropathy Questionnaire total scores (TQOL-DN). For the NIS-LL and TQOL-DN instruments, an increase in score indicates worsening impairment and decreased HR-QOL, respectively
After 18 months of treatment in the intent-to-treat (ITT) population, the proportion of patients with an NIS-LL response was numerically higher with tafamidis than with placebo, but the BGD was not statistically significant (Table 2). Likewise, the change from baseline in TQOL-DN scores was numerically, but not statistically, better with tafamidis than with placebo (Table 2) [11]. In a within-treatment comparison of the TQOL-DN in the ITT population, the change from baseline was not significant in the tafamidis group, whereas the total score deteriorated significantly from baseline in the placebo group (Table 2) [11]. The lack of statistically significant BGDs with regard to the co-primary endpoints was likely due to an increase in the number of patients who left the study to receive a liver transplant (reducing the power of the Fx-005 trial to detect BGDs in these endpoints).
In the efficacy evaluable (EE) population, significantly more tafamidis recipients than placebo recipients were NIS-LL responders, and HR-QOL (as assessed by TQOL-DN) was generally maintained with tafamidis, but deteriorated with placebo (Table 2) [11]. Of note, the analyses in the EE population were prespecified, because of the likelihood that many patients enrolled in the trial would undergo liver transplantation.
Tafamidis was favoured over placebo with regard to BGDs in changes from baseline to month 18 for all other pre-planned secondary disease progression endpoints (based on repeated measures analyses in the ITT population) [11]. Neurological function deteriorated to a significantly lesser extent with tafamidis than with placebo (increase in NIS-LL scores 2.81 vs. 5.83 points; p = 0.027), which was primarily due to more muscle weakness in the placebo group (p = 0.013). Nerve function (as measured by small-fibre function) was preserved in the tafamidis group, but deteriorated in the placebo group (p = 0.005), and nutritional status [as assessed by modified body mass index (mBMI)] improved with tafamidis, but deteriorated with placebo (p < 0.0001). Furthermore, after 18 months’ treatment, TTR was stabilized in 98% of patients in the tafamidis group but no patients in the placebo group (p < 0.0001).
Post-hoc analyses in the ITT population provide further support for the efficacy of tafamidis 20 mg/day in the treatment of TTR-FAP [15]. When adjusted for baseline NIS-LL disease severity, the change from baseline to month 18 in NIS-LL was significantly lower with tafamidis than with placebo (p < 0.05).
Extension studies
The beneficial effects of tafamidis are maintained during long-term treatment [12]. At the start of the Fx-006 trial (a 12-month open-label extension of Fx-005), 38 patients continued once-daily treatment with tafamidis 20 mg (tafamidis-tafamidis group) and 33 were switched from placebo to once-daily tafamidis 20 mg (placebo-tafamidis group).
Comparing the last 12 months of treatment with tafamidis (Fx-006) with the first 18 months of treatment (Fx-005), the tafamidis-tafamidis group did not show significant differences in the mean rate of change of NIS-LL response (0.8–0.11 points/month) or TQOL-DN (−0.03 to 0.25 points/month) [12]. Mean mBMI values declined during Fx-006, but remained above pretreatment levels [12].
Relative to treatment with placebo, the switch to tafamidis significantly reduced the mean rate of neurological deterioration as assessed by NIS-LL scores (0.34 vs. 0.16 points/month; p = 0.01), and significantly improved mean rates of change of TQOL-DN (0.61 vs. −0.16 points/month; p < 0.001) and mBMI (−1.77 vs. 5.99 units/month; p < 0.0001) [12].
Early initiation of tafamidis is associated with better long-term outcomes in terms of neurological function than commencing treatment later in the disease process [12]. The mean change from baseline in NIS-LL was significantly better in the tafamidis-tafamidis group after 30 months of treatment than in placebo-tafamidis group after 12 months of tafamidis treatment (3.0 vs 6.8 points; p = 0.04) [12].
