Abstract
Background
Hospital admissions in older adults are frequently drug related and avoidable. Clinical pharmacy interventions during hospital stay might reduce drug-related harm and reduce hospital visits. Moreover, several recent positive clinical pharmacy investigations incorporated a transitional care component to further improve medication use after discharge. It is currently unclear what the strength of evidence is and what the exact components should be of such clinical pharmacy interventions in older adults.
Objective
An evidence-based review was performed to determine the status of the evidence and also to explore whether a clinical pharmacy intervention incorporating transitional care was associated with reduced hospital visits after discharge.
Methods
Prospective controlled investigations were included if they contained a clinical pharmacy intervention that was initiated before discharge in older inpatients. Relevant quasi-experimental and randomized controlled trials were searched in MEDLINE. First, an evidence-based review was performed, including a description of the study design, characteristics, and outcomes. Major components of successful clinical pharmacy interventions were described and potential implications for clinical practice and research were determined. Second, the Fisher’s exact test was used to explore the association between transitional care and reduced hospital visits. Third, based on these findings, a medication review proposal was developed to improve medication use in older adults.
Results
Thirty-five studies were included, with 26 randomized controlled trials. Median patient follow-up after discharge was 90 days (interquartile range 37–180 days) and investigators enrolled a median of 210 (interquartile range 110–498) study participants. On average, patients were aged 77.5 years (interquartile range 73–82.2 years). Nine randomized controlled trials had sufficient power to detect a reduction in hospital visits after discharge; this was reduced in three randomized controlled trials. Post-discharge follow-up was not associated with reduced post-discharge hospital visits (20 randomized controlled trials: follow-up vs. no follow-up: 6/11 vs. 1/9, p = 0.070). There was a significant reduction in post-discharge hospital visits in patients aged 75 years or older (12 randomized controlled trials: follow-up vs. no follow-up: 5/7 vs. 0/5, p = 0.028). A medication review proposal was developed, consisting of six steps.
Conclusions
Three powered randomized controlled trials were identified that found a significant association between a pharmacist-led intervention in older adults and a reduction in post-discharge hospital visits. In clinical practice, an intervention consisting of medication reconciliation, review, counseling, and post-discharge follow-up should be provided to such high-risk inpatients. Regarding research priorities, large, multi-center randomized controlled trials should be performed to generate more evidence on the impact of clinical pharmacy interventions on the patient trajectory and economic outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Older inpatients are regularly (re)admitted to the hospital and medication harm might play an important role herein. |
Clinical pharmacists can reduce medication harm, improve overall medication use, and reduce post-discharge hospital visits in older inpatients, in particular when providing their services in a multi-faceted and multi-disciplinary manner. |
Post-discharge follow-up is important to extend the clinical pharmacy intervention beyond the hospital stay, to further impact the patient’s trajectory. |
1 Introduction
Improvements in medicine have made a large impact on life expectancy, which has never been higher. This has resulted in a longevity revolution, with a global doubling of the number of older people expected by 2050 [1, 2]. As part of these continuous improvements in healthcare, increasing numbers of patients have been granted access to a plethora of therapies. Not all therapies however are able to positively affect patient outcome. In particular, older adults might incur net harm by experiencing drug-related problems [3]. Drug-related problems can become an important cause of iatrogenic morbidity at a high age, underscoring the persistent importance of the old adage ‘first do no harm’ [4]. This burden of this drug-related problem is particularly high in older adults admitted to the hospital [5].
Pharmacists can play an important role in improving outcomes such as hospital admissions in very old adults, mainly through a process of identifying, preventing, and resolving drug-related problems [6, 7]. Patient-directed care provided by pharmacists has been introduced increasingly during the past decades [5, 8,9,10,11,12]. Importantly, several seminal investigations pointed towards improved post-discharge outcomes when the intervention contained a transitional component (e.g., telephone follow-up) [13, 14].
It is difficult to perform a meta-analysis on such investigations in older inpatients, given the broad definition of what constitutes a medical inpatient, but also because of different follow-up times, different definitions used for outcome measures, the actual content of the clinical pharmacy intervention, and whether the meta-analyzed outcome measure was initially a sufficiently powered primary outcome. Several meta-analyses have indeed concluded that it is difficult to draw robust conclusions given the high level of heterogeneity and the overall low quality of evidence [10, 11, 15,16,17,18]. As a result, equipoise remains about whether clinical pharmacy services in general can reduce overall healthcare use in older inpatients [14, 19]. Subsequently, there is a need for pragmatic information on how to best provide clinical pharmacy services in older inpatients based on the current body of evidence.
