Abstract
Aim
Our aim was to assess the efficacy and safety of mipomersen through a systematic review of the literature and a meta-analysis of the available clinical studies.
Methods
A systematic literature search in SCOPUS, PubMed Medline, ISI Web of Science and Google Scholar databases was conducted up to January 20, 2019, in order to identify clinical trials assessing the effect of mipomersen on lipoproteins, and the safety profile of mipomersen. Effect sizes for lipid changes were expressed as weighted mean differences (WMD) and 95% confidence intervals (CI). For safety analysis, odd ratios (OR) and 95% CI were calculated using the Mantel–Haenszel method. Data were pooled from 13 clinical studies comprising 49 arms, which included 1053 subjects overall, with 729 in the active-treated arm and 324 in the control arm.
Results
Meta-analysis of data suggested that mipomersen significantly reduced low-density lipoprotein cholesterol (WMD − 1.52, 95% CI − 1.85 to − 1.19; p < 0.001), total cholesterol (WMD − 1.55, 95% CI − 1.97 to − 1.13; p < 0.001), non-high-density lipoprotein cholesterol (non-HDL-C) (WMD − 1.66, 95% CI − 2.06 to − 1.27; p < 0.001), lipoprotein(a) (WMD − 0.99, 95% CI − 1.37 to − 0.62; p < 0.001), apolipoprotein B (WMD − 1.66, 95% CI − 2.04 to − 1.27; p < 0.001), triglycerides (WMD –0.61, 95% CI − 0.76 to − 0.46, p < 0.001), very-low-density lipoprotein cholesterol (WMD − 0.58, 95% CI − 0.73 to − 0.43; p < 0.001) and apolipoprotein A-I (WMD − 0.25, 95% CI − 0.51 to − 0.001; p = 0.049) without affecting HDL-C levels (WMD 0.11, 95% CI − 0.03 to 0.26; p = 0.124). However, treatment with mipomersen was positively associated with an increased risk of discontinuation of treatment (OR 3.02, 95% CI 1.96–4.65; p < 0.001), injection-site reaction (OR 11.41, 95% CI 7.88–16.52; p < 0.001), hepatic steatosis (OR 4.96, 95% CI 1.99–12.39; p = 0.001), hepatic enzymes elevation (OR 3.61, 95% CI 2.09–6.24; p < 0.001) and flu-like symptoms (OR 2.02, 95% CI 1.45–2.81; p < 0.001).
Conclusion
Despite favourable effects on the lipid profile, some concerns are reinforced from the safety profile. As a matter of fact, mipomersen therapy is more likely discontinued and associated with increased risk of injection-site reactions, hepatic steatosis, hepatic enzyme elevation, and flu-like symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mipomersen has recently been developed as adjunctive therapy for the treatment of homozygous familial hypercholesterolaemia. |
In 2013, the European Medicines Agency (EMA) refused mipomersen marketing authorization in Europe due to safety concerns. |
Despite favourable effects on the lipid profile, mipomersen is more likely discontinued and is associated with increased risk of injection-site reactions, hepatic steatosis, hepatic enzyme elevation, and flu-like symptoms. |
To date, there is no apparent reason to stop taking mipomersen when it is effective and well tolerated, even though a careful monitoring of liver function is needed. |
1 Background
Familial hypercholesterolaemia (FH) is highly prevalent throughout the world and represents a major public health concern [1, 2].
According to the latest research, FH is most commonly caused (> 95%) by mutations in the low-density lipoprotein (LDL) receptor gene (LDLR, MIM 606945), while secondary genes are, in descending order of frequency, apolipoprotein B (APOB, MIM 107730) (2.5%), proprotein convertase subtilisin/kexin type 9 (PCSK9, MIM 607786) (< 1%) and low-LDLR adaptor protein 1 (LDLRAP1, MIM 695747) (< 1%) [3], which lead to impaired low-density lipoprotein-cholesterol (LDL-C) uptake or increased LDL receptor (LDLR) degradation [4].
Regardless of whether the screening is clinical or genetic, the prevalence of heterozygous FH (HeFH) seems to be higher than previously thought, being estimated at 1 in 200–250 individuals [1, 5], while homozygous FH (HoFH) is much rarer, estimated at 1 in 160,000–300,000 [3]. The burden of markedly elevated LDL-C levels from birth underlies the sequelae of atherosclerotic cardiovascular disease complications, which are very early for untreated HoFH, typically occurring in childhood or at most within the second decade of life [1, 3, 6].
Current treatment of HoFH with conventional drugs consists of high doses of high-intensity statins, often in combination with ezetimibe, which have recently been shown to reduce CV and all-cause mortality, with minimal adverse effects and relatively low costs [3]. Combinations of statins with other cholesterol-lowering medications, including fibrates, bile acid sequestrants, probucol and niacin have been studied in HoFH and can be considered to further lower LDL-C levels, although their use may be limited by poor tolerability [3]. However, the LDL-C goal is often not reached with this therapeutic regimen, underlying the need for additional LDL-lowering options. One of these is PCSK9 inhibition, with promising results of a robust additional LDL-C reduction and relevant reduction in major adverse cardiac events (MACE) obtained by administering evolocumab 420 mg every 4 weeks, with or without LDL apheresis [7,8,9].
