Abstract
Lisdexamfetamine dimesylate (lisdexamfetamine; Elvanse®; Tyvense®), an orally-active dexamfetamine prodrug, is indicated in the EU for the treatment of attention-deficit hyperactivity disorder (ADHD) in children aged ≥ 6 years (including adolescents) when the response to previous methylphenidate (MPH) treatment is clinically inadequate. The original approval of the drug was based on the results of phase III trials in children and adolescents with ADHD who had an inadequate response to previous pharmacotherapy (e.g. MPH) or were treatment naïve. In these studies, short-term treatment with flexibly-dosed lisdexamfetamine demonstrated greater efficacy than atomoxetine, based on a prospective comparison, and osmotic-release oral system (OROS)-MPH, based on a post hoc comparison. Improvements in ADHD symptoms were accompanied by improvements in health-related quality of life and functioning that were maintained as long as treatment with lisdexamfetamine was continued in a long-term extension of one of these trials. In subsequent phase IV head-to-head studies in adolescents with ADHD and an inadequate response to previous pharmacotherapy, lisdexamfetamine demonstrated greater efficacy than OROS-MPH when both medications were force-titrated, but not when they were flexibly-titrated. Lisdexamfetamine was generally well tolerated, with an adverse event profile (e.g. decreased appetite, headache, weight reduction, insomnia and irritability) typical of that reported for other stimulants. Thus, lisdexamfetamine provides an alternative option for the treatment of children and/or adolescents with ADHD who have not responded adequately to previous ADHD pharmacotherapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Long-acting dexamfetamine prodrug that requires in vivo hydrolysis to release the active molecule |
Prodrug design prevents mechanical drug tampering |
Offers convenient, once-daily oral administration |
Efficacy demonstrated in well-designed studies |
Tolerability profile similar to that of other stimulants; most treatment-emergent adverse events are mild to moderate in severity |
1 Introduction
Attention-deficit hyperactivity disorder (ADHD) is a neurobehavioral disorder that, although usually first diagnosed in childhood, often lasts into adolescence and adulthood [1]. It is characterized by persistent core symptoms of hyperactivity, impulsivity and/or inattention, and is associated with impaired functioning [2, 3], a reduced quality of life [4] and a considerable economic impact [5]. ADHD is estimated to affect just under 5% of children and adolescents in Europe [6].
The approach to managing ADHD in school-age children and adolescents is often multimodal, combining nonpharmacological strategies, such as behavioural and psychoeducational interventions, with pharmacological treatments, such as the stimulants methylphenidate (MPH) and amphetamine and the non-stimulants atomoxetine and guanfacine extended release [3, 7, 8]. Stimulants are the most effective and commonly prescribed ADHD medications [9,10,11,12]; long-acting formulations are potentially advantageous over short-acting preparations, as they may improve adherence and reduce the likelihood for abuse [13,14,15].
Lisdexamfetamine dimesylate (lisdexamfetamine) is a long-acting amfetamine prodrug that is administered orally once daily [16, 17]. In the EU, lisdexamfetamine (Elvanse®; Tyvense®) is indicated as part of a comprehensive treatment programme for ADHD in children aged ≥ 6 years (including adolescents) when the response to previous MPH treatment is considered clinically inadequate [18]. The drug has also been approved for use in adults with ADHD under the EU decentralization procedure, with the first approvals in the UK, Sweden and Denmark [19]. In the USA, lisdexamfetamine (Vyvanse®) has been approved for the treatment of ADHD in patients aged 6 years and over [20].
This article provides an overview of the pharmacological properties of lisdexamfetamine and reviews, from an EU perspective, recent clinical data relevant to its use in the treatment of children and adolescents with ADHD who have not responded adequately to previous therapy.
2 Pharmacological Properties of Lisdexamfetamine
Lisdexamfetamine is a pharmacologically inactive and water soluble prodrug of dextroamfetamine (dexamfetamine, d-amfetamine), a non-catecholamine sympathomimetic amine with potent central stimulant activity [16, 18, 20, 21]. The mechanism whereby d-amfetamine improves ADHD symptoms is not fully understood, but is thought to be related to its activity as an indirect dopamine and noradrenaline agonist [16].
Lisdexamfetamine comprises l-lysine amino acid covalently bonded to d-amfetamine; this structure prevents mechanical drug tampering [16]. Orally administered lisdexamfetamine is absorbed into the circulation where the covalent bond is hydrolysed to release active d-amfetamine and l-lysine [18, 20]. The absorption of lisdexamfetamine is thought to involve a high-capacity system (peptide transporter 1 in the small intestine); the rate-limiting step of its conversion to d-amfetamine (primarily by red blood cells) also appears to involve a high-capacity system [22]. Studies have suggested that gastrointestinal pH and changes in normal gastrointestinal transit times are unlikely to affect the conversion of lisdexamfetamine to d-amfetamine [16].
In children aged 6–12 years with ADHD, the times to peak plasma concentration of the intact prodrug and d-amfetamine were ≈ 1 and ≈ 3.5 h, respectively; d-amfetamine demonstrated linear pharmacokinetics following single-dose administration of lisdexamfetamine 30–70 mg [18, 20]. In healthy adults, the pharmacokinetic parameters of d-amfetamine derived from lisdexamfetamine exhibited low interpatient/intersubject and intrasubject variability [20]; d-amfetamine steady-state concentrations were achieved by day 5 [21].
Lisdexamfetamine is not metabolized by CYP enzymes; metabolism of d-amfetamine involves hydroxylation, deamination and conjugation [18]. Renal excretion is the primary route of elimination of the intact prodrug as well as d-amfetamine and its metabolites; ≈ 96% of the lisdexamfetamine dose is recovered in the urine over a period of 120 h [18, 20]. The plasma elimination half-lives of lisdexamfetamine and d-amfetamine are 0.4 and 10.1 h, respectively [21]. In terms of clearance, lisdexamfetamine demonstrates similar pharmacokinetics in subpopulations of paediatric patients (children aged 6–12 years and adolescents aged 13–17 years with ADHD) and healthy adults [18].
In a study in otherwise healthy subjects with varying degrees of renal impairment, mean d-amfetamine clearance was higher in those with normal renal function compared with those with severely impaired renal function (glomerular filtration rate 15 to < 30 mL/min/1.73 m2) and those with end-stage renal disease (0.7 vs. 0.4 and 0.3 L/h/kg, respectively) [18, 20]. Studies have not been conducted in patients with hepatic impairment [18, 20].
