Abstract
Background
The risk-benefit balance of pharmacological treatment for children and adolescents with ADHD and the factors that moderate this relationship are unclear.
Methods
A systematic review and meta-analysis of randomised, placebo-controlled clinical trials (RPCCTs) investigating the efficacy of pharmacological treatment in children or adolescents with ADHD was carried out. Meta-analysis of treatment discontinuation, clinician-, parent- and teacher-rated efficacy and adverse events was performed. The effect of covariates was studied.
Results
Sixty-three studies were included. Ten drugs were investigated, with atomoxetine and methylphenidate the most frequently studied. RPCCTs had mostly a short duration (7.9 weeks). All-cause treatment discontinuation was lower with pharmacological treatment than placebo (OR = 0.68). Pharmacological treatment was more efficacious than placebo independently of the rater (clinician, standardised mean difference (SMD) 0.74; parent, SMD = 0.63; or teacher, SMD = 0.75). Evidence of publication bias was found for clinician-rated efficacy, especially in industry-sponsored RPCCT. Psychostimulants showed a higher efficacy and were associated with a better outcome on treatment discontinuation than non-stimulant drugs. Efficacy was smaller in RPCCTs for which a psychiatric comorbid disorder was an inclusion criterion, was larger in studies with a commercial sponsorship and showed a negative association with treatment length.
Conclusions
In the short term, pharmacological treatment provides moderate–high symptom relief, is safe and shows lower treatment discontinuation than placebo, suggesting a suitable risk-benefit balance, particularly with psychostimulants. The efficacy is lower in patients with a comorbid psychiatric disorder and should be assessed periodically, as it appears to reduce over time. Publication bias of clinician-rated efficacy in studies with a commercial sponsor is suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most prevalent psychiatric disorders in children and adolescents. It has been estimated to affect around 7% of children (Thomas et al. 2015). ADHD is characterised by symptoms of inattention, impulsivity and hyperactivity which interfere with patients’ life functioning resulting in academic underachievement (Daley and Birchwood 2010) and difficulties in peer and family relations (Harpin 2005; Hoza 2007). ADHD has been associated with an increased risk of drug use, car accidents, injuries and legal problems in adolescence and adulthood (Charach et al. 2011; Dalsgaard et al. 2015; Jerome et al. 2006; Langley et al. 2010; Mannuzza et al. 2008; Nigg 2013). According to the majority of clinical guidelines, pharmacological treatment is a cornerstone of the management of ADHD (Seixas et al. 2012). Unsurprisingly, pharmacological treatment of ADHD has become popular and its use has increased markedly over the last decades (Burcu et al. 2016; Visser et al. 2014).
Despite the popularity of pharmacological treatment of ADHD, there are concerns about its efficacy, as data on patient-centred endpoints such as the functional consequences of ADHD are scarce (Feldman and Reiff 2014; Molina et al. 2009). Conversely, most research has focused on ADHD symptom severity. This study endpoint has several limitations such as its subjective nature. Furthermore, ADHD symptom severity is assessed with rating scales whose interpretation is unclear as it is not known what is the repercussion of a change of the score of these scales on the clinical consequences of ADHD Besides, the outcome “ADHD symptom severity” frequently has a high risk of attrition bias (Cunill et al. 2016). This type of bias arises from the systematic differences between the characteristics of patients that drop out of the active group and those that leave the placebo group, which can lead to each group having different ADHD severity, causing bias in the efficacy results. There are also safety concerns due to the fact that pharmacological treatment has been associated with frequent adverse events, some of which can be severe (Graham et al. 2011; Perrin et al. 2008; Swanson and Volkow 2008). In this context, weighing efficacy against safety and establishing the risk-benefit balance of the interventions investigated are complicated.
This difficulty of assessing the risk-benefit relationship of pharmacological interventions for ADHD can, in part, be overcome by using the endpoint ‘all-cause treatment discontinuation’. This outcome is a pragmatic one that combines the evaluation of both efficacy and safety in a straightforward way: lower than placebo treatment discontinuation would indicate that the intervention’s efficacy outweighs its side effects, while a higher rate of treatment discontinuation would indicate that the symptom improvement with the medication does not compensate for its side effects (Stroup et al. 2003). Furthermore, treatment discontinuation is not affected by attrition bias because there are no missing data on this outcome.