At the end of Fx-006, TTR tetramers stabilized in the vast majority of patients in the tafamidis-tafamidis and placebo-tafamidis groups (94.1 and 93.3% patients), and there was no evidence of the development of tolerance to the TTR-stabilizing effects of tafamidis [12].
The importance of early versus delayed tafamidis treatment was also demonstrated in the longest prospective evaluation of tafamidis treatment available to date [16]. In this analysis, 71 patients who participated in the Fx-005 and Fx-006 trials continued to receive tafamidis for up to 5.5 years. Early treatment (i.e. >1 dose during Fx-005 or after switching from placebo in Fx-006) resulted in sustained delays in neurological progression (mean changes from baseline in NIS-LL 0.6, 1.3, 2.2, 3.5 and 5.3 points after 1, 2, 3.5, 4.5 and 5.5 years, respectively) and a nonsignificant decline in nutritional status (mean changes in mBMI 28.5, 28.2, 16.3, −15 and −7.8 kg/m2 × g/L after 1, 2, 3.5, 4.5 and 5.5 years) [16].
According to a post-hoc analysis of the Fx-005 and Fx-006 trials, mBMI is a relatively good indicator of disease progression in TTR-FAP patients [17]. At the completion of Fx-006, mBMI had improved by 16 and 28 kg/m2 × g/L in the tafamidis-tafamidis and placebo-tafamidis groups, respectively. The most marked increase in mBMI occurred in patients with low BMI at study entry.
Non-comparative trials
Tafamidis 20 mg once daily stabilized TTR tetramers in 95% of patients at 6 weeks (primary endpoint) and 100% of evaluable patients at months 6 and 12 in a two-part, 12-month, phase 2 noncomparative study in 19 TTR-FAP patients with various non-V30M mutations [13]. Throughout 12 months’ treatment, there was some degree of worsening of neurological function, but HR-QOL, mBMI, cardiac biomarker N-terminal pro-hormone brain natriuretic peptide, and echocardiographic parameters did not worsen to a clinically relevant extent.
Once-daily tafamidis 20 mg also stabilized TTR in an open-label phase 3 trial in Japanese patients with V30M or non-V30M mutations (n = 9 and 1, respectively) [18]. At week 8, all ten patients achieved TTR stabilization (primary endpoint), with nine and eight patients maintaining TTR stabilization at weeks 52 and 78, respectively. Tafamidis was also associated with a slowing of neurological progression (80, 60 and 40% of patients were NIS-LL responders at weeks 26, 52 and 78, with corresponding mean changes from baseline in NIS-LL scores of 2.1, 3.6 and 3.3 points). At weeks 26, 52 and 78, mean changes from baseline in TQOL-DN scores (11.8, 12.5 and 10.8 points) and mBMI (26.6, 64.9 and 53.7 units) indicated that tafamidis stabilized HR-QOL and nutritional status, respectively [18].
Studies in the clinical practice setting
Tafamidis 20 mg once daily was effective in the long-term treatment of TTR-FAP in the real-world setting [19–22]. In a large, single-centre analysis in Portugal (where the condition is endemic), 104 patients with TTR-FAP (mean disease duration 32 months) completed 24 months of tafamidis treatment, with 69 and 57% of these patients being responders at 1 and 2 years, respectively [19]. Moreover, tafamidis improved survival relative to natural TTR-FAP progression according to an analysis of long-term survival based on a Portuguese database of 3019 patients with the V30M mutation over the past 100 years (n = 371, 981 and 1667 for the tafamidis, liver transplantation and natural disease groups, respectively) [22]. Median survival was not reached in the tafamidis group, who had a 5-year survival rate of 99.70%. Relative to natural disease progression, survival was increased with both tafamidis and liver transplantation, as shown by hazard ratios of 0.04 (95% CI 0.02–0.12) and 0.15 (95% CI 0.12–0.19), respectively [22].