Lisdexamfetamine has a low potential for drug-drug interactions [20, 21]; any interactions involving the drug are likely to be due to d-amfetamine and its metabolites [18, 23]. In this respect, agents that acidify (e.g. ascorbic acid) and alkalinize (e.g. sodium bicarbonate) urine shorten and extend the half-life of amfetamine, respectively [18]. Lisdexamfetamine (amfetamine) should not be administered concomitantly or within 2 weeks after discontinuing monoamine oxidase inhibitor treatment because of the potential for precipitating hypertensive crisis [18].
3 Therapeutic Efficacy of Lisdexamfetamine
This section focuses on findings from six fully published trials of lisdexamfetamine: three phase III studies (nos. 317 [24], 325 [25] and 326 [26]) and three phase IV studies (nos. 404 [27], 405 [28, 29] and 406 [28, 30]) in children and adolescents [24,25,26,27] or adolescents only [28,29,30]. Five of these studies were conducted exclusively (325 and 404) or partly (317, 326 and 406) in Europe; study 405 was conducted entirely in the USA. Study 404 was primarily designed to evaluate the long-term tolerability of lisdexamfetamine (Sect. 4.2); efficacy was assessed as a secondary objective. The results of other, older phase II and III studies in children or adolescents with ADHD that were conducted entirely in the USA are not discussed, but are reviewed elsewhere [23].
3.1 Short-Term Treatment
The short-term efficacy of lisdexamfetamine in children and/or adolescents with ADHD has been assessed in four randomized, double-blind, placebo- or active-controlled, multicentre trials of 6–9 weeks’ duration (studies 317, 325, 405 and 406) [24, 25, 28,29,30].
Eligible patients were males and females aged 6–17 (13–17 [28]) years with a primary diagnosis of ADHD (according to DSM-IV-TR criteria) and symptoms of at least moderate severity [baseline ADHD rating scale version IV (ADHD-RS-IV) total score ≥ 28]. All four studies excluded patients who had failed to (fully [28,29,30]) respond to previous MPH or amfetamine therapy as well as those who were well controlled on their current ADHD medication [24, 25, 29, 30]. Study 317 exclusively enrolled patients who had responded inadequately to previous MPH therapy [24]. In contrast, inadequate response to prior MPH was not a specified inclusion criterion in studies 325 [25], 405 [29] and 406 [30]; therefore, these three studies could have enrolled patients who had a better than minimal, but less than satisfactory, response to their current ADHD medication (e.g. MPH) or were treatment-naïve.
Atomoxetine, a long-acting oral non-stimulant, was included as an active comparator in study 317 [24]; osmotic-release oral system (OROS)-MPH, a long-acting oral stimulant, was included as an active comparator in studies 405 [28] and 406 [28], and as an active reference arm in (placebo-controlled) study 325 [25]. All study medications were administered once daily at dosages approved for use in Europe and/or the USA. The most notable difference between the two regions is that the maximum licensed dosage of OROS-MPH is higher in the USA than in Europe (72 vs. 54 mg/day). However, in the UK, for example, OROS-MPH dosages of up to 108 mg/day can be prescribed under the direction of a specialist [31].
Studies 317 [24], 325 [25] and 405 [28] used a flexible-titration design, whereas study 406 [28] adopted a force-titration design. In the flexible-dose trials [24, 25, 28], study medications were titrated (at weekly intervals) to the optimal dosage over a period of 4 [24, 25] or 5 [28] weeks; the optimum dosage produced an ‘acceptable’ response [defined as a ≥ 30% reduction in ADHD-RS-IV total score from baseline and a Clinical Global Impressions-Improvement (CGI-I) rating of 1 (very much improved) or 2 (much improved), with tolerable adverse effects]. Participants who achieved an acceptable response were maintained on their optimal dosage for the remaining 3 [25, 28] or 5 [24] weeks of double-blind treatment. In the fixed-dose trial [28], the dosages of the study medications were progressively increased (at weekly intervals) to the maximum licenced dosages in the USA over a period of 4 weeks and then maintained at that level for the remaining 2 weeks of double-blind treatment.
Efficacy analyses were performed on the full analysis set (FAS); effect sizes (ESs) of 0.2, 0.5 and 0.8 were deemed to be small, medium and large, respectively.
Study 325 participants who received ≥ 4 weeks’ double-blind treatment, reached the last visit of the dose-optimization period, and completed a 1-week post-treatment washout period, were eligible to enter an extension (study 326 [26]; Sect. 3.2).
3.1.1 Comparisons with Placebo
In studies 325 [25], 405 [28] and 406 [28], flexible- or fixed-dosing with lisdexamfetamine was more effective than placebo in reducing ADHD symptoms in children and/or adolescents who had previously responded inadequately (i.e. less than optimally) to MPH or amfetamine therapy. At endpoint [25] or end-of-study [28], lisdexamfetamine was superior to placebo with respect to the reduction from baseline in the investigator-rated ADHD-RS-IV total score and the proportion of patients achieving a CGI-I rating of 1 or 2, which were typically the primary and key secondary efficacy measures, respectively (Table 1). Findings for the ADHD-RS-IV hyperactivity/impulsivity and inattentiveness subscale scores reflected those for the ADHD-RS-IV total score [25, 28].
Improvements relative to placebo in ADHD symptoms in patients receiving lisdexamfetamine were rapid in onset and large in magnitude in study 325 [25]. The difference (lisdexamfetamine–placebo) in the least-squares mean (LSM) change from baseline in ADHD-RS-IV total score was significant (p < 0.001) at the first post-baseline visit (week 1) and all subsequent post-baseline visits [25]. The lisdexamfetamine ES versus placebo based on change from baseline to endpoint in ADHD-RS-IV total score was 1.80 [25].
Patients receiving lisdexamfetamine in this study experienced significant improvements in ADHD symptoms compared with patients receiving placebo, irrespective of their age, sex or baseline disease severity [32]. Moreover, the beneficial effect of lisdexamfetamine was similar in subgroups of patients categorized according to whether or not they had previously received ADHD medication, including MPH, and was similar to that seen in the overall study population (Fig. 1) [33].
Post hoc subgroup analyses of the primary efficacy measure in study 325 according to previous treatment for ADHD [33]. Effect sizes relative to placebo are shown below the bars. ADHD attention-deficit hyperactivity disorder, ADHD-RS-IV ADHD rating scale IV, OROS osmotic-release oral system
Lisdexamfetamine also demonstrated greater efficacy than placebo in terms of the proportion of patients achieving a clinically significant response (defined as a ≥ 30% reduction from baseline in ADHD-RS-IV total score and a CGI-I score of 1 or 2) at endpoint (i.e. the last on-treatment visit with valid data; 74.2 vs. 10.7%; p < 0.001) [post hoc analysis] [34].