The endpoint ‘treatment discontinuation’ has been used for comparing competing interventions for several psychiatric disorders, including major depression (Anderson and Tomenson 1995; McGrath et al. 2006), agitation in patients with Alzheimer disease (Schneider et al. 2006), opioid dependence (Johnson et al. 2000; Oviedo-Joekes et al. 2009) and schizophrenia (Lieberman et al. 2005). The effect of pharmacological interventions on all-cause treatment discontinuation in ADHD has also been investigated in adults (Cunill et al. 2016). In these patients, it has been shown that all-cause treatment discontinuation was higher with pharmacological treatment than with placebo, suggesting a poor risk-benefit relationship of pharmacological treatment in adults with ADHD.
The efficacy and safety of pharmacological treatment have frequently been studied in children and adolescents with ADHD. Most studies have focussed on efficacy outcomes, with particular attention on ADHD symptom improvement (e.g. No authors 2015; Hirota et al. 2014; Otasowie et al. 2014; Punja et al. 2016; Schwartz and Correll 2014, and Storebø et al. 2015). The efficacy on clinical global impression (Ruggiero et al. 2014) and on neuropsychological outcomes (Coghill et al. 2014; Tamminga et al. 2016) has also been investigated. Numerous studies have addressed primarily pharmacological treatment safety (Bushe and Savill 2013; Coughlin et al. 2015; Mick et al. 2013; Otasowie et al. 2014; Storebø et al. 2015). Nevertheless, so far, no placebo-controlled study has placed particular attention to all-cause treatment discontinuation.
This study aimed to compare all-cause treatment discontinuation between pharmacological treatment and placebo in children and adolescents with ADHD. In addition, the efficacy on ADHD symptom severity and safety was investigated. Furthermore, as between-study variability in efficacy, safety and treatment discontinuation is expected, we investigated the potential sources of such variability by determining the effect of study design- (e.g. the presence of a lead-in phase or comorbidity as an inclusion criterion), patient- (e.g. age, gender, or prior stimulant treatment), intervention- (e.g. type of drug, administration of concomitant psychotherapy) and sponsor-related covariates on study outcomes. These covariates were chosen as they have previously been shown to modify the effect size of interventions used to treat ADHD and other psychiatric disorders (Cunill et al. 2015, 2016; Leucht et al. 2012; Yildiz et al. 2011). This information may be useful for designing future clinical trials and multiple treatment meta-analyses (MTM) in this field and for tailoring ADHD treatment to a patient’s characteristics.
Methods
Design
A systematic review with meta-analysis (SRMA) was performed. This study used the same methodology as a previous SRMA in adults with ADHD (Cunill et al. 2016) with two main differences: it focused on children and adolescents with ADHD, and the efficacy on ADHD symptom severity was assessed separately for each type of rater. This study was registered with the international prospective register of systematic reviews (PROSPERO): CRD42015019045.
Inclusion and exclusion criteria
We included (1) randomised placebo-controlled clinical trials (RPCCTs) with a parallel design; (2) enrolling children or adolescents with ADHD as diagnosed using the DSM-III or subsequent editions; (3) investigating the efficacy and safety of pharmacological interventions approved by the Food and Drug Administration (FDA), the European Medicines Agency (EMA) or those recommended by outstanding clinical guidelines (Bolea-Alamañac et al. 2014; Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA) 2011; National Institute for Health and Clinical Excellence (NICE) 2008; Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management 2011) (methylphenidate, dexmethylphenidate, dexamphetamine, mixed amphetamine salts, atomoxetine, bupropion, modafinil, guanfacine, clonidine, imipramine and desipramine); and (4) with a double-blind phase lasting at least 3 weeks. We excluded studies that (1) were not carried out in an outpatient setting; (2) were written in languages other than English, French, German or Spanish; (3) did not report all-cause treatment discontinuation; (4) were published only as abstracts; or (5) used a cross-over design. We excluded cross-over studies because this type of study has a higher risk of bias than parallel ones due to the possibility of a carry-over effect of the interventions investigated and because the statistical analysis is not performed by the ITT approach. Furthermore, the data needed to combine cross-over studies in meta-analysis are not usually available in a way that takes advantage of their cross-over design (Elbourne et al. 2002).