A multicenter observational study in Italy included 61 patients with TTR-FAP (28% with the V30M mutation) who were treated with tafamidis for 3 years [20]. Of note, 72% of patients had stage 1 disease (the approved indication for tafamidis in the EU [14]), with 20 and 8% having stage 2 and 3 of the disease, respectively [20]. During the first 6 months of treatment, neurological function worsened, but progression slowed significantly after this timepoint. After 3 years’ treatment, irrespective of mutation type and disease stage, one-third of patients had neurological stability, as defined by the NIS-LL score [20]. Among 34 patients with cardiac involvement at baseline, 15% had progression of cardiac disease, and new onset of cardiomyopathy occurred in 30% of patients. Overall, functional progression of disease while receiving tafamidis continued in 43% of patients. With regard to mBMI at baseline, higher mBMI values were associated with better preservation of neurological function than lower values [20].
In a retrospective analysis of data from the global disease registry (the Transthyretin Amyloidosis Outcomes Survey), 257 patients treated with tafamidis for 24 months displayed less neurological disease progression and greater improvement in HR-QOL than matched controls (significance of BGDs not reported) [21].
What is the tolerability profile of tafamidis?
Oral tafamidis 20 mg once daily is generally well tolerated, based on results from clinical trials [11–13, 16, 18] and the clinical practice setting [19, 20]. Among tafamidis and placebo groups in the Fx-005 trial, 92 and 97% of patients, respectively, experienced ≥1 treatment-emergent adverse events (TEAEs) throughout 18 months’ treatment, with most being mild to moderate in severity [11]. In the corresponding groups, 6 and 5% of patients discontinued treatment due to a TEAE.
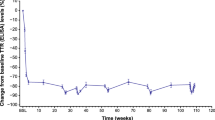
TEAEs that occurred in ≥5% of tafamidis-treated patients and with higher (≥2%) incidence than in the placebo group included diarrhoea, urinary tract infection, pain in extremity, upper abdominal pain, myalgia, punctate keratitis and vaginal infection (Fig. 1) [11]. Serious TEAEs occurred in 9.2 and 7.9% of tafamidis and placebo recipients, respectively. There were no clinically relevant changes in laboratory parameters.
Most common treatment-emergent adverse events (reported in ≥5% of tafamidis recipients and with a ≥2% higher incidence than in the placebo group) in an 18-month, phase 3 trial of once-daily tafamidis 20 mg in patients with early stage V30M transthyretin familial amyloid polyneuropathy [11]
During long-term treatment (up to 5.5 years), no new safety issues or tafamidis-related adverse effects were identified [16]. Three patients (4%) discontinued treatment due to a TEAEs that was considered to be possibly related to tafamidis (urticaria, renal impairment) or underlying TTR-FAP (diarrhoea) [16].
Tafamidis was also well tolerated in the clinical practice setting [19, 20]. No new safety concerns, including renal, thyroid and liver function abnormalities, were identified during 2 years of tafamidis treatment in the Portuguese study [19]. In the 3-year study in Italy, no patients discontinued treatment due to a treatment-related adverse event [20]. Overall, eight patients (13%) reported TEAEs, including two cases of urinary tract infection, and one case each of diarrhoea, gastroenteritis, and angular stomatitis. Three serious TEAEs were considered unlikely to be related to tafamidis and consistent with disease-associated cardiac morbidity: one patient died as a result of cardiac failure and two had rapid cardiac function worsening [20].
What is the current clinical role of tafamidis?