After administration of lisdexamfetamine in the early morning (07:00 h), improvements relative to placebo in ADHD-related symptoms and problem behaviours were maintained throughout the day and were ongoing in the early evening (18:00 h; last assessment), as evaluated using an abbreviated version of the Connors’ Parent Rating Scale-Revised (CPRS-R) in study 325 [35]. At endpoint (i.e. the last on-treatment visit with valid data), the difference in LSM change from baseline in CPRS-R total score significantly (p < 0.001) favoured lisdexamfetamine over placebo at all three time-of-day assessments [10:00 h (ES = 1.42), 14:00 h (1.41) and 18:00 h (1.30)]. Compared with patients receiving placebo, those receiving lisdexamfetamine experienced significant (p < 0.001) improvements in all four CPRS-R subscale scores averaged across the day [ADHD index (ES = 1.54), oppositional (0.95), hyperactivity (1.22) and cognitive (1.21)] [35].
In addition to providing symptomatic relief, treatment with lisdexamfetamine resulted in improvements in health-related quality of life (HRQOL) and daily functioning, as assessed using the Child Health and Illness Profile-Child Edition: Parent Report Form (CHIP-CE:PRF) and the Weiss Functional Impairment Rating Scale-Parent Report (WFIRS-P) instruments, respectively, in study 325 [36]. At endpoint (i.e. the last on-treatment visit with valid data), improvements from baseline in T-scores of four domains of the CHIP-CE:PRF in which mean T-scores at baseline were at least one population standard deviation (SD) below the normative mean, were significant (p < 0.05) with lisdexamfetamine versus placebo [achievement (ES = 1.280), risk avoidance (1.079), resilience (0.421) and satisfaction (0.365)]. No significant difference was seen in the only other domain of this instrument (comfort), in which T-scores at baseline were closer to the normative mean. Compared with placebo-treated patients, lisdexamfetamine-treated patients experienced significant (p < 0.001) improvements from baseline to endpoint in WFIRS-P total score (ES = 0.924) and four of the six domains [learning and school (ES = 1.249), family (0.730), social activities (0.643) and risky activities (0.640)]. No significant differences were seen in the other two domains of this instrument (life skills and child’s self-concept) [36].
3.1.2 Comparison with Atomoxetine
In study 317, flexible dosing with lisdexamfetamine was associated with a more rapid and robust improvement than atomoxetine in ADHD symptoms in children and adolescents who had previously responded inadequately to OROS-MPH therapy [24].
The median time to first clinical response (CGI-I rating of 1 or 2; primary efficacy measure) was significantly shorter in lisdexamfetamine-treated than atomoxetine-treated patients (Table 1). In addition, at all post-baseline visits, significantly (p < 0.01) more lisdexamfetamine than atomoxetine recipients achieved a CGI-I rating of 1 or 2 [24], including week 9 (Table 1). Indeed, at week 9, treatment response rates were significantly (p < 0.01) higher for lisdexamfetamine than atomoxetine in all prespecified responder analyses based on a single ADHD-RS-IV or CGI-I criterion [24, 37]. A sustained response was defined as a reduction from baseline in ADHD-RS-IV total score (of ≥ 25, ≥ 30 or ≥ 50%) or a CGI-I rating of 1 or 2 throughout weeks 4–9; significantly (p < 0.05) more lisdexamfetamine-treated than atomoxetine-treated patients met these criteria (66.1 vs 51.1% for ≥ 25% decrease in ADHD-RS-IV total score; 61.4 vs. 47.4% for ≥ 30% decrease in ADHD-RS-IV total score; 41.7 vs. 23.7% for ≥ 50% decrease in ADHD-RS-IV total score; and 52.0 vs. 39.3% for CGI-I rating of 1 or 2) [37].
Lisdexamfetamine-treated versus atomoxetine-treated patients experienced significantly (p < 0.001) greater reductions from baseline in ADHD-RS-IV total score at all post-baseline visits [24], including week 9 (Table 1); the ES by week 9 was 0.56 [24]. Lisdexamfetamine recipients experienced significantly (p < 0.001) greater improvements in both ADHD-RS-IV subscales compared with atomoxetine recipients [hyperactivity/impulsivity (ES = 0.53) and inattention (0.53)] [24].
As well as a more marked treatment response, lisdexamfetamine was associated with a more pronounced improvement in daily functioning [38]. Lisdexamfetamine-treated versus atomoxetine-treated patients experienced significantly (p < 0.05) greater improvements from baseline to week 9 in WFIRS-P total score (ES = 0.27) and two of the six domains [learning and school (ES = 0.43) and social activities (0.34)] [38].
3.1.3 Comparisons with OROS-MPH
Comparisons of lisdexamfetamine with OROS-MPH in children and/or adolescents who had previously responded inadequately to MPH or amfetamine therapy have yielded somewhat inconsistent results. Thus, lisdexamfetamine provided significantly greater improvements versus OROS-MPH on the primary and key secondary efficacy measures in study 406 (a force-titration trial; pre-specified comparison) and study 325 (a flexible-titration trial; post hoc comparison), but not study 405 (a flexible-titration trial; pre-specified comparison) (Table 1).
Based on the change from baseline to end-of-study in ADHD-RS-IV total score, the ESs for lisdexamfetamine versus placebo were 0.82 and 1.16 in studies 406 and 405, respectively; the corresponding ESs for OROS-MPH were 0.50 and 0.97 [18, 28]. Of note, the lisdexamfetamine ESs versus OROS-MPH (0.33 and 0.20 in studies 406 and 405, respectively) were lower than the estimated ES of ≥ 0.35 for which these studies were powered [18, 28].
Although study 325 was not designed to formally compare lisdexamfetamine with OROS-MPH, the lisdexamfetamine ESs versus OROS-MPH were 0.54 and 0.377–0.435, based on post hoc analyses of ADHD-RS-IV and CPRS-R total scores, respectively [35, 36]. The two active treatments (lisdexamfetamine 30–70 mg/day and OROS-MPH 18–54 mg/day) had a similar positive impact on HRQOL and daily functioning in this study [36].
3.2 Long-Term Treatment
Patients from Europe were eligible to enter study 326, provided they had completed study 325 [26] (Sect. 3.1). Patients from the USA could also enter the extension directly, provided they satisfied inclusion/exclusion criteria applied for study 325, albeit the exclusion criteria were broadened to include failure to respond to previous amfetamine therapy alongside failure to respond to previous OROS-MPH therapy—the latter being a key exclusion criterion in study 325 [26] (Sect. 3.1).