Procedures
The following datasets were searched (the last search was performed on 1 February 2016): Medline, the Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO, www.clinicaltrials.gov, www.clinicaltrialsregister.eu, www.controlled-trials.com, FDA, EMA and laboratory web pages (see Online Resource 1 for search strategies). Abstracts of potentially relevant studies were inspected, and the full articles of those studies deemed suitable were acquired. The reference list of retrieved studies and relevant guidelines (Bolea-Alamañac et al. 2014; Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA) 2011; National Institute for Health and Clinical Excellence (NICE) 2008; Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management 2011) were also examined to identify any further studies. For each study included, a citation search was performed in the ISI Web of Knowledge to identify any missed study that may have cited it. Three reviewers (MR, RC, XC) performed independently duplicate data extraction from the studies selected. Data extraction was compared and disagreements were resolved by consensus. Where relevant information was not available, study authors were e-mailed and missing information was requested.
The primary study endpoint was all-cause treatment discontinuation, defined as the proportion of patients randomised to study interventions who discontinued the treatment assigned due to any cause. The secondary endpoints were (1) discontinuation due to lack of efficacy (LoE); (2) discontinuation due to adverse event (AEs); (3) clinician-, (4) parent- and (5) teacher-rated ADHD symptom severity, with preference given to change scores over endpoint ones (the decision of providing separate efficacy estimates by type of rater was post hoc and was based on our prior study (Cunill et al. 2016) that found that the effect size was significantly influenced by the type of rater); (6) the number of patients experiencing any AE; and (7) serious AEs.
The following covariates were collected: number of study sites; comorbidity as inclusion criterion (yes vs. no); placebo lead-in period (yes vs. no); patients’ compensation for participating in the study (yes vs. no); age; gender (% men); prior treatment with stimulants (% treated); clinician- (% scale maxima), parent- (% scale maxima) and teacher-rated ADHD severity (% scale maxima); oppositional defiant disorder (ODD) (% with comorbid ODD); type of drug (stimulant vs. non-stimulant); dosage (fixed vs. flexible); treatment duration (weeks); administration of concomitant psychotherapy during the study (yes vs. no); and sponsorship (independent vs. commercial) (this last covariate was included post hoc). Methylphenidate, dexmethylphenidate and amphetamine derivatives were classified as psychostimulants and the remaining drugs as non-stimulants. Given that in RPCCTs, ADHD severity is usually assessed using several scales, the baseline score was standardised, calculating the percent of scale maxima, which consists of the re-expression of that score as if the scale ranged from 0 to 100. We used the Cochrane Collaboration instrument (Higgins and Green 2011a) to ascertain the risk of bias of the studies included. This instrument rates the risk of bias on the basis of description and suitability in seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias. Some domains are assessed at study-level (sequence generation, allocation concealment, selective outcome reporting and other sources of bias) and the remaining ones at outcome-level (blinding, incomplete outcome data). This tool involves assigning a judgement relating to the risk of bias for each entry in terms of ‘low’, ‘high’ or ‘unclear’ risk.
Statistical analysis
Odds ratios (OR) and standardised mean differences (SMD) were calculated for binary and continuous outcomes, respectively. Studies with multiple and correlated comparisons were analysed as follows. When two different doses of the same drug were investigated, the results for each study outcome were combined and, for this study, one single effect estimate was calculated. Conversely, when two different drugs or formulations of the same drug were compared with a placebo group, we analysed the two pharmacological interventions separately but the number of patients in the placebo group was divided by two to avoid overcounting (Higgins and Green 2011b). Between-study statistical heterogeneity was assessed using Cochran’s Q test (Cochran 1954) and the I 2 index (Higgins et al. 2003). The I 2 index shows the percentage of variation in the pooled estimate that is not random but can be attributed to heterogeneity. This index allows for classifying statistical heterogeneity according to prespecified cutoffs (<25%, low heterogeneity; 25 to 50%, moderate; 50–75%, high; >75%, very high). The study-specific estimates were pooled by means of the inverse variance method using a random effects model. Sensitivity analyses were performed by excluding those studies rated as having a ‘high risk of bias’ and by removing each study once from the analysis to detect whether one study had an undue influence on the meta-analyses results. Funnel plots were generated and Begg’s and Egger’s tests were performed for potential publication bias detection (Begg and Mazumdar 1994). For outcomes showing statistical heterogeneity, we studied the effect of covariates using meta-regression (Berkey et al. 1995). The ratio of ORs (ROR) and the difference of SMD (diff SMD) were calculated for dichotomous and continuous outcomes, respectively. Statistical analyses were performed with Revman 5.2 (Nordic and Cochrane Centre 2014) and Stata statistical software, release 12.38 (StataCorp 2011).