Most recently, two additional drugs, lomitapide and mipomersen, have been developed and approved as adjunctive therapies for the treatment of HoFH [10]. These drugs reduce the production and secretion of apoB-containing lipoproteins [11, 12], rather than increasing their clearance from the circulation. In particular, mipomersen is an antisense oligonucleotide directed against apolipoprotein-B 100 (apo B-100) mRNA in the liver, ultimately resulting in decreased serum levels of apo B-100-containing lipoproteins such as LDL and lipoprotein(a) [Lp(a)] [13].
Even though mipomersen has been approved by the US Food and Drug Administration (FDA) as adjunct therapy for HoFH patients aged ≥ 12 years in the United States, in 2013 the European Medicines Agency (EMA) refused its marketing authorization in Europe because of safety concerns [14, 15]. Consequently, we aimed to perform a meta-analysis on clinical evidence available to date to better define its efficacy and tolerability profile.
2 Methods
The study was designed according to guidelines of the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [16], and was registered in the PROSPERO database (Registration number CRD42019121505). Because of the study design (meta-analysis), neither Institutional Review Board (IRB) approval, nor patient informed consent were required.
2.1 Search Strategy
PubMed, SCOPUS, Google Scholar and ISI Web of Science by Clarivate databases were searched, with no language restriction, using the following search terms: ‘Mipomersen’ AND (‘Clinical trial’ OR ‘Clinical study’). The wild-card term (*) was used to increase the sensitivity of the search strategy, which was limited to studies in humans. The reference lists of identified papers were manually checked for additional relevant articles. In particular, additional searches for potential trials included the references of review articles on that issue and the abstracts from selected congresses on the subject of the meta-analysis. Literature was searched from inception to January 20, 2019.
All paper abstracts were screened by two reviewers (FF and AFGC) in an initial process to remove ineligible articles. The remaining articles were obtained in full-text and assessed again by the same two researchers, who evaluated each article independently and carried out data extraction and quality assessment. Disagreements were resolved by discussion with a third party (NF).
2.2 Study Selection Criteria
Original studies were included if they met the following criteria: (1) being a clinical trial with either multicentre or single-centre design, (2) having an appropriate controlled design for mipomersen treatment, (3) investigating the effect of mipomersen on plasma lipids, (4) testing the safety of mipomersen short and middle-term administration, (5) reporting all the adverse events that occurred during the treatment.
Exclusion criteria were (1) lack of a control group for mipomersen administration, and (2) lack of sufficient information about the prevalence and nature of the adverse events. Studies were also excluded if they contained overlapping subjects with other studies.
2.3 Data Extraction
Data abstracted from the eligible studies were (1) first author’s name; (2) year of publication; (3) study design; (4) main inclusion criteria and underlying disease; (5) treatment duration; (6) study groups; (7) number of participants in the active and control group; (8) age and sex of study participants; and (9) discontinuation of treatment and adverse events occurring during the trials. Missing or unpublished data were sought by trying to contact authors or sponsors via e-mail and repeated messages were sent in case of no response. All data extraction and database typing were reviewed by the principal investigator (AFGC) before the final analysis, and doubts were resolved by mutual agreement among the authors.
2.4 Quality Assessment
A systematic assessment of risk of bias in the included studies was performed using the Cochrane criteria [17]. The following items were used: adequacy of sequence generation, allocation concealment, blinding addressing of dropouts (incomplete outcome data), selective outcome reporting, and other probable sources of bias [18]. Risk-of-bias assessment was performed independently by two reviewers (FF and AFGC); disagreements were resolved by a consensus-based discussion.
2.5 Data Synthesis
Meta-analysis was entirely conducted using Comprehensive Meta-Analysis (CMA) V3 software (Biostat, NJ, USA) [19].
Net changes in the investigated parameters (change scores) were calculated by subtracting the value at baseline from the value after intervention, in both the active-treated and the control group. All values were collated as percent change from baseline. Standard deviations (SDs) of the mean difference were obtained as per the following, reported by Follmann and colleagues: SD = square root [(SDpre-treatment)2 + (SDpost-treatment)2 − (2R × SDpre-treatment × SDpost-treatment)], assuming a correlation coefficient (R) = 0.5 [20]. If the outcome measures were reported in median and range (or 95% confidence interval [CI]), mean and SD values were estimated using the method described by Wan et al. [21]. Where standard error of the mean (SEM) was only reported as a dispersion measure, SD was estimated using the following formula: SD = SEM × square root (n), with n being the number of subjects. To avoid a double-counting problem, in trials comparing multiple treatment arms versus a single control group, the number of subjects in the control group was divided by the required comparisons. Studies’ findings were combined using a fixed-effect model or a random-effect model (using the DerSimonian–Laird method) and the generic inverse variance method, based on the level of interstudy heterogeneity, which was quantitatively assessed using the Higgins index (I2) [22]. Effect sizes for lipid changes were expressed as weighted mean differences (WMD) and 95% CI. For safety analysis, odd ratios (OR) and 95% CI intervals were calculated using the Mantel–Haenszel method [23]. Safety analysis was performed by excluding studies with zero events in both arms. If one or more outcomes could not be extracted from a study, the study was removed only from the analysis involving those outcomes. Adverse events were considered for the analysis only if occurring in at least two of the included clinical trials. In order to evaluate the influence of each study on the overall effect size, sensitivity analysis was conducted using the leave-one-out method (i.e. removing one study at a time and repeating the analysis) [24]. Two-sided p values ≤ 0.05 were considered as statistically significant for all tests.