The primary aim of this research was hence to perform an evidence-based review on the content of successful clinical pharmacy services in older inpatients and the impact on hospital visits. In addition, we also specifically aimed to further explore the potential value of providing post-discharge follow-up to reduce hospital visits. Finally, based on the review results, the goal was to provide a practical medication review proposal for clinical pharmacists dealing with older inpatients.
2 Methods
2.1 Data Source
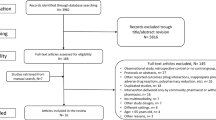
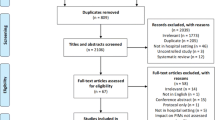
An evidence-based review of the literature was performed. The data search was based on search terms previously reported by Kaboli et al. [20]. Studies were retrieved from the bibliographic database MEDLINE using the following search terms: clinical pharmacy, clinical pharmacists, hospital pharmacists, pharmacy services, pharmaceutical care, outcome, healthcare utilization, hospital utilization, morbidity, readmissions, and hospital visits. Searches were limited to English articles published from inception to July 2019. Snowball sampling was used to identify additional publications for review.
2.2 Study Selection
One reviewer selected the publications (LVDL). In case of any doubt, consensus was reached with two other researchers (JH and KW) about whether to include the publication for further review.
First, relevance to the research questions was evaluated based on screening the title and abstract. Second, articles were included in the review if the following criteria were met: a prospective controlled study design, with a clinical pharmacy intervention component, that was initiated before discharge. Both randomized controlled trials (RCTs) and quasi-experimental (QE) studies were eligible for inclusion. Only primary study results were included for review, and the average age of study participants had to be at least 65 years, by design or owing to the age of enrolled study participants.
Studies in which children were enrolled or patients were exclusively admitted to intensive care units or surgical wards were excluded. Investigations pertaining to a specific drug treatment (e.g., only warfarin or antimicrobial therapies) were excluded as well.
2.3 Data Extraction and Synthesis
A data collection form was used to extract the following information from the included studies: author, year, country and region, study design (RCT or QE), and study population (sample size and age). Regarding study methods, the following data were retrieved: number of study arms, mono- or multi-centric design, and whether a primary outcome was defined and a prior sample size estimation was performed for any of the reported outcomes. We also evaluated whether post-discharge hospital visits had been included as one of the study outcomes and whether the study intervention contained a transitional intervention component (i.e., post-discharge follow-up, however provided).
Regarding the study results, we obtained mortality rates and documented whether the study had reached a statistically significant result for its primary outcome. We also documented whether a reduction in admissions, all-cause readmissions, or emergency department visits had been reported. In addition, information on cost benefits or balanced cost savings were retrieved. Balanced cost savings were defined as the costs of hospital care minus the costs of the clinical pharmacy intervention.
We also determined whether hospital visits were reduced after discharge. This was defined as a statistically significant reduction in readmissions and/or emergency department visits and was documented for each study, if applicable. In addition, to provide readers with baseline event rates, data were extracted for these three outcomes for control and intervention groups at 30, 60, 90, 180, and 365 days after discharge, if available. No formal quality assessment of the included studies was performed.
2.4 Data Analysis
Normality of continuous variables was ascertained by visual inspection of the histograms and QQ-plots. Parametric data were shown as mean (± standard deviation) and non-parametric continuous data as median (interquartile range [IQR] = Q1−Q3), as appropriate. Counts were summarized as n (%).
In general, smaller studies have been associated with an overestimation of the effect size and are more heterogeneous than larger studies [21]. This might render it more difficult to interpret the strength of evidence of such investigations. To evaluate whether study size changed over the years, a Kruskal–Wallis, one-way analysis of variance was used.
To explore the association of a transitional care component with post-discharge hospital visits, the Fisher’s exact test was used. Only data from RCTs were used for this exploratory analysis. First, the impact of a transitional component on post-discharge hospital visits was estimated in all RCTs. Second, the exploratory analysis was repeated in RCTs in which the average age of the study population was at least 75 years.
Results were considered to be statistically significant if the two-tailed p-value was < 0.05. Statistical analysis was performed with IBM SPSS Statistics for Windows version 20.0 (IBM Corporation, Armonk, NY, USA).