Oral tafamidis is the first disease-modifying drug to be approved in the EU to slow the progression of peripheral neurological impairment in adults with early-stage TTR amyloidosis. It acts by kinetically stabilizing the TTR tetramer, thereby inhibiting monomer formation, misfolding and amyloidogenesis. According to the recent European TTR-FAP guidelines [4], tafamidis is recommended as the first-line treatment for stage 1 disease (defined as being able to walk unaided outside), regardless of patient age or the presence of contraindications for liver transplantation (Fig. 2). Its use during the early stages of the disease has the potential to delay the need for liver transplantation [4–6]. If stage 1 disease progresses during treatment with tafamidis, second-line treatment is liver transplantation in patients with no contraindications to its use. In patients with contraindications to liver transplantation, second-line treatment options include enrolment in a clinical trial of a therapy that has shown promising results (e.g. gene silencing approaches, such as antisense oligonucleotides and RNA interference therapeutics [7, 23]) or the off-label use of diflunisal (Fig. 2) [4].
Patient care guidelines for the treatment of stage 1 transthyretin familial amyloid polyneuropathy (TTR-FAP), as suggested by Adams et al. [4]
In the Fx-005 trial in adults with early-stage V30M TTR-FAP, tafamidis 20 mg once daily significantly slowed the deterioration of neurological function and maintained HR-QOL in the EE population. The lack of a significant treatment difference in the ITT population was largely due to the high percentage of patients who discontinued treatment in order to undergo liver transplantation (21% vs. a pre-trial estimate of 10%) [11]. Long-term extension studies have demonstrated that the beneficial effects of tafamidis on neurological deterioration and HR-QOL are maintained. Results of long-term studies in the clinical practice setting support those of clinical trials, with a substantial proportion of patients treated with tafamidis achieving neurological stability. Furthermore, results indicate that tafamidis should be initiated as soon as possible after diagnosis in order to optimise clinical outcomes. While the V30M mutation is the most common, and only patients with this mutation were included in the Fx-005 trial, evidence indicates that tafamidis is also effective in patients with non-V30M mutations. Tafamidis is generally well tolerated, including during long-term treatment.
Results of a recent phase 2 study demonstrated that tafamidis produces TTR stabilization in patients with TTR amyloid cardiomyopathy [24]. Further research is currently being conducted to determine the safety and efficacy of tafamidis in patients with amyloid cardiomyopathy resulting from TTR genetic variants or wild-type TTR, including a global phase 3 trial (NCT01994889) and its long-term safety extension (NCT02791230) [25].
References
Adams D, Théaudin M, Cauquil C, et al. FAP neuropathy and emerging treatments. Curr Neurol Neurosci Rep. 2014;14(3):435.
Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol. 2011;10(12):1086–97.
Coelho T, Merlini G, Bulawa CE, et al. Mechanism of action and clinical application of tafamidis in hereditary transthyretin amyloidosis. Neurol Ther. 2016;5(1):1–25.
Adams D, Suhr OB, Hund E, et al. First European concensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Neurology. 2016;29(Suppl 1):S14–26.
Ando Y, Coelho T, Berk JL, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31.
Hawkins PN, Ando Y, Dispenzeri A, et al. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–38.
Sekijima Y. Recent progress in the understanding and treatment of transthyretin amyloidosis. Clin Pharm Ther. 2014;39(3):225–33.
Ericzon BG, Wilczek HE, Larsson M, et al. Liver transplantation for hereditary transthyretin myloidosis: after 20 years still the best therapeutic alternative? Transplantation. 2015;99(9):1847–54.
Suhr OB, Larsson M, Ericzon BG, et al. Survival after transplantation in patients with mutations other than Val30Met: extracts from the FAP World Transplant Registry. Transplantation. 2016;100(2):373–81.
Bulawa CE, Connelly S, DeVit M, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci. 2012;109(24):9629–34.
Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–92.
Coelho T, Maia LF, Martins da Silva A, et al. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260(11):2802–14.
Merlini G, Planté-Bordeneuve V, Judge DP, et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis. J Cardiovasc Transl Res. 2013;6(6):1011–20.
Vyndaqel 20 mg soft capsules (tafamidis meglumine): summary of product characteristics. London: European Medicines Agency; 2016.