2.6 Publication Biases
Potential publication biases were explored using visual inspection of Begg’s funnel plot asymmetry, Begg’s rank correlation test, and Egger’s weighted regression test [25]. The Duval and Tweedie ‘trim and fill’ method was used to adjust the analysis for the effects of publication bias [26]. Two-sided p values ≤ 0.05 were considered statistically significant.
3 Results
3.1 Flow and Characteristics of the Included Studies
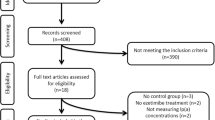
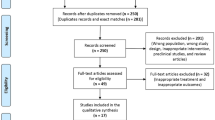
After database searches performed strictly according to inclusion and exclusion criteria, 247 published articles were identified, and the abstracts reviewed. Of these, 192 were excluded because they were non-original articles. Another 37 were eliminated because they did not meet the inclusion criteria. Thus, 18 articles were carefully assessed and reviewed. An additional five studies were excluded because of incomplete data (n = 2) or substantial sample overlap (n = 3) (Appendix 1, see electronic supplementary material [ESM]). Finally, 13 studies were eligible and included in the meta-analysis [27,28,29,30,31,32,33,34,35,36,37,38,39,40]. The study selection process is shown in Fig. 1. Data were pooled from 13 clinical trials comprising 49 treatment arms, which included 1053 subjects, with 729 in the active-treated arm and 324 in the control arm.
Eligible studies were published between 2006 and 2019. Follow-up periods ranged between 3 and 60 weeks and different treatment schedules were tested. All selected trials were designed with parallel groups and were multicentre [27, 31,32,33, 36,37,38] or single-centre [28, 30, 35, 39, 40] clinical studies. Enrolled subjects were adults and young adults with a diagnosis of HeFH [27, 28, 33, 37, 39] or HoFH [38], patients with coronary heart disease, [27, 31,32,33] or with a good status of health [30, 35, 36, 40]. The baseline characteristics of the evaluated studies are summarized in Table 1.
3.2 Risk of Bias Assessment
Almost all of the included studies were characterized by sufficient information regarding sequence generation, allocation concealment, personal and outcome assessments. All studies showed low risk of bias as regards incomplete outcome data and selective outcome reporting. Details of the quality of bias assessment are reported in Table 2.
3.3 Effect of Mipomersen on Lipids
Meta-analysis of data suggested that mipomersen significantly reduced LDL-C (WMD − 1.52, 95% CI − 1.85 to − 1.19; p < 0.001; I2 = 73.6%) (Fig. 2), total cholesterol (TC) (WMD − 1.55, 95% CI − 1.97 to − 1.13; p < 0.001; I2 = 80.3%), non-high density lipoprotein cholesterol (non-HDL-C) (WMD − 1.66, 95% CI − 2.06 to − 1.27; p < 0.001; I2 = 77%), Lp(a) (WMD − 0.99, 95% CI − 1.37 to − 0.62; p < 0.001; I2 = 77.3%), Apo B (WMD − 1.66, 95% CI − 2.04 to − 1.27; p < 0.001; I2 = 80.1%) (Fig. 3), HDL-C (WMD 0.11, 95% CI − 0.03 to 0.26; p = 0.124; I2 = 18.4%), triglycerides (TG) (WMD − 0.61, 95% CI − 0.76 to − 0.46; p < 0.001; I2 = 45.1%], very-low-density lipoprotein cholesterol (VLDL-C) (WMD − 0.58, 95% CI − 0.73 to − 0.43; p < 0.001; I2 = 18.1%) and Apo A-I (WMD − 0.25, 95% CI − 0.51 to − 0.001; p = 0.049; I2 = 54.8%), without affecting HDL-C levels (WMD 0.11, 95% CI − 0.03 to 0.26; p = 0.124; I2 = 18.4%) (Fig. 4). The effect sizes were robust in the leave-one-out sensitivity analysis and not mainly driven by a single study (Figs. S1, S2, see ESM).