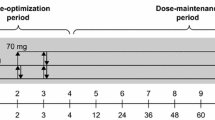
Study 326 consisted of a 26-week open-label period of treatment with lisdexamfetamine followed by a 6-week, double-blind, placebo-controlled, randomized withdrawal period and a 1-week washout period [26]. The first 4 weeks of the open-label period was a dose-optimization period; participants then continued to receive their optimal dosage of lisdexamfetamine (i.e. that which produced an ‘acceptable’ response; see Sect. 3.1) during a 20-week dose-maintenance period and a 2-week fixed-dose period. Patients confirmed as responders (i.e. those with an ADHD-RS-IV total score ≤ 22 and/or Clinical Global Impressions-Severity (CGI-S) score of < 3 who did not require dosage adjustments or experience unacceptable adverse events during the fixed-dose period) were then randomized to continue receiving their optimal dosage of lisdexamfetamine or to switch to placebo during the randomized withdrawal period [26]. The primary efficacy measure was the proportion of patients meeting treatment failure criteria at the endpoint of the randomized withdrawal period (i.e. the last post-randomization visit with valid data); treatment failure was defined as a ≥ 50% increase in ADHD-RS-IV total score and a ≥ 2-point increase in CGI-S score compared with baseline [26]. Efficacy outcomes during the open-label and randomized withdrawal periods were assessed in the FAS [26].
Data from this study demonstrated that the efficacy of lisdexamfetamine in terms of improving ADHD symptoms, HRQOL and functional impairment was maintained during long-term treatment and, furthermore, showed that treatment needed to be continued in order for the beneficial effects to be maintained [26, 39].
Of the 276 patients who entered the extension, 236 (86%) were from Europe. At the baseline of the open-label period (defined as the baseline of study 325 for European patients or the first visit in study 326 for US patients), the mean ADHD-RS-IV total score was 40.6. At the endpoint of the open-label period (i.e. the last visit with valid data), the mean change from baseline in ADHD-RS-IV total score was −26.6 (p < 0.001). In terms of the clinical response rate, ≈ 80% of patients had a CGI-I rating of 1 or 2 at endpoint [26]. CHIP-CE: PRF T-scores in all domains improved significantly (p < 0.001) from baseline to endpoint, as did WFIRS-P total score and scores in all domains; these improvements occurred mainly during the first 8 weeks of treatment. Overall, the pattern and magnitude of improvements in CHIP-CE: PRF domain T-scores and WFIRS-P domain scores in the open-label period was consistent with that observed in study 325 [39].
Of the 157 patients who entered the randomized withdrawal period, 78 and 79 received lisdexamfetamine and placebo, respectively. At the endpoint of the randomized withdrawal period, fewer lisdexamfetamine recipients than placebo recipients met the treatment failure criteria [15.8 vs. 67.5% (p < 0.001)]; similar results were seen regardless of patient age, sex or region of origin. Most treatment failures occurred during the first 2 weeks [26].
Following improvement in all domains of the CHIP-CE: PRF and WFIRS-P during the open-label period, HRQOL and functional impairment scores deteriorated in the placebo group, but not in the lisdexamfetamine group, during the randomized withdrawal period. Differences between the lisdexamfetamine and placebo groups in LSM changes from baseline to endpoint were significant (p < 0.01) in three of the five CHIP-CE: PRF domains [achievement (ES = 0.696), risk avoidance (0.829) and satisfaction (0.636)] as well as in three of the six WFIRS-P domains [family (ES = 0.859), learning and school (0.716) and risky activities (0.506)] and in WFIRS-P total score (ES = 0.908) [39].
Data from study 404, the longest lisdexamfetamine clinical trial to be performed to date, demonstrated that the efficacy of the drug in terms of improving ADHD symptoms was maintained over a 2-year period [27]. This was an open-label, multicentre study in 314 children and adolescents aged 6–17 years with ADHD, of whom 124 had participated in a previous lisdexamfetamine trial (317, 325 or 326) and 190 were directly enrolled. Using the same flexible-titration design employed in the previous trials, the patient’s optimal dosage of lisdexamfetamine between 30 and 70 mg/day was determined during a 4-week dose-optimization period; this was followed by a 100-week dose maintenance period. Efficacy analyses were performed using the FAS [27]. The mean change in ADHD-RS-IV from baseline to the last on-treatment assessment (LOTA) was − 25.8 for the total score, and − 12.6 and − 13.1 for the hyperactivity/impulsivity and inattention subscale scores, respectively. At LOTA, 77.9% of patients were classified as responders, based on a CGI-I score of 1 or 2; 77.3% of patients were classified as responders, based on a CGI-I score of 1 or 2 and a ≥ 30% reduction from baseline in ADHD-RS-IV total score; and 69.2% of patients were classified as responders, based on a CGI-I score of 1 or 2 and a ≥ 50% reduction from baseline in ADHD-RS-IV total score [27].
4 Tolerability of Lisdexamfetamine
4.1 Short-Term Tolerability
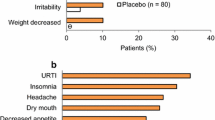
As has been described previously [40], short-term treatment with lisdexamfetamine is generally well tolerated, with an adverse event profile typical of that for stimulants. The most frequently reported treatment-emergent adverse events (TEAEs) in the four phase III or IV trials of 6–9 weeks’ duration in children and/or adolescents with ADHD discussed in Sect. 3.1 included decreased appetite (25.2–53.3% vs. 2.7–10.0% with placebo), headache (13.3–15.2 vs. 7.7–20.0%), weight reduction (10.6–21.9 vs. ≤ 1.1%), insomnia (7.8–14.4 vs. ≤ 2.7%), irritability (5.0–20.1 vs. 6.4 and 9.9%) and nausea (5.0–10.8 vs. 2.7–4.4%); most TEAEs were mild to moderate in severity [24, 25, 28].
Serious TEAEs were reported by ≤ 2.7% of lisdexamfetamine-treated and placebo-treated recipients [24, 25, 28]. However, no serious TEAEs were reported in study 317 [24]; none of the serious TEAEs that occurred in lisdexamfetamine recipients in study 325 were considered to be related to the study drug [25]. TEAEs leading to discontinuation were reported by 4.5–7.6% of lisdexamfetamine recipients compared with 0.9–3.6% of placebo recipients [24, 25, 28].