2.5 Medication Review Proposal
Data from positive RCTs were collected and compiled into a preliminary proposal. Additional information was then retrieved from recent reviews on improving medication use in older adults and was added to the proposal. The following reviews and guidance documents were selected by the authors: detailed information on deprescribing as provided by Scott et al.; step-based information from the Dutch Structured Tool to Reduce Inappropriate Prescribing (STRIP); the comprehensive approach of the Northern Irish Integrated Medicines Management (IMM) model, and the American Geriatrics Society Guiding Principles on multi-morbidity in older adults, all of which were further supplemented by our own experiences [22,23,24,25,26,27,28]. Importantly, the American Geriatrics Society guidance document strongly promotes determining patient concerns and defining therapy goals before moving forward to the following steps in the algorithm. Consensus was reached among all authors concerning the final proposal.
2.6 Ongoing Investigations
The online databases MEDLINE and ClinicalTrials.gov were searched using the same search terms as detailed above to identify ongoing relevant investigations. The following data were extracted: authors, country (region), setting, design, participants, inclusion criteria, primary outcome, estimated sample size, usual care, intervention components, expected duration, and recruitment status.
3 Results
3.1 Literature Overview
The literature search resulted in 35 publications (n = 13,003 participants), with nine studies having a QE design (n = 3845) and 26 an RCT design (n = 9158). A summary of the main trial components is provided in Table 1.
The sample sizes did not differ significantly over the years (1994–2019) (p = 0.772). Most studies were monocentric (n = 27) and were performed in Europe (n = 20). Median patient follow-up after discharge was 90 days (IQR 37–180 days) and study investigators enrolled a median of 210 (IQR 110–498) study participants. Across all studies, patients were aged on average 77.5 years (IQR 73–82.2 years); 21 studies enrolled participants with an average age of 75 years or older.
Mortality was high in both control and intervention groups in this review. Overall, studies reported similar mortality rates in control and intervention groups (median mortality rate of 16% [IQR 8.6–2.7] and 16% [IQR 8.8–2.2], respectively). Approximately half of all patients were readmitted to the hospital at 1 year after discharge (control 53.2% [IQR 48.6–57.9]; intervention 49.4% [IQR 34.9–58.8]). A summary of hospital visits and mortality rates is provided in Table 2.
Three studies did not have a clearly defined primary outcome. In 20 of the 32 remaining studies, a statistically significant result for the primary outcome measure was reached (QE 6; RCT 14). Hospital visits after discharge were evaluated in 27 investigations (QE 7; RCT 20), and in 14 as part of the primary outcome (QE 2; RCT 12). A positive effect of a clinical pharmacy intervention on post-discharge hospital visits was reported in 12 individual investigations (QE 5; RCT 7).
Out of 26 RCTs, 22 had sufficient power to detect an impact of the clinical pharmacy intervention on the reported primary outcome. In nine of these 22 studies, the primary endpoint also contained a clinical outcome pertaining to hospital visits after discharge, which was reduced in the RCTs of Ravn-Nielsen et al., Gillespie et al., and López Cabezas et al. [13, 14, 29]. The same positive RCTs also showed balanced cost savings [14, 29, 30].
Post-discharge follow-up was not associated with reduced post-discharge hospital visits in a total of 20 RCTs (follow-up vs. no follow-up: 6/11 vs. 1/9, p = 0.070). However, there was a reduction in post-discharge hospital visits when selecting the 12 RCTs, where the average study participants’ age was at least 75 years (follow-up vs. no follow-up: 5/7 vs. 0/5, p = 0.028).
3.2 Medication Review Proposal
Commonly, clinical pharmacy interventions in complex older inpatients followed a multi-faceted approach, consisting of multiple single components [24]. The Lund IMM model deserves more attention in this regard [24, 31,32,33,34]. It entails the systematic provision of pharmaceutical care during hospital stay and was explicitly provided in the investigation by Gillespie et al. and by default, also in the investigations of Ravn-Nielsen et al. and López Cabezas et al. [13, 14, 29]. According to the Lund IMM model, pharmacists are expected to promote a correct medication reconciliation and to perform a medication review using the best possible medication list. A motivational interview technique can be applied to elicit desired changes in patients (and caretakers) to further strengthen the effect of the clinical pharmacy intervention regarding appropriate medication use [13, 32]. Importantly, to increase the persistence of the intervention after hospital discharge, a post-discharge follow-up can be provided. Such a transitional component was shown by Ravn-Nielsen et al. to be essential in reducing the number of readmissions, when compared to a clinical pharmacy intervention without follow-up after discharge [13]. Clinical pharmacists can use a simple phone call to evaluate the drug regimen and resolve any outstanding issues or confusion regarding the patient’s therapy.
This holistic Lund IMM approach was used as a template upon which the medication review proposal was based. The medication review proposal in older adults consists of the following six steps: ascertaining patient concerns and defining therapy goals, medication (and medical history) reconciliation, actual medication review, patient education, promoting safe transition, and post-discharge follow-up. The detailed proposal is summarized in Table 3.