Visual inspection of Begg’s funnel plot revealed a slight asymmetry, suggesting potential publication bias for the effect of mipomersen on serum HDL-C concentrations (Fig. 5). This asymmetry was imputed to six potentially missing studies on the right side of the funnel plot, which increased the estimated effect size to 0.16 (95% CI 0.01–0.30), reaching statistical significance. In addition, Duval and Tweedie’s ‘trim and fill’ method yielded three potentially missing studies on the left side of the funnel plot, increasing the effect size on TC to − 1.69 (95% CI − 2.10 to − 1.29); two potentially missing studies on the left side of the plot increasing the effect size on TG to − 0.64 (95% CI − 0.79 to − 0.49); three potentially missing studies on the left side of the plot increasing the effect size on LDL-C to − 1.65 (95% CI − 1.98 to − 1.32); one potentially missing study on the left side of the plot increasing the effect size on non HDL-C to − 1.74 (95% CI − 2.14 to − 1.34); four potentially missing studies on the left side of the funnel increasing the effect size on VLDL to − 0.61 (95% CI − 0.75 to − 0.46); two potentially missing studies on the left side of the plot increasing the effect size on Lp(a) to − 1.11 (95% CI − 1.48 to − 0.73), four potentially missing studies on the left side of the plot increasing the effect size on Apo B to − 1.83 (95% CI − 2.21 to − 1.45) and seven potentially missing studies on the left side of the plot that lowered the effect size on Apo A-I to − 0.45 (95% CI − 0.68 to − 0.21). However, Begg’s rank correlation confirmed the presence of publication bias only for LDL-C (p = 0.03), non-HDL-C (p = 0.047) and Apo B (p = 0.03) and similar results were not observed even with Egger’s linear regression method (p > 0.05 always).
The classic fail-safe N test suggested that 1311 studies with negative results would be needed to bring the estimated effect size on TC to a non-significant level (p > 0.05); 147 studies with negative results would be needed to bring the estimated effect size on TG to a non-significant level (p > 0.05); 2476 studies with negative results would be needed to bring the estimated effect size on non LDL-C to a non-significant level (p > 0.05); 170 studies with negative results would be needed to bring the estimated effect size on VLDL to a non-significant level (p > 0.05); 419 studies with negative results would be needed to bring the estimated effect size on Lp(a) to a non-significant level (p > 0.05); 2789 studies with negative results would be needed to bring the estimated effect size on Apo B to a non-significant level (p > 0.05) and 27 studies with negative results would be needed to bring the estimated effect size on Apo A-I to a non-significant level (p > 0.05).
3.4 Safety Analysis
Primary outcomes were adverse events leading to discontinuation of treatment, death, MACE (i.e. acute myocardial infarction, coronary artery disease, angina pectoris, unstable angina, supraventricular extrasystoles, cardiac failure, ischaemic stroke), injection-site reaction (i.e. injection-site erythema, discolouration, pain, swelling, pruritus, haematoma, induration, discomfort, inflammation, nodule), fatigue, headache, dizziness, flu, flu-like symptoms, nasopharyngitis, cough, muscle symptoms (i.e. muscle fatigue, myalgia, muscle spasms, muscle stiffness), back pain, pain in extremity, chest pain, hepatic steatosis, nausea, constipation, diarrhoea, upper abdominal pain, lower abdominal pain, upper respiratory tract infection, urinary tract infection, creatinine elevation, hepatic enzymes elevation and C-reactive protein (CRP) elevation.
Mipomersen was positively associated with an increased risk of discontinuation of treatment, injection-site reaction, hepatic steatosis, hepatic enzymes elevation and flu-like symptoms (Fig. 6; Table S1 [see ESM]). These findings were robust in the leave-one-out sensitivity analyses (Fig. S3, see ESM). The incidence of the other adverse events did not differ between groups (Table S1, see ESM).
Visually, the funnel plot of standard error by log odds ratio was slightly asymmetric for risk of injection-site reaction and hepatic steatosis. This asymmetry was imputed to ten potentially missing studies on the left side of the funnel plot that lowered the estimated risk of injection-site reaction to 9.71 (95% CI 6.84–13.79) and two potentially missing studies on the left side of the plot that lowered the estimated risk of hepatic steatosis to 3.92 (95% CI 1.74–8.84) (Fig. S4, see ESM). In addition, Duval and Tweedie’s ‘trim and fill’ method yielded three potentially missing studies on the right side of the funnel plot, increasing the estimated risk of hepatic enzymes elevation to 4.46 (95% CI 2.64–7.53), and two potentially missing studies always on the right side of the plot, increasing the estimated risk of flu-like symptoms to 2.07 (95% CI 1.49–2.87) (Fig. S5, see ESM). However, Egger’s linear regression and Begg’s rank correlation did not confirm the presence of any publication bias in the current meta-analysis (p > 0.05 always). Finally, the classic fail-safe N test suggested that 55 studies with negative results would be needed to bring the estimated risk of treatment discontinuation to a non-significant level (p > 0.05); 1037 studies with negative results would be needed to bring the estimated risk for injection-site reaction to a non-significant level (p > 0.05); 12 studies with negative results would be needed to bring the estimated risk of hepatic steatosis to a non-significant level (p > 0.05); 28 studies with negative results would be needed to bring the estimated risk of hepatic enzymes elevation to a non-significant level (p > 0.05) and 34 studies with negative results would be needed to bring the estimated risk of flu-like symptoms to a non-significant level (p > 0.05).