As is typical for stimulants, lisdexamfetamine treatment is associated with small increases in BP and pulse rate; these increases are most pronounced at the highest dosage evaluated (70 mg/day) [40]. In the four phase III or IV trials, the mean changes from baseline to endpoint or end-of-study in SBP and DBP were 0.7–2.4 and 0.1–3.3 mmHg, respectively, in lisdexamfetamine 30–70 mg/day recipients compared with − 1.5 to 1 and − 1.2 to 1.2 mmHg, respectively, in placebo recipients; the mean changes in pulse rate were 3.6–6.7 and − 0.6 to 2.0 beats per minute (bpm) on lisdexamfetamine and placebo, respectively [24, 25, 28]. In general, mean changes in BP and pulse rate in lisdexamfetamine-treated patients have not been clinically meaningful; similarly, lisdexamfetamine therapy has not generally been associated with clinically relevant changes in mean ECG parameters [40].
Lisdsexamfetamine treatment was associated with modest decreases in bodyweight. In the four phase III or IV trials, mean decreases from baseline to endpoint or end-of-study in bodyweight of 1.3–2.1 kg in lisdexamfetamine 30–70 mg/day recipients contrasted with mean increases of 0.7–1.14 kg in placebo recipients [24, 25, 28]. Of note, anorexia was reported as a TEAE in 10.8% of lisdexamfetamine recipients (versus 1.8% of placebo recipients) in study 325 [25].
The adverse event profile of lisdexamfetamine was generally similar to that of atomoxetine in study 317 [24] and that of OROS-MPH in studies 325 [25], 405 [28] and 406 [28]. Increases in SBP, DBP and pulse rate in lisdexamfetamine recipients were also generally consistent with those reported in atoxomoxetine recipients (0.6 mmHg, 1.3 mmHg and 3.7 bpm, respectively [24]) and OROS-MPH recipients (0.3–2.6 mmHg, 1.7–3.3 mmHg and 3.4–7.6 bpm, respectively [25, 28]). However, decreases in bodyweight in patients receiving lisdexamfetamine were larger than those in patients receiving atomoxetine (0.15 kg [24]) or, to a lesser extent, those in patients receiving OROS-MPH (1.07–1.34 kg) [25, 28]. Additionally, more lisdexamfetamine than atomoxetine recipients met the outlier criterion for weight reduction (≥ 7% decrease from baseline: 26.8 vs. 4.5%) [24].
4.2 Long-Term Tolerability
The long-term tolerability of lisdexamfetamine has been evaluated in four studies in which children or adolescents with ADHD received flexible dosages of the drug (30–70 mg/day): three 26- to 52-week open-label extensions [study 302 (a continuation of two US-based phase II and III studies; n = 272), study 306 (a continuation of another US-based phase III study; n = 265) and study 326 (a continuation of study 325; n = 276; Sect. 3.2)] [40]; and a 104-week open-label safety study (study 404; n = 314; Sect. 3.2) [27].
The most frequently reported TEAEs in these open-label studies were similar to those reported in the short-term randomized trials; they included decreased appetite (21–54%), headache (18–22%) weight reduction (16–20%), insomnia (12–19%) and irritability (10–13%) [27, 40]. Moreover, most TEAEs were mild or moderate in severity [27, 40]. The most frequently reported treatment-related adverse events (TRAEs) in study 404 included decreased appetite (49.4%), weight decreased (18.2%), insomnia (13.1%), initial insomnia (8.9%), irritability (8.6%), nausea (6.7%), headache (5.7%) and tic (5.1%) [27].
Serious TEAEs were reported by 1–4% of patients in the extension studies [40] and ≈ 9% of patients in the safety study [27]. In study 404, six adolescents or children reported a total of seven syncopal episodes, of which three were considered to be treatment-related [27]. Psychiatric TEAEs of special interest (i.e. psychosis, mania, suicidal events and aggression events) occurred infrequently in this study and included a suicide attempt that was not considered to be related to the study drug [27]. TEAEs leading to discontinuation were reported by 6–16% of patients [27, 40]; the most common TEAEs leading to treatment cessation in study 404 were decreased appetite (2.2% of patients), irritability (1.3%), depressed mood (1.3%), insomnia (1.0%) and tic (1.0%) [27].
As regards cardiovascular parameters in these long-term studies, mean increases in SBP (0.7–3.4 mmHg), DBP (0.6–3.2 mmHg) and pulse rate (1.4–7.0 bpm) were modest and consistent with findings for lisdexamfetamine in short-term trials [27, 40], as were mean changes in QTcF (− 1.1 to 1.8 ms) [27, 40]. Additionally, in study 404, potentially clinically important (PCI) vital signs rates were generally similar to those reported in previous lisdexamfetamine clinical trials. For example, 22.4 and 38.8% of children aged 6–12 years experienced systolic BP ≥ 125 mmHg and diastolic BP ≥ 80 mmHg, respectively; 15.2 and 21.4% of adolescents aged 13–17 years experienced systolic BP ≥ 135 mmHg and diastolic BP ≥ 85 mmHg, respectively [27].
In study 326, the mean reduction from baseline to OLP endpoint in bodyweight was modest (2.24 kg), albeit 15% of patients reported anorexia as a TEAE [26].
4.2.1 Effect on Development in Children
The impact of 2 years’ treatment with lisdexamfetamine on growth [27, 41], sexual maturation [41] and cognitive function [42] in children and adolescents with ADHD has been evaluated in study 404.
Although mean bodyweight and height increased over the course of the study (by 2.1 kg and 6.1 cm, respectively), mean bodyweight, height and BMI z-scores decreased over the first 36 weeks of the study and then stabilized [27, 41]. The changes from baseline to the LOTA in mean z-scores for bodyweight, height and BMI were significantly less than zero (− 0.51, − 0.24 and − 0.59, respectively; nominal p < 0.0001). Similar proportions of patients were within 1 SD of the CDC population norms for bodyweight, height and BMI at both baseline and LOTA, although the proportion of patients who were > 1 SD below the CDC population norms increased over the course of the study (from 5.1% at baseline to 22.1% at week 84 for bodyweight; from 8.2% at baseline to 12.6% at week 96 for height; and from 8.3% at baseline to 28.8% at week 96 for BMI) [41]. Overall, these findings were consistent with previous longer-term investigations of stimulants, including an exploratory analysis of growth outcomes in children treated with lisdexamfetamine for up to 15 months in the USA [43].
Long-term treatment with lisdexamfetamine in study 404 was not associated with any clinically concerning trends in pubertal development (assessed by Tanner stages) [41] nor was it associated with deterioration in cognitive function (assessed using the Cambridge Neuropsychological Test Automated Battery) [42].