3.3 Ongoing Investigations
Five RCTs were identified, all of which take place in Europe. In total, 6840 study participants will be randomized. Relevant data are summarized in Table 4.
4 Discussion
In our evidence-based review, we found that multiple investigations established a role for clinical pharmacists in improving medication use and reducing hospital visits after discharge. The average study population of the included studies largely corresponded with a complex and multi-morbid patient profile, who regularly experience a high burden of amenable drug-related problems [35,36,37]. Importantly, the number of sufficiently powered investigations, which were dedicated to improving clinical outcome in older inpatients, was limited. Our evidence-based review also showed that setting up an RCT is feasible, in complex older inpatients even when aiming for an improvement in clinical outcome. Clinical outcome in this specific setting was mostly defined as a reduction in post-discharge hospital visits. The clinical relevance of fewer hospital contacts is largely related to its association with the patient’s clinical condition (e.g., management of heart failure) and has also been used as an indirect metric of the quality of care [38]. We found a positive association between providing post-discharge follow-up and a reduction in hospital visits in RCTs that enrolled participants aged on average 75 years or older.
Only the three following studies, out of 35 investigations, concerned RCTs that had sufficient power to detect a statistically significant difference concerning their clinical endpoints. First, the work of Gillespie et al. should be highlighted as their RCT (n = 386) was one of the first that was powered to detect the impact of a clinical pharmacy intervention in octogenarian Swedish inpatients [14]. The authors detected a moderate reduction of 16% in hospital visits during a 12-month follow-up using a Poisson regression analysis (relative risk 0.84, 95% confidence interval 0.72–0.99), driven in part by the reduction in emergency department visits and drug-related readmissions. Second, their Danish counterparts Ravn-Nielsen et al. showed afterwards that a multi-faceted intervention during a hospital stay significantly reduced the same composite primary endpoint at 180 days after discharge in a Danish patient sample (usual care vs. extended intervention 48.8% vs. 39.7%; hazard ratio 0.75, 95% confidence interval 0.62–0.90), which corresponded to a number needed to treat of 12 [13]. Theirs was the largest RCT to date (n = 1467). Third, in their RCT (n = 134), Lopez Cabezas et al. found that a comparable intervention, aiming to improve medication use during hospital stay while also providing active telephone follow-up after hospital discharge, was significantly associated with fewer readmissions (hazard ratio 0.56, 95% confidence interval 0.32–0.97) [29]. These positive RCTs convincingly showed that outcome can indeed be improved and furthermore that the costs of the clinical pharmacy interventions were at least balanced.
Taken together, these reports add weight to the hypothesis that a ward-based comprehensive intervention improves outcome when performed in acutely admitted older adults, with post-discharge follow-up provided by phone. A medication review algorithm was subsequently derived and we hypothesize that using such an approach would be of value to healthcare professionals when providing standardized comprehensive medication reviews.
We believe the findings of our evidence-based review are valid because of the broad inclusion criteria, the explicit documentation of trial design, and the additional analysis regarding transitional care. Importantly, this was however not a systematic review and the quality of the included investigations was not ascertained explicitly, which is a limitation. We cannot exclude that potentially eligible investigations might have been missed. This however fell beyond the scope of performing an evidence-based review. Furthermore, owing to the heterogeneity in designs, settings, interventions, and outcomes, no meta-analysis was performed, hence the preclusion of broad statements on the impact of clinical pharmacy services on clinical outcomes. Some additional considerations for clinical practice and research are proposed below.
4.1 Implications for Clinical Practice
As described in a majority of the included investigations in this review, clinical pharmacists regularly worked in a team setting. Pharmacists should hence proactively participate in ward-based services in older inpatients as members of a multi-disciplinary team [5, 39]. In the case of high-risk patient groups such as geriatric inpatients, pharmacists can perform structured medication reviews and provide recommendations to the prescriber, who remains in charge of coordinating the clinical assessment and therapy plan as was the case in the three positive RCTs. Importantly, working outside of a multi-disciplinary team might lead to failure of a pharmacist approach in medical inpatients, as recently discussed by Petrovic et al. [5]. Moreover, the integration of multiple healthcare providers, including pharmacists but also physical therapists, nurses, and psychologists, with complementary skills, seems warranted to fully impact outcome of the older inpatient [40].