4 Discussion
By analysing data from 13 randomized control studies including a total of 1053 patients, this meta-analysis showed that mipomersen significantly reduced TC (− 21.4%); LDL-C (− 26.4%); TG (− 16.2%); Lp(a) (− 22.7%); VLDL-C (− 19.6%); and non-HDL-C (− 28.1%) without affecting HDL-C levels (+ 1.4%). These findings strengthen those previously reported by Panta et al., based on 462 patients [41].
Despite favourable effects on the lipid profile, including Lp(a), some concerns are reinforced from the safety profile. As a matter of fact, mipomersen therapy is more likely discontinued and associated with increased risk of injection-site reactions, hepatic steatosis, hepatic enzyme elevation, and flu-like symptoms. Elevated liver enzymes can be attenuated by reducing the mipomersen dose, reducing dosing frequency, or temporarily stopping mipomersen [27, 32, 35, 42]. However, there is limited evidence on the effect of mipomersen on hepatic steatosis or fibrosis assessed by liver biopsies [43], and so longer-term follow-up studies with mipomersen are needed to evaluate the longer-term risk for permanent hepatic injury and adverse histological change.
Interestingly, only one of the currently available controlled clinical trials enrolled patients with HoFH [38]; consequently, the current pharmacological indication of mipomersen seems not to be supported by an adequate body of evidence.
Results from clinical trials conducted with drugs affecting serum LDL-C levels have shown that every 1 mmol/L (39 mg/dL) reduction in LDL-C levels is associated with a 19% reduction in coronary mortality as well as a 22% reduction in MACE [44]. In accordance with the latest European guidelines [45], LDL-C targets in HoFH are < 2.5 mmol/L (< 100 mg/dL) [< 3.5 mmol/L (< 135 mg/dL) in children], or < 1.8 mmol/L (< 70 mg/dL) in adults with clinical atherosclerotic cardiovascular disease. However, the majority of FH patients do not reach their LDL-C guideline recommended goals, although mipomersen has been shown to increase the attainment rates. Nevertheless, the MACE rate in the mipomersen trials was 9.5 events/1000 months of treatment, which is equivalent to an 11.4% annualized event rate [46]. Conversely, the lomitapide studies reported a rate of 1.7 MACE events/1000 months (equivalent to a 2.0% annualized event rate) [47, 48], and the TAUSSIG (Trial Assessing Long-Term Use of PCSK9 Inhibition in Subjects with Genetic LDL Disorders) trial had a MACE rate of 1.8 events/1000 months (equivalent to a 2.1% annualized event rate) [49]. For this reason, lomitapide and the PCSK9 inhibitor evolocumab may represent an optimal therapeutic opportunity for homozygous FH patients as an alternative to mipomersen. As a matter of fact, both TESLA (Trial Evaluating PCSK9 Antibody in Subjects With LDL Receptor Abnormalities) and TAUSSIG clinical trials showed that the PCSK9 inhibitor evolocumab promotes a 20–30% reduction in LDL-C in HoFH patients on top of conventional lipid-lowering therapies, and also lomitapide seems to be pretty promising [49,50,51,52], with a better safety profile than mipomersen. In addition, the use of evolocumab as an adjunctive therapy for HoFH subjects seems to be further supported by the markedly elevated PCSK9 levels that HoFH patients have in comparison with HeFH or non-FH subjects [53].
Our analysis also shows that mipomersen reduces Lp(a) by 22.7%, which represents an independent risk factor for the development of cardiovascular disease [54]. This effect, which has just been investigated by Nandakumar et al. [55], seems to be due to an increased fractional catabolic rate (FCR) of Lp(a) and could definitely contribute to the reduction of the CV risk, especially in patients with high Lp(a) levels at baseline. However, in the light recent statements by Burgess et al., this essay has to be critically evaluated [56].
Finally, mipomersen antisense inhibition of apoB synthesis reduces plasma concentrations of apolipoprotein C-III (apo C-III) and apo C-III-containing lipoproteins by which TG and VLDL-C decrease [57]. Remarkably, this is of particular importance since lower concentrations of apoC-III and LDL with apoC-III have been associated with reduced risk of cardiovascular disease regardless of other traditional risk factors [57, 58].
In conclusion, the current meta-analysis demonstrates the positive effects of mipomersen on lipid profile, but also emphasizes the multiple adverse effects exerted by mipomersen with uncertainty regarding long-term effects, such as risk for hepatic injury. To date, there is no apparent reason to stop taking mipomersen when it is effective and well tolerated, even though a careful monitoring of liver function is needed. Furthermore, the PCSK9 monoclonal antibodies should be considered as a valid alternative to mipomersen for the treatment of HoFH patients. As a matter of fact, the development of the PCSK9 inhibitor evolocumab showed efficacy similar to mipomersen with respect to LDL-C levels, with a better safety profile.