5 Dosage and Administration of Lisdexamfetamine
In the EU, the starting dose of lisdexamfetamine is 30 mg administered once daily in the morning, with or without food; however, patients can commence on the lower dosage of 20 mg/day if considered appropriate by the treating clinician [18]. The dosage may subsequently be titrated upwards in increments of 10 or 20 mg/day at ≈ 1-week intervals; the maximum recommended dosage is 70 mg/day [18]. The maximum recommended dosage in patients with severe renal insufficiency is 50 mg/day; further dosage reduction should be considered in patients undergoing dialysis, as neither lisdexamfetamine nor d-amfetamine are dialysable [18]. Treatment should be stopped if there is no improvement in symptoms after appropriate dosage adjustment over a 1-month period [18].
Local prescribing information should be consulted for more detailed information regarding posology and method of administration, contraindications, warnings and precautions, drug interactions and use in special patient populations.
6 Place of Lisdexamfetamine in the Management of ADHD in Children and Adolescents
Lisdexamfetamine is the first and currently only oral stimulant (d-amfetamine) prodrug for the treatment of ADHD [44]; it was originally developed with the aim of providing a longer duration of action and a reduced potential for abuse compared with existing oral stimulant preparations [16, 17, 21, 23]. In particular, the prodrug design (covalently bonded l-lysine and d-amfetamine) prevents mechanical drug tampering [45].
By utilizing prodrug technology rather than a combination of immediate-release and delayed-release beads or an OROS to achieve a prolonged duration of action, lisedexamfetamine differs mechanistically from other long-acting oral stimulant preparations that can be administered once daily. However, it offers the same dosing convenience and potential to improve adherence and reduce diversion and abuse compared with short-acting stimulants, which require more frequent administration [9, 13, 23, 44]. Moreover, exposure to d-amfetamine following lisdexamfetamine administration appears to be largely unaffected by gastrointestinal factors, such as variations in pH and motility (Sect. 2); this may be advantageous in terms of the consistency of drug delivery compared with long-acting beaded formulations of amfetamines, as the absorption of amfetamines from the latter varies with gastrointestinal pH [13, 46]. Regarding the long duration of action of lisdexamfetamine that permits once daily dosing, ongoing clinical efficacy has been observed up to 13 h postdose in children [25, 35].
In terms of treating paediatric ADHD within the EU, lisdexamfetamine is indicated in children and adolescents aged ≥ 6 years when the response to previous MPH treatment is considered clinically inadequate (Sect. 1). However, it may be appropriate to continue treatment in adolescents whose symptoms persist into adulthood (and who have shown clear benefit from treatment) [18]. This licenced use is largely consistent with recent guidance issued by the National Institute for Health and Care Excellence (NICE) in the UK that addresses children aged ≥ 5 years and young people with ADHD [7]. According to this advice, MPH is the first-line treatment; lisdexamfetamine can be considered for those patients whose ADHD symptoms are not responding adequately to MPH. Atomoxetine or guanfacine can be offered to patients if they cannot tolerate MPH or lisdexamfetamine or if their symptoms have not responded to separate 6-week trials of MPH and lisdexamfetamine, having tried alternative formulations and adequate doses. Additionally, d-amfetamine can be considered for those patients whose ADHD symptoms are responding to lisdexamfetamine but who cannot tolerate the longer effect profile [7].
The approval of lisdexamfetamine as a second-line therapy for paediatric ADHD in the EU was based on the then-available results of three phase III studies in children and adolescents aged 6–17 years with ADHD that were conducted exclusively or partly in Europe [18]. In study 325, short-term treatment with flexibly-dosed lisdexamfetamine produced significantly greater improvements than placebo in ADHD symptoms, HRQOL and daily functioning in patients who had a better than minimal, but less than satisfactory, response to their current ADHD medication or were treatment-naïve (Sect. 3.1). In post hoc subgroup analyses, the beneficial effect of lisdexamfetamine on ADHD symptoms was seen in patients previously treated with MPH (Sect. 3.1). In study 326 (an open-label extension of study 325), the beneficial effects of lisdexamfetamine therapy were maintained during long-term treatment, albeit the benefits were only sustained while treatment was continued (Sect. 3.2). In study 317, short-term treatment with flexibly-dosed lisdexamfetamine was associated with a more rapid and robust treatment response than atomoxetine (also administered once daily) in children and adolescents who had previously responded inadequately to MPH therapy (Sect. 3.1.2); these results are highly relevant to clinical practice in the EU, where both agents are considered to be second-line treatments (see above).
Lisdexamfetamine was generally well tolerated, both during short-term (up to 9 weeks’) and long-term (up to 2 years’) treatment, with an adverse event profile (e.g. decreased appetite, headache, weight reduction, insomnia and irritability) typical of that reported for other stimulants. Moreover, most TEAEs were mild or moderate in severity (Sect. 4).
Data for lisdexamfetamine relating to four specific tolerability concerns associated with stimulant use, namely weight loss/growth suppression, cardiovascular safety, abuse potential and sleep disturbances, have been reviewed in more detail elsewhere [40]. Long-term use of lisdexamfetamine has been associated with a growth-suppressive effect in children (Sect. 4.2.1); as with other stimulants, height and weight should be assessed before, and continuously during, treatment [40]. Long-term treatment with lisdexamfetamine does not, however, appear to adversely affect pubertal development or cognitive function (Sect. 4.2.1). Cardiovascular-related serious TEAEs and discontinuations, and ECG abnormalities, have been reported only rarely in clinical trials of lisdexamfetamine in patients with ADHD of all ages [40]. Moreover, observed increases in BP and pulse rate, for example in children and adolescents (Sect. 4.1), have been small and, in general, not clinically significant [40]. Nonetheless, cardiovascular status (including BP and pulse rate) should be assessed before, and monitored continuously during, treatment [18]. As with other stimulants, use of lisdexamfetamine should generally be avoided in children or adolescents with structural cardiac abnormalities or other serious heart problems [18].
The potential for abuse should also be assessed before, and monitored continuously during, treatment [18]. The possibility that stimulants will be misused is a problem particularly pertinent to adolescents and adults with ADHD [47]. Post-marketing survey data suggest that, among adults, the rate of non-medical use of lisdexamfetamine is generally lower than that for short-acting stimulant formulations and equivalent to or lower than that for long-acting stimulant formulations [40]. These findings are consistent with the results of phase I studies in adult stimulant abusers, which are suggestive of a lower potential for abuse of lisdexamfetamine than a short-acting d-amfetamine preparation [40]. Notwithstanding insomnia is one of the most common TEAEs associated with the use of stimulants, the overall impact of these ADHD medications on sleep is unclear. The limited data available suggest that lisdexamfetamine does not contribute to sleep disturbances in children or impair sleep quality in adults [40].