Hospital-wide implementation of clinical pharmacy services, while potentially useful, is not common in Europe. This is currently not feasible in many hospitals because of insufficient staffing or funding, but also because of other priorities of the hospital pharmacy management and hospital boards [41]. Hence, it may then be reasonable to target a high-risk population, such as older adults acutely admitted to the hospital, who are more likely to derive a meaningful and clinical benefit from a clinical pharmacy service. Most commonly, this will pertain to adults, aged at least 65 years, who have been acutely admitted to an internal medicine or geriatric care ward, as was the case in the majority of studies included in this review.
In larger hospitals, it might be easier to find the necessary resources. In the case of limited resources, it could be efficient to divert scarce means to the period directly prior to discharge to promote appropriate medication use in the high-risk period after hospital discharge [13, 17].
4.2 Implications for Research
Several pertinent questions remain regarding the clinical benefits of clinical pharmacy services in daily clinical practice. Most investigations in our review were monocentric, were not powered for clinical outcomes, did not ascertain patient-reported quality of life, and did not enroll the very old adults. It is furthermore unclear how study findings should be implemented into clinical practice as academic investigations might be limited in their external validity, e.g., regarding staff allocation and time investment per patient [42]. For example, in Belgium, there is one hospital pharmacist available per 150 beds, with additional governmental funding to support clinical pharmacy services (0.25 full-time equivalent per 250 beds) [43]. In contrast, the clinical pharmacy intervention as described by Ravn-Nielsen et al. required on average 2 h per patient [13]. Their intervention was however proven to be cost effective; a significant reduction in readmissions was not associated with an increase in cost. In contrast, a trend toward a total cost reduction of €1657 (p = 0.1083) was found per patient in their cost-consequence analysis in favor of the extended clinical pharmacy intervention [30].
In the reviewed studies, patient’s family members or caretakers were rarely engaged, which can be considered to be a missed opportunity. In a 2017 meta-analysis, Rodakowski et al. showed the importance of actively drawing upon their presence and influence in the care trajectory of older inpatients after hospital discharge [44]. They found a 25% reduction in hospital readmissions at 90 days. In particular in very old inpatients, we propose to involve family members and caretakers to increase the impact of a clinical pharmacy intervention.
Five relevant ongoing RCTs have been identified. These investigations can be expected to shed more light on the impact of clinical pharmacy services in older inpatients. In particular, MEDBRIDGE and IMMENSE could be expected to provide robust information [32, 45]. Both studies will enroll exclusively older adults and apply a comprehensive clinical pharmacy intervention, which will include post-discharge follow-up. Importantly, both are also sufficiently powered to detect an impact on hospital visits after discharge.
In sum, more data should be collected on the impact of clinical pharmacy services on the older patient’s trajectory after a hospital stay. Although several quasi-experimental study designs were retrieved in our literature search, the majority of results were still derived from RCTs. We propose that new investigations should maximally apply the RCT design and primarily aim to improve clinical outcome, including drug-related, disease-specific (e.g., heart failure-related hospitalizations), and all-cause readmissions. This proposal corresponds largely to the research priorities as proposed recently by an international consortium of experts [46].
5 Conclusions
A literature review was performed and 35 studies were identified. Three sufficiently powered RCTs found a significant association between a pharmacist-led intervention performed in older, acutely admitted medical inpatients and a reduction in post-discharge hospital visits. In clinical practice, a comprehensive pharmacist intervention consisting of medication reconciliation, review, counseling, and post-discharge follow-up should be provided to high-risk inpatients. Regarding research priorities, large multi-center RCTs should be performed to collect information on the impact of clinical pharmacy interventions on the patient trajectory and economic outcomes in very old inpatients.
References
Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–208.
Cohen JE. Human population: the next half century. Science. 2003;302(5648):1172–5.
Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–54.
Scott IA, Anderson K, Freeman CR, Stowasser DA. First do no harm: a real need to deprescribe in older patients. Med J Aust. 2014;201(7):390–2.
Petrovic M, Somers A, Onder G. Optimization of geriatric pharmacotherapy: role of multifaceted cooperation in the hospital setting. Drugs Aging. 2016;33(3):179–88.
Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–43.
American College of Clinical Pharmacy. The definition of clinical pharmacy. Pharmacotherapy. 2008;28(6):816–7.
Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
Clyne B, Fitzgerald C, Quinlan A, Hardy C, Galvin R, Fahey T, et al. Interventions to address potentially inappropriate prescribing in community-dwelling older adults: a systematic review of randomized controlled trials. J Am Geriatr Soc. 2016;64(6):1210–22.
Renaudin P, Boyer L, Esteve MA, Bertault-Peres P, Auquier P, Honore S. Do pharmacist-led medication reviews in hospitals help reduce hospital readmissions? A systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(6):1660–73.