Results
Studies, patients and intervention characteristics
Sixty-three studies that enrolled 11,788 patients were included (see online resources 2 and 3 for flow diagram and reference of the studies included). Twelve studies compared two drugs with placebo; therefore, 75 drug vs. placebo comparisons were analysed. Table 1 and online resource 4 show the characteristics of the studies, patients and interventions investigated. Most (84.1%) studies were multiple sites and were conducted in the USA (79.4%). A placebo lead-in phase was included in 16.1% of studies. Compensation for participating was given infrequently (4.8%). The majority of studies had a commercial sponsor (83.6%).
Patients had an average age of 10.5 years and most of them were male (75.8%) and had an ADHD combined subtype (73.3%). Baseline ADHD symptoms were of moderate severity. Although comorbidity was an inclusion criterion in only a few studies (17.7%), comorbid ODD was frequent among the patients included (34.9%).
Ten drugs were studied, namely, atomoxetine (23 studies, 3881 patients, mean dose = 1.3 mg/kg/day), bupropion (2 studies, 139 patients, dose up to 6.0 mg/kg/day), clonidine (4 studies, 368 patients, dose up to 0.6 mg/day), desipramine (2 studies, 103 patients, mean dose = 4.1 mg/kg/day), dexmethylphenidate (3 studies, 421 patients, mean dose = 22 mg/day), guanfacine (8 studies, 1860 patients, mean dose = 3.5 mg/day), lisdexamfetamine (3 studies, 773 patients, mean dose = 54.4 mg/day), methylphenidate (20 studies, 2394 patients, mean dose = 1.1 mg/kg/day), MAS (3 studies, 900 patients, mean dose = 21.6 mg/day) and modafinil (6 studies, 956 patients, mean dose = 389.7 mg/day). Dosage was flexible in two thirds of the studies. Treatment lasted an average of 7.9 weeks, ranging from 3 to 18 weeks. Psychotherapy was infrequently administered (12.7%).
No study included was deemed free of bias for all outcomes analysed (Table 1 and online resource 5). The outcomes more frequently scored as having a high risk of bias were parent- and teacher-rated efficacy, with attrition bias the most common source of potential bias identified. Such bias was judged to be likely either because treatment discontinuation was high or because between-group differences in treatment discontinuation were found. Under these circumstances, no imputation method was considered to address the missing data in a suitable way.
Treatment discontinuation, efficacy and safety
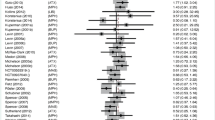
Treatment discontinuation (Fig. 1) was lower among patients who received pharmacological treatment than among those who received placebo (19.7 vs. 26%, OR = 0.68 [0.58, 0.79], p < 0.00001, I 2 = 50.7%). To further analyse this outcome, the risk difference and the number needed to treat were calculated (RD = −0.05 [−0.08, −0.03], p < 0.0001; NNT = 20). Discontinuation due to LoE was lower among patients receiving pharmacological treatment than among those receiving placebo (5.0 vs. 14.5%, OR = 0.30 [0.25, 0.35], p < 0.0001, I 2 = 12.3%, online resource 6), while discontinuation due to AEs was higher (4.5 vs. 1.7%, OR = 1.82 [1.37, 2.40], p < 0.0001, I 2 = 0%, online resource 7).
Forest plot of the effect of pharmacological treatment on all-cause treatment discontinuation in children and adolescents with ADHD. On the right (values >1.0), placebo is better than pharmacological treatment. Abbreviations: ATX atomoxetine, BUP bupropion, CLO clonidine, DMI desipramine, dMPH dexmethylphenidate, GUA guanfacine, LIS lisdexamfetamine, MAS mixed amphetamine salts, MOD modafinil, MPH methylphenidate
Pharmacological treatment was more efficacious than placebo for reducing ADHD symptom severity irrespective of who the rater was the clinician (SMD = 0.74 [0.65, 0.84], p < 0.00001, I 2 = 73.5%, Fig. 2), the parent (SMD = 0.63 [0.54, 0.72], p < 0.00001, I 2 = 41.3%, Fig. 3) or the teacher (SMD = 0.75 [0.64, 0.86], p < 0.00001, I 2 = 52.9%, Fig. 4).