The two phase IV studies of lisdexamfetamine and OROS-MPH in adolescents who had previously responded inadequately to MPH or amfetamine therapy (Sect. 3.1.3) are the largest head-to-head trials to date comparing representatives of amphetamine and MPH stimulant classes [28]. Short-term treatment with lisdexamfetamine produced significantly greater improvements than OROS-MPH in ADHD symptoms when forced-titrated to a target dosage (study 406), but not when flexibly-titrated (study 405). This apparent inconsistency may not be surprising given that force-titration studies are generally considered to be better able to detect differences between active medications than flexible-titration studies [28]. Nonetheless, the result of study 405 contrasts with that of a post hoc analysis of study 325—a trial that, although not designed to be a formal head-to-head comparison, showed that flexibly-dosed lisdexamfetamine did produce significantly greater improvements than OROS-MPH in ADHD symptoms [Sect. 3.1.3]. The reasons for this inconsistency are unclear, but may be related to between-study differences in the maximum permitted dosage of OROS-MPH (54 mg/day in study 325 vs. 72 mg/day in study 405), the severity of ADHD symptoms at baseline (ADHD-RS-IV total score ≈ 40–41 in study 325 vs. ≈ 37–38 in study 405) and the age range of the participants (6–17 years in study 325 vs. 13–17 years in study 405) [Sect. 3]. An indirect comparison performed prior to the full publication of studies 405 and 406 found that lisdexamfetamine had greater efficacy than extended-release formulations of MPH (including OROS-MPH) in reducing symptoms in children and adolescents with ADHD, and that both of these stimulant preparations were more effective than the non-stimulants atomoxetine and guanfacine extended release [11]. Of note, there were no clear differences between these treatments regarding all-cause and adverse event-related discontinuation rates [11].
Available economic evidence for children and adolescents with ADHD indicates that pharmacotherapy is generally a cost-effective option compared with behavioural therapy or placebo/no treatment [5]. Against this background, a pharmacoeconomic analysis of study 317 indicates that, from the perspective of the National Health Service in the UK, lisdexamfetamine provides a cost-effective option relative to atomoxetine for children and adolescents with ADHD who are inadequate responders to MPH [48].
In conclusion, lisdexamfetamine, an oral amphetamine prodrug that has the convenience of once-daily administration, a potentially low liability for abuse compared with short-acting formulations of stimulants, and a tolerability profile consistent with that of other stimulants, provides an alternative option for the treatment of children and/or adolescents with ADHD who have not responded adequately to previous ADHD pharmacotherapies.
Data Selection Lisdexamfetamine: 196 records identified
Duplicates removed | 26 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 10 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 98 |
Cited efficacy/tolerability articles | 24 |
Cited articles not efficacy/tolerability | 25 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Lisdexamfetamine, lis-dexamfetamine, Elvanse, NRP-104, NRP104, Attention-deficit hyperactivity disorder, Attention deficit hyperactivity disorder, ADHD, child, adolescent. Records were limited to those in English language. Searches last updated 5 June 2018 | |
References
Adesman A. The diagnosis and management of attention-deficit/hyperactivity disorder in pediatric patients. Prim Care Companion J Clin Psychiatry. 2001;3(2):66–77.
Barkley RA. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York: Guilford Press; 2006.
American Academy of Pediatrics. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–22.
Danckaerts M, Sonuga-Barke EJS, Banaschewski T, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19(2):83–105.
Wu E, Hodgkins P, Ben-Hamadi R, et al. Cost effectiveness of pharmacotherapies for attention-deficit hyperactivity disorder. CNS Drugs. 2012;26(7):581–600.
Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8.
National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. NICE guideline [NG87]. 2018. https://www.nice.org.uk. Accessed 5 June 2018.
Huss M, Chen W, Ludolph AG, et al. Guanfacine extended release: a new pharmacological treatment option in Europe. Clin Drug Investig. 2016;36:1–25.
Shier AC. Pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: clinical strategies. J Cent Nerv Syst Dis. 2013;5:1–17.
Luan R, Mu Z, Yue F, et al. Efficacy and tolerability of different interventions in children and adolescents with attention deficit hyperactivity disorder. Front Psychiatry. 2017;8:229. https://doi.org/10.3389/fpsyt.2017.00229.
Joseph A, Ayyagar R, Xie M, et al. Comparative efficacy and safety of attention-deficit/hyperactivity disorder pharmacotherapies, including guanfacine extended release: a mixed treatment comparison. Eur Child Adolesc Psychiatry. 2017;26:875–97.
Riera M, Castells X, Tobias A, et al. Discontinuation of pharmacological treatment of children and adolescents with attention deficit hyperactivity disorder: meta-analysis of 63 studies enrolling 11,788 patients. Psychopharmacology. 2017;234(17):2657–71.
Lopez FA, Leroux JR. Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges. Atten Defic Hyperact Disord. 2013;5(3):249–65.
Hodgkins P, Shaw M, McCarthy S, et al. The pharmacology and clinical outcomes of amphetamines to treat ADHD. CNS Drugs. 2012;26(3):245–68.
Banaschewski T, Coghill D, Santosh P, et al. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry. 2006;15(8):476–95.
Weber J, Siddiqui MA. Lisdexamfetamine dimesylate. CNS Drugs. 2009;23(5):419–25.
Steer C, Froelich J, Soutullo C, et al. Lisdexamfetamine dimesylate. CNS Drugs. 2012;26(8):691–705.
Shire Pharmaceutical Contracts Limited. Elvanse 20, 30, 40, 50, 60 & 70 mg capsules, hard. UK summary of product characteristics 2017. https://www.medicines.org.uk/emc/product/2979/smpc. Accessed 5 June 2018.
Frampton JE. Lisdexamfetamine: a review in ADHD in adults. CNS Drugs. 2016;30(4):343–54.
Shire US inc. Vyvanse® (lisdexamfetamine dimesylate) capsules, for oral use. US prescribing information. 2015. https://www.accessdata.fda.gov. Accessed 5 June 2018.
Blick SK, Keating GM. Lisdexamfetamine. Paediatr Drugs. 2007;9(2):129–35.
Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr. 2010;6:317–27.
Najib J. Lisdexamfetamine in the treatment of adolescents and children with attention-deficit/hyperactivity disorder. Adolesc Health Med Ther. 2012;3:51–66.
Dittmann RW, Cardo E, Nagy P, et al. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs. 2013;27(12):1081–92.
Coghill D, Banaschewski T, Lecendreux M, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23(10):1208–18.