Gray SL, Hart LA, Perera S, Semla TP, Schmader KE, Hanlon JT. Meta-analysis of interventions to reduce adverse drug reactions in older adults. J Am Geriatr Soc. 2018;66(2):282–8.
Spinewine A, Fialova D, Byrne S. The role of the pharmacist in optimizing pharmacotherapy in older people. Drugs Aging. 2012;29(6):495–510.
Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med. 2018;178(3):375–82.
Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol. 2008;65(3):303–16.
Thomas R, Huntley AL, Mann M, Huws D, Elwyn G, Paranjothy S, et al. Pharmacist-led interventions to reduce unplanned admissions for older people: a systematic review and meta-analysis of randomised controlled trials. Age Ageing. 2014;43(2):174–87.
Hohl CM, Wickham ME, Sobolev B, Perry JJ, Sivilotti ML, Garrison S, et al. The effect of early in-hospital medication review on health outcomes: a systematic review. Br J Clin Pharmacol. 2015;80(1):51–61.
Cheema E, Alhomoud FK, Kinsara ASA, Alsiddik J, Barnawi MH, Al-Muwallad MA, et al. The impact of pharmacists-led medicines reconciliation on healthcare outcomes in secondary care: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2018;13(3):e0193510.
Walsh KA, O’Riordan D, Kearney PM, Timmons S, Byrne S. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists’ interventions in secondary care. Age Ageing. 2016;45(2):201–9.
Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–64.
IntHout J, Ioannidis JP, Borm GF, Goeman JJ. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J Clin Epidemiol. 2015;68(8):860–9.
Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–34.
Drenth-van Maanen AC, Leendertse AJ, Jansen PAF, Knol W, Keijsers C, Meulendijk MC, et al. The Systematic Tool to Reduce Inappropriate Prescribing (STRIP): combining implicit and explicit prescribing tools to improve appropriate prescribing. J Eval Clin Pract. 2018;24(2):317–22.
Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. J Eval Clin Pract. 2007;13(5):781–8.
Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60(10):E1–25.
Walgraeve K, Van der Linden L, Flamaing J, Fagard K, Spriet I, Tournoy J. Feasibility of optimizing pharmacotherapy in heart failure patients admitted to an acute geriatric ward: role of the clinical pharmacist. Eur Geriatr Med. 2018;9(1):103–11.
Van der Linden L, Hias J, Dreessen L, Milisen K, Flamaing J, Spriet I, et al. Medication review versus usual care to improve drug therapies in older inpatients not admitted to geriatric wards: a quasi-experimental study (RASP-IGCT). BMC Geriatr. 2018;18(1):155.
Van der Linden L, Decoutere L, Walgraeve K, Milisen K, Flamaing J, Spriet I, et al. Combined use of the Rationalization of Home Medication by an Adjusted STOPP in Older Patients (RASP) list and a pharmacist-led medication review in very old inpatients: impact on quality of prescribing and clinical outcome. Drugs Aging. 2017;34(2):123–33.
Lopez Cabezas C, Falces Salvador C, Cubi Quadrada D, Arnau Bartes A, Ylla Bore M, Muro Perea N, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farm Hosp. 2006;30(6):328–42.
Rasmussen MK, Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, et al. Cost-consequence analysis evaluating multifaceted clinical pharmacist intervention targeting patient transitions of care from hospital to primary care. JACCP. 2019;2(2):123–30.
Scott MG, Scullin C, Hogg A, Fleming GF, McElnay JC. Integrated medicines management to medicines optimisation in Northern Ireland (2000–2014): a review. Eur J Hosp Pharm Sci Pract. 2015;22(4):222–8.
Johansen JS, Havnes K, Halvorsen KH, Haustreis S, Skaue LW, Kamycheva E, et al. Interdisciplinary collaboration across secondary and primary care to improve medication safety in the elderly (IMMENSE study): study protocol for a randomised controlled trial. BMJ Open. 2018;8(1):e020106.
Hellstrom LM, Bondesson A, Hoglund P, Midlov P, Holmdahl L, Rickhag E, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. Eur J Clin Pharmacol. 2011;67(7):741–52.
Bondesson A, Holmdahl L, Midlov P, Hoglund P, Andersson E, Eriksson T. Acceptance and importance of clinical pharmacists’ LIMM-based recommendations. Int J Clin Pharm. 2012;34(2):272–6.
Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168(17):1890–6.
Lavan A, Eustace J, Dahly D, Flanagan E, Gallagher P, Cullinane S, et al. Incident adverse drug reactions in geriatric inpatients: a multicentred observational study. Ther Adv Drug Saf. 2018;9(1):13–23.