Forest plot of the effect of pharmacological treatment on clinician-rated ADHD symptoms in children and adolescents with ADHD. On the right (values >0.0), pharmacological intervention is better than placebo. Abbreviations: ATX atomoxetine, BUP bupropion, CLO clonidine, DMI desipramine, dMPH dexmethylphenidate, GUA guanfacine, LIS lisdexamfetamine, MAS mixed amphetamine salts, MOD modafinil, MPH methylphenidate
Forest plot of the effect of pharmacological treatment on parent-rated ADHD symptoms in children and adolescents with ADHD. On the right (values >0.0), pharmacological intervention is better than placebo. Abbreviations: ATX atomoxetine, BUP bupropion, CLO clonidine, DMI desipramine, dMPH dexmethylphenidate, GUA guanfacine, LIS lisdexamfetamine, MAS mixed amphetamine salts, MOD modafinil, MPH methylphenidate
Forest plot of the effect of pharmacological treatment on teacher-rated ADHD symptoms in children and adolescents with ADHD. On the right (values >0.0), pharmacological intervention is better than placebo. Abbreviations: ATX atomoxetine, BUP bupropion, CLO clonidine, DMI desipramine, dMPH dexmethylphenidate, GUA guanfacine, LIS lisdexamfetamine, MAS mixed amphetamine salts, MOD modafinil, MPH methylphenidate
AEs were common, being more frequent among patients receiving pharmacological interventions (72.2 vs. 56.9%, OR = 2.09 [1.85, 2.37], p < 0.00001, I 2 = 27.4%, Fig. 5). Conversely, SAEs were infrequent and no differences were found between pharmacological interventions and placebo (1.8 vs. 1.5%, OR = 1.00 [0.62, 1.63], p = 0.99, I 2 = 0%, online resource 8).
Forest plot of the effect of pharmacological treatment on any adverse event in children and adolescents with ADHD. On the right (values >1.0), placebo is better than pharmacological intervention. Abbreviations: ATX atomoxetine, BUP bupropion, dAMP dexamphetamine, DMI desipramine, dMPH dexmethylphenidate, GUA guanfacine, LIS lisdexamfetamine, MAS mixed amphetamine salts, MOD modafinil, MPH methylphenidate
Sensitivity and publication bias analysis
The results of the effect of pharmacological treatment on treatment discontinuation, efficacy and safety remained similar after excluding each study once and after excluding those studies rated as having a high risk of bias (online resource 9). The analysis of funnel plots along with Egger’s and Begg’s tests did not support this meta-analysis being influenced by publication bias with the exception of clinician-rated ADHD symptom severity (online resource 10). For this outcome, the funnel plot showed that the majority of studies had a similar standard error and were distributed within the same stratum, with more studies outside the funnel than at the bottom of it. Egger’s and Begg’s tests for clinician-rated-ADHD symptom severity were statistically significant. We repeated the study of publication bias limiting the analyses to industry-sponsored RPCCT and similar results were found.
Effect of covariates on treatment discontinuation, efficacy and safety
Table 2 shows the effect of covariates on study outcomes. RPCCTs that investigated psychostimulants had a more favourable outcome on all-cause treatment discontinuation than those investigating non-stimulant drugs. This outcome was less favourable among studies using a fixed dosage compared to those using a flexible one. The effect of these covariates remained statistically significant after performing a multivariate meta-regression analysis (psychostimulants vs. non-stimulants: ROR = 0.63 p = 0.002, fixed vs. flexible dosage: ROR = 1.60, p = 0.002). The proportion of variance that remained unexplained by the covariates analysed was moderate (I 2 residual = 34.7%).
The pooled OR of LoE-induced discontinuation was higher when a comorbid disorder was an inclusion criterion, while it was lower when patients’ baseline clinician-rated ADHD severity was higher (online resource 11) and when a psychostimulant was investigated. The efficacy of pharmacological treatment on clinician-rated ADHD symptom severity was smaller in RPCCTs for which comorbidity was an inclusion criterion, in studies enrolling older patients, in those with a higher proportion of patients with a history of stimulant treatment and those of longer duration, and was larger in studies that had a commercial sponsor (online resources 12, 13 and 14). The efficacy on parent-rated ADHD symptom severity was positively associated with baseline severity (online resource 15). The efficacy on teacher-rated ADHD symptom severity was larger in RPCCTs that investigated psychostimulant drugs and was negatively associated with study duration (online resource 16). Pharmacological treatment had a less favourable outcome on AEs in RPCCTs with a higher proportion of patients with ODD (online resource 17). Multivariate meta-regression was not performed for these outcomes because the number of studies was too small for such analyses.