Coghill DR, Banaschewski T, Lecendreux M, et al. Maintenance of efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: randomized-withdrawal study design. J Am Acad Child Adolesc Psychiatry. 2014;53(6):647–57.e1.
Coghill DR, Banaschewski T, Nagy P, et al. Long-term safety and efficacy of lisdexamfetamine dimesylate in children and adolescents with ADHD: a phase IV, 2-year, open-label study in Europe. CNS Drugs. 2017;31:625–38.
Newcorn JH, Nagy P, Childress AC, et al. Randomized, double-blind, placebo-controlled acute comparator trials of lisdexamfetamine and extended-release methylphenidate in adolescents with attention-deficit/hyperactivity disorder. CNS Drugs. 2017;31:999–1014.
Shire. Effectiveness of Vyvanse compared to Concerta in adolescents with attention-deficit/hyperactivity disorder [ClinicalTrials.gov identifier NCT01552915]. US National Institutes of Health, ClinicalTrials.gov [online]. 2014. https://clinicaltrials.gov/ct2/show/NCT01552915. Accessed 5 June 2018.
Shire. Effectiveness of Vyvanse compared to Concerta in adolescents with attention-deficit/hyperactivity disorder [ClinicalTrials.gov identifier NCT01552902]. US National Institutes of Health, ClinicalTrials.gov [online]. 2015. https://clinicaltrials.gov/ct2/show/NCT01552902. Accessed 5 June 2018.
Nottinghamshire Area Prescribing Committee. Methylphenidate. Information sheet for primary care prescribers. 2016. http://www.nottsapc.nhs.uk. Accessed 5 June 2018.
Lecendreux M, Banaschewski T, Soutullo C, et al. Efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: effect of age, sex and baseline disease severity. In: 21st European Congress of Psychiatry; 2014.
Coghill DR, Banaschewski T, Lecendreux M, et al. Post hoc analyses of the impact of previous medication on the efficacy of lisdexamfetamine dimesylate in the treatment of attention-deficit/hyperactivity disorder in a randomized, controlled trial. Neuropsychiatr Dis Treat. 2014;10:2039–47.
Soutullo C, Banaschewski T, Lecendreux M, et al. A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs. 2013;27(9):743–51.
Coghill DR, Banaschewski T, Lecendreux M, et al. Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial. Eur Child Adolesc Psychiatry. 2014;23(2):61–8.
Banaschewski T, Soutullo C, Lecendreux M, et al. Health-related quality of life and functional outcomes from a randomized, controlled study of lisdexamfetamine dimesylate in children and adolescents with attention deficit hyperactivity disorder. CNS Drugs. 2013;27(10):829–40.
Dittmann RW, Cardo E, Nagy P, et al. Treatment response and remission in a double-blind, randomized, head-to-head study of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit hyperactivity disorder. CNS Drugs. 2014;28(11):1059–69.
Nagy P, Häge A, Coghill DR, et al. Functional outcomes from a head-to-head, randomized, double-blind trial of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention/deficit/hyperactivity disorder and an inadequate response to methylphenidate. Eur Child Adolesc Psychiatry. 2016;25(2):141–9.
Banaschewski T, Johnson M, Lecendreux M, et al. Health-related quality of life and functional outcomes from a randomized-withdrawal study of long-term lisdexamfetamine dimesylate treatment in children and adolescents with attention deficit/hyperactivity disorder. CNS Drugs. 2014;28(12):1191–203.
Coghill D, Caballero B, Sorooshian S, et al. A systematic review of the safety of lisdexamfetamine dimesylate. CNS Drugs. 2014;28(6):497–511.
Banaschewski T, Johnson M, Nagy P, et al. Growth and puberty in a 2-year open-label study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. CNS Drugs. 2018;32(5):455–67.
Coghill DR, Banaschewski T, Bliss C, et al. Cognitive function of children and adolescents with attention-deficit/hyperactivity disorder in a 2-year open-label study of lisdexamfetamine dimesylate. CNS Drugs. 2018;32(1):85–95.
Faraone SV, Spencer TJ, Kollins SH. Effects of lisdexamfetamine dimesylate treatment for ADHD on growth. J Am Acad Child Adolesc Psychiatry. 2010;49(1):24–32.
Goodman DW. Lisdexamfetamine dimesylate. The first prodrug stimulant. Psychiatry (Edgemont). 2007;4(8):39–45.
Faraone SV. Lisdexamfetamine dimesylate: the first long-acting prodrug stimulant treatment for attention deficit/hyperactivity disorder. Expert Opin Pharmacother. 2008;9:1565–74.
Ermer J, Adeyi B, Pucci M. Pharmacokinetic variability of long-acting stimulants in the treatment of children and adults with attention-deficit hyperactivity disorder. CNS Drugs. 2010;24(12):1009–25.
Weyandt LL, Marraccini ME, Gudmundsdottir BG, et al. Pharmacological interventions for adolescents and adults with ADHD: stimulant and nonstimulant medications and misuse of prescription stimulants. Psychol Res Behav Manag. 2014;7:223–49.
Zimovetz EA, Beard SM, Hodgkins P, et al. A cost-utility analysis of lisdexamfetamine versus atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and inadequate response to methylphenidate. CNS Drugs. 2016;30(10):985–96.
Shire. Comparison of lisdexamfetamine dimesylate with atomoxetine HCl in attention-deficit/hyperactivity disorder (ADHD) subjects with an inadequate response to methylphenidate [ClinicalTrials.gov identifier NCT01106430]. US National Institutes of Health, ClinicalTrials.gov [online]. 2014. https://clinicaltrials.gov/ct2/show/study/NCT01106430. Accessed 5 June 2018.
Acknowledgements
During the peer review process, the manufacturer of lisdexamfetamine was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
James Frampton is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: D.R. Coghill, Department of Paediatrics, Faculty of Medicine, Dentistry and Health Science, University of Melbourne, Melbourne, Australia; S. Cortese, Centre for Innovation in Mental Health, Academic Unit of Psychology, University of Southampton, Southampton, UK; R. Jain, Department of Psychiatry, Texas Tech Health Sciences Center School of Medicine, Midland, TX, USA; M. Johnson, Gillberg Neuropsychiatry Center, University of Gothenburg, Gothenburg, Sweden; J. Najib, LIU, Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Brooklyn, NY, USA; C.R. Steer, Paediatric Department, Victoria Hospital, Kirkcaldy, UK.
Rights and permissions
About this article
Cite this article
Frampton, J.E. Lisdexamfetamine Dimesylate: A Review in Paediatric ADHD. Drugs 78, 1025–1036 (2018). https://doi.org/10.1007/s40265-018-0936-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0936-0