Keohane D, Schwartz J, Gundapaneni B, et al. Tafamidis delays disease progression in TTR Familial Amyloid Polyneuropathy: supportive analyses from a pivotal trial [poster no. 59]. In: European Conference on Rare Diseases & Orphan Products; 2016.
Waddington Cruz M, Amass L, Keohane D, et al. Early intervention with tafamidis provides long-term (5.5-year) delay of neurologic progression in transthyretin hereditary amyloid polyneuropathy. Amyloid. 2016;23(3):178–83.
Suhr OB, Conceicao IM, Karayal ON, et al. Post hoc analysis of nutritional status in patients with transthyretin familial amyloid polyneuropathy: impact of tafamidis. Neurol Ther. 2014;3(2):101–12.
Ando Y, Sekijima Y, Obayashi K, et al. Effects of tafamidis treatment on transthyretin (TTR) stabilization, efficacy, and safety in Japanese patients with familial amyloid polyneuropathy (TTR-FAP) with Val30Met and non-Val30Met: a phase III, open-label study. J Neurol Sci. 2016;362:266–71.
Coelho T, da Silva AM, Alves C, et al. Familial amyloid polyneuropathy treatment with tafamidis: evaluation of one- and two-year treatment in Porto, Portugal. Orphanet J Rare Dis. 2015;10(Suppl 1):O25.
Cortese A, Vita G, Luigetti M, et al. Monitoring effectiveness and safety of tafamidis in transthyretin amyloidosis in Italy: a longitudinal multicenter study in a non-endemic area. J Neurol. 2016;263(5):916–24.
Stewart M, Keohane D, Short S, et al. Positive real-world effectiveness of tafamidis for delaying disease progression in transthyretin familial amyloid polyneuropathy [abstract]. Orphanet J Rare Dis. 2015;10(Suppl 1):4.
Ines M, Coelho T, Conceicao I, et al. Long-term survival of 3000 transthyretin familial amyloid polyneuropathy patients over 100 years [abstract no. O1209]. Eur J Neurol. 2016;23(Suppl 2):44.
Adams D, Cauquil C, Labeyrie C, et al. TTR kinetic stabilizers and TTR gene silencing: a new era in therapy for familial amyloidotic polyneuropathies. Expert Opin Pharmacother. 2016;17(6):791–802.
Maurer MS, Grogan DR, Judge DP, et al. Tafamidis in transthyretin amyloid cardiomyopathy: effects on transthyretin stabilization and clinical outcomes. Circ Heart Fail. 2015;8(3):519–26.
US National Institutes of Health. ClinicalTrials.gov. 2016. http://www.clinicaltrials.gov/. Accessed 28 Nov 2016.
Scott LJ. Tafamidis: a review of its use in familial amyloid polyneuropathy. Drugs. 2014;74(12):1371–8.
Acknowledgements
The review was updated from Drugs 2014;74(12):1371–8 [26], and was reviewed by: A. Castano, Division of Cardiology, Department of Medicine, Columbia University Medical Center, New York, USA; M. Waddington Cruz, Amyloidosis Research and Treatment Center, University Hospital, Federal University of Rio De Janeiro, Rio De Janeiro, Brazil. During the peer review process, the manufacturer of tafamidis was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
K. McKeage, K.A. Lyseng-Williamson and L.J. Scott are contracted/salaried employees of Adis/Springer, are responsible for the article content and declare no conflicts of interest.
Additional information
The original publication of this article Figure 2 is incorrect; this error has now been corrected.
An erratum to this article is available at http://dx.doi.org/10.1007/s40267-017-0376-z.
Rights and permissions
About this article
Cite this article
McKeage, K., Lyseng-Williamson, K.A. & Scott, L.J. Tafamidis in transthyretin amyloidosis: a guide to its use in delaying peripheral neurological impairment in patients with stage 1 polyneuropathy. Drugs Ther Perspect 33, 47–53 (2017). https://doi.org/10.1007/s40267-016-0368-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0368-4