References
Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Borén J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjærg-Hansen A, European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478-90a. https://doi.org/10.1093/eurheartj/eht273.
Vallejo-Vaz AJ, Kondapally Seshasai SR, Cole D, Hovingh GK, Kastelein JJ, Mata P, Raal FJ, Santos RD, Soran H, Watts GF, Abifadel M, Aguilar-Salinas CA, Akram A, Alnouri F, Alonso R, Al-Rasadi K, Banach M, Bogsrud MP, Bourbon M, Bruckert E, Car J, Corral P, Descamps O, Dieplinger H, Durst R, Freiberger T, Gaspar IM, Genest J, Harada-Shiba M, Jiang L, Kayikcioglu M, Lam CS, Latkovskis G, Laufs U, Liberopoulos E, Nilsson L, Nordestgaard BG, O’Donoghue JM, Sahebkar A, Schunkert H, Shehab A, Stoll M, Su TC, Susekov A, Widén E, Catapano AL, Ray KK. Familial hypercholesterolaemia: a global call to arms. Atherosclerosis. 2015;243(1):257–9. https://doi.org/10.1016/j.atherosclerosis.2015.09.021.
Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen-Thiessen E, Tybjærg-Hansen A, Watts GF, Averna M, Boileau C, Borén J, Catapano AL, Defesche JC, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Ray KK, Stalenhoef AF, Stroes E, Taskinen MR, Wiegman A, Wiklund O, Chapman MJ, European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35(32):2146–57. https://doi.org/10.1093/eurheartj/ehu274.
Brautbar A, Leary E, Rasmussen K, Wilson DP, Steiner RD, Virani S. Genetics of familial hypercholesterolemia. Curr Atheroscler Rep. 2015;17(4):491. https://doi.org/10.1007/s11883-015-0491-z.
Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97(11):3956–64. https://doi.org/10.1210/jc.2012-1563.
Reiner Ž. Management of patients with familial hypercholesterolaemia. Nat Rev Cardiol. 2015;12(10):565–75. https://doi.org/10.1038/nrcardio.2015.92.
Landmesser U, Chapman MJ, Stock JK, Amarenco P, Belch JJF, Borén J, Farnier M, Ference BA, Gielen S, Graham I, Grobbee DE, Hovingh GK, Lüscher TF, Piepoli MF, Ray KK, Stroes ES, Wiklund O, Windecker S, Zamorano JL, Pinto F, Tokgözoglu L, Bax JJ, Catapano AL. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J. 2018;39(14):1131–43. https://doi.org/10.1093/eurheartj/ehx549.
Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA, TESLA Investigators. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–50. https://doi.org/10.1016/S0140-6736(14)61374-X.
Raal FJ, Hovingh GK, Blom D, Santos RD, Harada-Shiba M, Bruckert E, Couture P, Soran H, Watts GF, Kurtz C, Honarpour N, Tang L, Kasichayanula S, Wasserman SM, Stein EA. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 2017;5(4):280–90. https://doi.org/10.1016/S2213-8587(17)30044-X.
Rader DJ, Kastelein JJ. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129(9):1022–32. https://doi.org/10.1161/CIRCULATIONAHA.113.001292.
Crooke ST, Geary RS. Clinical pharmacological properties of mipomersen (Kynamro), a second generation antisense inhibitor of apolipoprotein B. Br J Clin Pharmacol. 2013;76(2):269–76. https://doi.org/10.1111/j.1365-2125.2012.04469.x.
Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356(2):148–56.
Thomas T, Ginsberg H. Development of apolipoprotein B antisense molecules as a therapy for hyperlipidemia. Curr Atheroscler Rep. 2010;12(1):58–65. https://doi.org/10.1007/s11883-009-0078-7.
Levin AA. Treating disease at the RNA Level with oligonucleotides. N Engl J Med. 2019;380(1):57–70. https://doi.org/10.1056/NEJMra1705346.
Parham JS, Goldberg AC. Mipomersen and its use in familial hypercholesterolemia. Expert Opin Pharmacother. 2019;20(2):127–31. https://doi.org/10.1080/14656566.2018.1550071.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.0. 2. Chichester: Wiley; 2009 (Ref Type: Report; 2010).
Fogacci F, Banach M, Mikhailidis DP, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group; International Lipid Expert Panel (ILEP), et al. Safety of red yeast rice supplementation: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;143:1–16.
Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis version 3. Englewood: Biostat; 2005. p. 104.
Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–73.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;19(14):135. https://doi.org/10.1186/1471-2288-14-135.
Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20:123–9. https://doi.org/10.1111/1469-0691.12494.
Haenszel W, Hon NB. Statistical approaches to the study of cancer with particular reference to case registers. J Chronic Dis. 1956;4(6):589–99.
Fogacci F, Banach M, Cicero AFG. Resveratrol effect on patients with non-alcoholic fatty liver disease: a matter of dose and treatment length. Diabetes Obes Metab. 2018;20(7):1798–9. https://doi.org/10.1111/dom.13324.