Discussion
An appreciable number of RPCCTs investigating the efficacy and safety of pharmacological treatment of ADHD have been carried out, particularly with methylphenidate, atomoxetine and guanfacine. Nevertheless, most of these studies were short term. Overall, pharmacological treatment improved ADHD symptoms in children and adolescents with ADHD of moderate severity. The effect size was medium–large irrespective of who the efficacy rater was. Although AEs were frequent, yet rarely serious, all-cause treatment discontinuation was lower with the active intervention than with placebo. These results suggest that, in the short term, the efficacy of pharmacological treatment on ADHD symptoms outweighs its AEs. The results of this study contrast with those of adults with ADHD for whom all-cause treatment discontinuation was higher with pharmacological treatment than with placebo, probably because the efficacy in this population was small (Cunill et al. 2016).
This study also found that several patient-, intervention-, study design- and sponsor-related covariates modified the efficacy, safety and discontinuation of pharmacological treatment for children and adolescents with ADHD, the most prominent of which was the type of pharmacological treatment. Psychostimulants achieved larger symptom improvement as assessed by teachers than non-stimulant drugs (diff SMD = 0.23). This difference may explain why psychostimulants also show a better outcome on both LoE-induced (ROR = 0.58) and all-cause treatment discontinuation (ROR = 0.61). These results, along with the fact that no differences were found on safety outcomes, would support that psychostimulants have a more suitable risk-benefit relationship than non-stimulant drugs. That psychostimulants are more efficacious for reducing ADHD symptoms than non-stimulant drugs has also been found in direct comparisons between methylphenidate and atomoxetine in children (Newcorn et al. 2008). Also in adults, it has recently been found that psychostimulants are more efficacious than non-stimulants, but the difference was slightly lower (diff. SMD = 0.18) and was not associated with a better outcome on treatment discontinuation (Cunill et al. 2016). The type of dose regime also moderated the effect on all-cause treatment discontinuation. Flexible dose regimes attained more favourable outcomes than fixed ones, probably because this type of dose regime adapts better to the patient’s needs.
Also notable was the moderating effect of treatment length and comorbidity. Both clinician- and teacher-rated efficacy reduced progressively over time. A similar modifying effect of time has been found in other studies with children (MTA Cooperative Group 2004) and adults with ADHD (Cunill et al. 2016) and may be suggestive of tolerance to pharmacological treatment. The progressive reduction of the efficacy could explain the finding that the larger the proportion of patients with prior treatment with stimulants the lower the clinician-rated efficacy, though this finding could also be due to the fact that patients with a history of stimulant treatment are more likely to be non-responders. Regarding comorbidity, we found that studies for which a comorbid psychiatric disorder was an inclusion criterion had both lower efficacy as rated by clinicians and worse outcomes on discontinuation due to inefficacy. This may be a consequence of the symptom overlap between ADHD and comorbid disorders that can make the assessment of symptom severity more difficult. Alternatively, since pharmacological treatment does not directly target the comorbid disorder, it is likely that inattention and impulsivity symptoms secondary to it are little improved by these drugs. This finding is clinically relevant because comorbidity is highly frequent in patients with ADHD (Jensen and Steinhausen 2015; Larson et al. 2011).
A weak effect of baseline severity on treatment efficacy was found. A positive association between parent-rated baseline severity and efficacy and by a negative association between clinician-rated baseline severity and discontinuation due to inefficacy suggests that the higher the baseline severity the larger the room for improvement.
Finally, age and type of sponsor moderated one outcome: clinician-rated efficacy. The efficacy of pharmacological treatment reduced with age. This finding could be explained by an increased probability of previous stimulant treatment in older patients. Concerning the type of sponsor, we found larger clinician-rated efficacy in studies that had a commercial sponsor. This result along with the finding of a possible risk of publication bias for this outcome, particularly in industry-sponsored studies, could indicate that negative results on clinician-rated efficacy are not published leading to commercial studies to have a larger efficacy results. This finding could contribute to the understanding of vested interest bias in industry-sponsored RPCCT (Lundh et al. 2017).