El Morabet N, Uitvlugt EB, van den Bemt BJF, van den Bemt P, Janssen MJA, Karapinar-Carkit F. Prevalence and preventability of drug-related hospital readmissions: a systematic review. J Am Geriatr Soc. 2018;66(3):602–8.
Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–95.
Koshman SL, Charrois TL, Simpson SH, McAlister FA, Tsuyuki RT. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Arch Intern Med. 2008;168(7):687–94.
Onder G, van der Cammen TJ, Petrovic M, Somers A, Rajkumar C. Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing. 2013;42(3):284–91.
Frontini R, Miharija-Gala T, Sykora J. EAHP survey 2010 on hospital pharmacy in Europe: parts 4 and 5. Clinical services and patient safety. Eur J Hosp Pharm. 2013;20:69–73.
Van der Linden L, Hias J, Walgraeve K, Flamaing J, Spriet I, Tournoy J. Clinical pharmacy services on geriatric care wards: catch 22 of implementation and research. Drugs Aging. 2018;35(5):375–7.
Somers A, Spinewine A, Spriet I, Steurbaut S, Tulkens P, Hecq JD, et al. Development of clinical pharmacy in Belgian hospitals through pilot projects funded by the government. Acta Clin Belg. 2019;74(2):75–81.
Rodakowski J, Rocco PB, Ortiz M, Folb B, Schulz R, Morton SC, et al. Caregiver integration during discharge planning for older adults to reduce resource use: a metaanalysis. J Am Geriatr Soc. 2017;65(8):1748–55.
Kempen TGH, Bertilsson M, Lindner KJ, Sulku J, Nielsen EI, Hogberg A, et al. Medication Reviews Bridging Healthcare (MedBridge): study protocol for a pragmatic cluster-randomised crossover trial. Contemp Clin Trials. 2017;61:126–32.
Tan ECK, Sluggett JK, Johnell K, Onder G, Elseviers M, Morin L, et al. Research priorities for optimizing geriatric pharmacotherapy: an international consensus. J Am Med Dir Assoc. 2018;19(3):193–9.
Lipton HL, Bird JA. The impact of clinical pharmacists’ consultations on geriatric patients’ compliance and medical care use: a randomized controlled trial. Gerontologist. 1994;34(3):307–15.
Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberal H. A pharmacy discharge plan for hospitalized elderly patients: a randomized controlled trial. Age Ageing. 2001;30(1):33–40.
Al-Rashed SA, Wright DJ, Roebuck N, Sunter W, Chrystyn H. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657–64.
Naunton M, Peterson GM. Evaluation of home-based follow-up of high-risk elderly patients discharged from hospital. J Pharm Pract Res. 2003;33(3):176–82.
Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother. 2004;2(4):257–64.
Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc. 2007;55(5):658–65.
Triller DM, Hamilton RA. Effect of pharmaceutical care services on outcomes for home care patients with heart failure. Am J Health Syst Pharm. 2007;64(21):2244–9.
Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [NCT00351676]. Med Care. 2009;47(6):642–50.
Koehler BE, Richter KM, Youngblood L, Cohen BA, Prengler ID, Cheng D, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211–8.
Eggink RN, Lenderink AW, Widdershoven JW, van den Bemt PM. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharm World Sci. 2010;32(6):759–66.
Lisby M, Thomsen A, Nielsen LP, Lyhne NM, Breum-Leer C, Fredberg U, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic Clin Pharmacol Toxicol. 2010;106(5):422–7.
Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Qual Saf. 2011;20(9):738–46.
Elliott RA, Tran T, Taylor SE, Harvey PA, Belfrage MK, Jennings RJ, et al. Impact of a pharmacist-prepared interim residential care medication administration chart on gaps in continuity of medication management after discharge from hospital to residential care: a prospective pre- and post-intervention study (MedGap Study). BMJ Open. 2012;2(3):e000918.
Scullin C, Hogg A, Luo R, Scott MG, McElnay JC. Integrated medicines management: can routine implementation improve quality? J Eval Clin Pract. 2012;18(4):807–15.
Barker A, Barlis P, Berlowitz D, Page K, Jackson B, Lim WK. Pharmacist directed home medication reviews in patients with chronic heart failure: a randomised clinical trial. Int J Cardiol. 2012;159(2):139–43.
Marusic S, Gojo-Tomic N, Erdeljic V, Bacic-Vrca V, Franic M, Kirin M, et al. The effect of pharmacotherapeutic counseling on readmissions and emergency department visits. Int J Clin Pharm. 2013;35(1):37–44.