Sahebkar A, Pirro M, Reiner Ž, et al. A systematic review and meta-analysis of controlled trials on the effects of statin and fibrate therapies on plasma homocysteine levels. Curr Med Chem. 2016;23(39):4490–503.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Reeskamp LF, Kastelein JJP, Moriarty PM, Duell PB, Catapano AL, Santos RD, Ballantyne CM. Safety and efficacy of mipomersen in patients with heterozygous familial hypercholesterolemia. Atherosclerosis. 2019;280:109–17.
Waldmann E, Vogt A, Crispin A, Altenhofer J, Riks I, Parhofer KG. Effect of mipomersen on LDL-cholesterol in patients with severe LDL-hypercholesterolaemia and atherosclerosis treated by lipoprotein apheresis (the MICA-study). Atherosclerosis. 2017;259:20–5. https://doi.org/10.1016/j.atherosclerosis.2017.02.019.
Waldmann E, Vogt A, Crispin A, Altenhofer J, Riks I, Parhofer KG. Corrigendum to: “Effect of mipomersen on LDL-cholesterol in patients with severe LDL-hypercholesterolaemia and atherosclerosis treated by lipoprotein apheresis (The MICA-Study)” [Atherosclerosis 259 (2017 Apr) 20-25]. Atherosclerosis. 2018;275:461–2. https://doi.org/10.1016/j.atherosclerosis.2018.05.020.
Flaim JD, Grundy JS, Baker BF, McGowan MP, Kastelein JJ. Changes in mipomersen dosing regimen provide similar exposure with improved tolerability in randomized placebo-controlled study of healthy volunteers. J Am Heart Assoc. 2014;3(2):e000560. https://doi.org/10.1161/JAHA.113.000560.
Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2013;62(23):2178–84. https://doi.org/10.1016/j.jacc.2013.07.081.
McGowan MP, Tardif JC, Ceska R, Burgess LJ, Soran H, Gouni-Berthold I, Wagener G, Chasan-Taber S. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One. 2012;7(11):e49006. https://doi.org/10.1371/journal.pone.0049006.
Stein EA, Dufour R, Gagne C, Gaudet D, East C, Donovan JM, Chin W, Tribble DL, McGowan M. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 2012;126(19):2283–92. https://doi.org/10.1161/CIRCULATIONAHA.112.104125.
Visser ME, Wagener G, Baker BF, Geary RS, Donovan JM, Beuers UH, Nederveen AJ, Verheij J, Trip MD, Basart DC, Kastelein JJ, Stroes ES. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur Heart J. 2012;33(9):1142–9. https://doi.org/10.1093/eurheartj/ehs023.
Akdim F, Tribble DL, Flaim JD, Yu R, Su J, Geary RS, Baker BF, Fuhr R, Wedel MK, Kastelein JJ. Efficacy of apolipoprotein B synthesis inhibition in subjects with mild-to-moderate hyperlipidaemia. Eur Heart J. 2011;32(21):2650–9. https://doi.org/10.1093/eurheartj/ehr148.
Akdim F, Stroes ES, Sijbrands EJ, Tribble DL, Trip MD, Jukema JW, Flaim JD, Su J, Yu R, Baker BF, Wedel MK, Kastelein JJ. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol. 2010;55(15):1611–8. https://doi.org/10.1016/j.jacc.2009.11.069.
Akdim F, Visser ME, Tribble DL, Baker BF, Stroes ES, Yu R, Flaim JD, Su J, Stein EA, Kastelein JJ. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol. 2010;105(10):1413–9. https://doi.org/10.1016/j.amjcard.2010.01.003.
Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9719):998–1006. https://doi.org/10.1016/S0140-6736(10)60284-X.
Visser ME, Akdim F, Tribble DL, Nederveen AJ, Kwoh TJ, Kastelein JJ, Trip MD, Stroes ES. Effect of apolipoprotein-B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lipid Res. 2010;51(5):1057–62. https://doi.org/10.1194/jlr.M002915.
Kastelein JJ, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, Chuang E, Graham MJ, Crooke RM. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114(16):1729–35.
Panta R, Dahal K, Kunwar S. Efficacy and safety of mipomersen in treatment of dyslipidemia: a meta-analysis of randomized controlled trials. J Clin Lipidol. 2015;9(2):217–25. https://doi.org/10.1016/j.jacl.2014.12.006.
Santos RD, Duell PB, East C, Guyton JR, Moriarty PM, Chin W, Mittleman RS. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur Heart J. 2015;36(9):566–75. https://doi.org/10.1093/eurheartj/eht549.
Hashemi N, Odze RD, McGowan MP, Santos RD, Stroes ES, Cohen DE. Liver histology during Mipomersen therapy for severe hypercholesterolemia. J Clin Lipidol. 2014;8(6):606–11. https://doi.org/10.1016/j.jacl.2014.08.002.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78 (Epub 2005 Sep 27; Erratum in: Lancet. 2005 Oct 15-21;366(9494):1358. Erratum in: Lancet. 2008 Jun 21;371(9630):2084).
Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT, ESC Scientific Document Group. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. https://doi.org/10.1093/eurheartj/ehw272.
Duell PB, Santos RD, Kirwan BA, Witztum JL, Tsimikas S, Kastelein JJP. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol. 2016;10(4):1011–21. https://doi.org/10.1016/j.jacl.2016.04.013.
Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AM, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ, Phase 3 HoFH Lomitapide Study investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381(9860):40–6. https://doi.org/10.1016/S0140-6736(12)61731-0.
Blom DJ, Averna MR, Meagher EA, du Toit Theron H, Sirtori CR, Hegele RA, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Larrey D, Bloedon LT, Foulds P, Rader DJ, Cuchel M. Long-term efficacy and safety of the microsomal triglyceride transfer protein inhibitor lomitapide in patients with homozygous familial hypercholesterolemia. Circulation. 2017;136(3):332–5. https://doi.org/10.1161/CIRCULATIONAHA.117.028208.
Raal FJ, Hovingh GK, Blom D, Santos RD, Harada-Shiba M, Bruckert E, Couture P, Soran H, Watts GF, Kurtz C, Honarpour N, Tang L, Kasichayanula S, Wasserman SM, Stein EA. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 2017;5(4):280–90. https://doi.org/10.1016/S2213-8587(17)30044-X.
Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA, TESLA Investigators. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–50. https://doi.org/10.1016/S0140-6736(14)61374-X.
Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128(19):2113–20. https://doi.org/10.1161/CIRCULATIONAHA.113.004678.
Lambert G, Chatelais M, Petrides F, Passard M, Thedrez A, Rye KA, Schwahn U, Gusarova V, Blom DJ, Sasiela W, Marais AD. Normalization of low-density lipoprotein receptor expression in receptor defective homozygous familial hypercholesterolemia by inhibition of PCSK9 with alirocumab. J Am Coll Cardiol. 2014;64(21):2299–300. https://doi.org/10.1016/j.jacc.2014.07.995.
Raal F, Panz V, Immelman A, Pilcher G. Elevated PCSK9 levels in untreated patients with heterozygous or homozygous familial hypercholesterolemia and the response to high-dose statin therapy. J Am Heart Assoc. 2013;2(2):e000028. https://doi.org/10.1161/JAHA.112.000028.
Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgözoglu L, Tybjærg-Hansen A, European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–53. https://doi.org/10.1093/eurheartj/ehq386.
Nandakumar R, Matveyenko A, Thomas T, Pavlyha M, Ngai C, Holleran S, Ramakrishnan R, Ginsberg HN, Karmally W, Marcovina SM, Reyes-Soffer G. Effects of mipomersen, an apolipoprotein B100 antisense, on lipoprotein (a) metabolism in healthy subjects. J Lipid Res. 2018;59(12):2397–402. https://doi.org/10.1194/jlr.P082834.
Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, Willeit P, Young R, Surendran P, Karthikeyan S, Bolton TR, Peters JE, Kamstrup PR, Tybjærg-Hansen A, Benn M, Langsted A, Schnohr P, Vedel-Krogh S, Kobylecki CJ, Ford I, Packard C, Trompet S, Jukema JW, Sattar N, Di Angelantonio E, Saleheen D, Howson JMM, Nordestgaard BG, Butterworth AS, Danesh J, European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD) Consortium. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619–27. https://doi.org/10.1001/jamacardio.2018.1470.
Furtado JD, Wedel MK, Sacks FM. Antisense inhibition of apoB synthesis with mipomersen reduces plasma apoC-III and apoC-III-containing lipoproteins. J Lipid Res. 2012;53(4):784–91. https://doi.org/10.1194/jlr.P021717.
Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, Willeit J, Kiechl S, Mayr M. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol. 2017;69(7):789–800. https://doi.org/10.1016/j.jacc.2016.11.065.
Acknowledgements
Funding
This paper was written independently; no company or institution supported it financially. No professional writer was involved in the preparation of this meta-analysis.
Declaration of interest
Arrigo F.G. Cicero provided scientific consultancies for Mylan and Menarini; Alberto Corsini has received honoraria from AstraZeneca, AMGEN, Sanofi, Recordati, Novartis, MSD, Mediolanum, DOC, Mylan and Pfizer; Nicola Ferri has received honoraria from Daichii-Sankyo, DOC, Mylan and Pfizer; Federica Fogacci has served as a consultant to Mylan; Peter P. Toth has served on the speakers bureau of Amarin, Amgen, Kowa, Merck, Novo-Nordisk, Regeneron, Sanofi, and has served as a consultant to Amarin, Amgen, AstraZeneca, Kowa, Merck, Nov-Nordisk, and Theravance; Massimiliano Ruscica has no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fogacci, F., Ferri, N., Toth, P.P. et al. Efficacy and Safety of Mipomersen: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Drugs 79, 751–766 (2019). https://doi.org/10.1007/s40265-019-01114-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01114-z