Eisenhower C. Impact of pharmacist-conducted medication reconciliation at discharge on readmissions of elderly patients with COPD. Ann Pharmacother. 2014;48(2):203–8.
Basger BJ, Moles RJ, Chen TF. Impact of an enhanced pharmacy discharge service on prescribing appropriateness criteria: a randomised controlled trial. Int J Clin Pharm. 2015;37(6):1194–205.
Khalil H, Bell B, Chambers H, Sheikh A, Avery AJ. Professional, structural and organisational interventions in primary care for reducing medication errors. Cochrane Database Syst Rev. 2017;10:CD003942.
O’Sullivan D, O’Mahony D, O’Connor MN, Gallagher P, Gallagher J, Cullinan S, et al. Prevention of adverse drug reactions in hospitalised older patients using a software-supported structured pharmacist intervention: a cluster randomised controlled trial. Drugs Aging. 2016;33(1):63–73.
Tong EY, Roman C, Mitra B, Yip G, Gibbs H, Newnham H, et al. Partnered pharmacist charting on admission in the general medical and emergency short-stay unit: a cluster-randomised controlled trial in patients with complex medication regimens. J Clin Pharm Ther. 2016;41(4):414–8.
Roblek T, Deticek A, Leskovar B, Suskovic S, Horvat M, Belic A, et al. Clinical-pharmacist intervention reduces clinically relevant drug-drug interactions in patients with heart failure: a randomized, double-blind, controlled trial. Int J Cardiol. 2016;15(203):647–52.
Cossette B, Ethier JF, Joly-Mischlich T, Bergeron J, Ricard G, Brazeau S, et al. Reduction in targeted potentially inappropriate medication use in elderly inpatients: a pragmatic randomized controlled trial. Eur J Clin Pharmacol. 2017;73(10):1237–45.
Gustafsson M, Sjolander M, Pfister B, Jonsson J, Schneede J, Lovheim H. Pharmacist participation in hospital ward teams and hospital readmission rates among people with dementia: a randomized controlled trial. Eur J Clin Pharmacol. 2017;73(7):827–35.
Nielsen TRH, Honore PH, Rasmussen M, Andersen SE. Clinical effects of a pharmacist intervention in acute wards: a randomized controlled trial. Basic Clin Pharmacol Toxicol. 2017;121(4):325–33.
Bonetti AF, Bagatim BQ, Mendes AM, Rotta I, Reis RC, Favero MLD, et al. Impact of discharge medication counseling in the cardiology unit of a tertiary hospital in Brazil: a randomized controlled trial. Clinics (Sao Paulo). 2018;73:e325.
Rottman-Sagebiel R, Cupples N, Wang CP, Cope S, Pastewait S, Braden H, et al. A pharmacist-led transitional care program to reduce hospital readmissions in older adults. Fed Pract. 2018;35(12):42–50.
Graabaek T, Hedegaard U, Christensen MB, Clemmensen MH, Knudsen T, Aagaard L. Effect of a medicines management model on medication-related readmissions in older patients admitted to a medical acute admission unit: a randomized controlled trial. J Eval Clin Pract. 2019;25(1):88–96.
Topinkova E, Baeyens JP, Michel JP, Lang PO. Evidence-based strategies for the optimization of pharmacotherapy in older people. Drugs Aging. 2012;29(6):477–94.
Wauters M, Elseviers M, Vaes B, Degryse J, Dalleur O, Vander Stichele R, et al. Too many, too few, or too unsafe? Impact of inappropriate prescribing on mortality, and hospitalization in a cohort of community-dwelling oldest old. Br J Clin Pharmacol. 2016;82(5):1382–92.
Van der Linden L, Hias J, Spriet I, Walgraeve K, Flamaing J, Tournoy J. Medication review in older adults: importance of time to benefit. Am J Health Syst Pharm. 2019;76(4):247–50.
Kini V, Ho PM. Interventions to improve medication adherence: a review. JAMA. 2018;320(23):2461–73.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript. The first draft was written by the first author (LVDL) and all authors commented on previous versions of the manuscripts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
Lorenz Van der Linden and Isabel Spriet have received a clinical scholarship from the Clinical Research Fund of UZ Leuven.
Conflict of interest
Lorenz Van der Linden, Julie Hias, Karolien Walgraeve, Johan Flamaing, Jos Tournoy, and Isabel Spriet have no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Van der Linden, L., Hias, J., Walgraeve, K. et al. Clinical Pharmacy Services in Older Inpatients: An Evidence-Based Review. Drugs Aging 37, 161–174 (2020). https://doi.org/10.1007/s40266-019-00733-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-019-00733-1