No statistical heterogeneity was found for discontinuation due to AEs or serious AEs. This is likely to be due to the relatively low incidence of these events, which results in rather imprecise study effect estimates. In these circumstances, statistical heterogeneity is unlikely. Conversely, statistical heterogeneity was found for the outcome ‘any AE’. One covariate, the prevalence of ODD, was negatively associated with any AE. This finding may reveal that patients with ODD show worse treatment tolerability or, alternatively, that these patients are more likely to complain about mild AEs that may go unnoticed in patients without this comorbidity.
It must be noted that the covariate analyses of all-cause treatment discontinuation, clinician-, parent- and teacher-rated efficacy showed residual heterogeneity, which indicates that the covariates investigated were unable to explain a significant proportion of the between-study variance. Conversely, for discontinuation due to LoE and any AE, there was no residual heterogeneity, which may be due to the fact that statistical heterogeneity was relatively low for these outcomes.
This study has several limitations. The inclusion of RPCCTs with low quality may have biased our results. Such a possibility is unlikely because excluding RPCCTs with a high risk of bias has yielded similar results to the primary analysis. Publication bias is a meta-analysis-specific source of bias, of which we found no evidence except for clinician-rated efficacy. A high number of covariate analyses has been carried out; thus, the risk of false positive associations must be taken into account. The effect of covariates may be confounded by the influence of other covariates due to the observational nature of meta-regression analyses. Confusion can be controlled using multivariate meta-regression, but we could only use this method for all-cause treatment discontinuation. Since we analysed aggregated data, the possibility of ecological bias cannot be ruled out. For these reasons, the findings of the meta-regression analysis should be confirmed with ad hoc studies. The external validity of this study may be compromised by the short duration of interventions investigated and the fact that psychotherapy was infrequently provided, which contrasts with the clinical practice and clinical guidelines recommendations (Seixas et al. 2012). It is rather simplistic to classify drugs as ‘psychostimulants’ and ‘non-stimulants’, as the psychostimulant effect is a continuum that ranges from none to strong. For this reason, it may be argued that we, like others (Cunill et al. 2016; Faraone and Glatt 2010), have classified bupropion and modafinil as ‘non-stimulant’ drugs when these drugs have shown mild stimulant effects in some studies. It would probably be more accurate to classify the studied drugs according to their degree of psychostimulant effect but, to our knowledge, this classification does not exist. Similarly, the effect of a relevant covariate such as dose has not been investigated because to do so the equivalent dose for each drug is needed and such equivalence is not known. It must be noted that the results of the covariate analysis cannot be applied to cross-over trials as this type of studies was excluded from this meta-analysis.
The finding that the incidence of AEs and discontinuation due to AEs was higher with pharmacological treatment than placebo suggests the possibility that study interventions were identified, which could cause blinding failure. The use of active placebo that mimics some AEs of study medications has been proposed to reduce the possibility of unblinding. Nevertheless, this type of comparators is ethically arguable as the administration of nocebo may collide with the principle of non-maleficence. Putting it altogether highlights the importance of using objective outcomes to limit performance and detection bias instead of relying on subjective outcomes like ADHD symptom severity.
Study strengths include the large sample size and remarkable representativeness of all ages, genders and psychiatric comorbidities. Furthermore, the primary outcome was a pragmatic one whose interpretation is straightforward. Finally, the use of meta-regression has the advantage of enabling the investigation of sponsor- and study design-related covariates, such as the presence of a lead-in phase, as they show between-study variability, and thus their modifying effect cannot be studied alternatively.
Conclusions
Numerous RPCCTs of short duration have investigated the efficacy of pharmacological treatment for children and adolescents with ADHD, particularly of methylphenidate, atomoxetine and guanfacine. In the short term, pharmacological treatment of ADHD achieves medium–high symptom relief and an improvement in treatment discontinuation when compared to placebo, suggesting a favourable risk-benefit relationship.
Psychostimulants seem more efficacious and show a better outcome on treatment discontinuation than non-stimulant drugs. Patients with a comorbid psychiatric disorder appear to benefit less from pharmacological treatment than those without psychiatric comorbidities. The efficacy should be assessed routinely, as it seems to reduce over time. RPCCTs with a commercial sponsor show larger clinician-rated efficacy probably due to publication bias.
References
Anderson IM, Tomenson BM (1995) Treatment discontinuation with selective serotonin reuptake inhibitors compared with tricyclic antidepressants: a meta-analysis. BMJ 310:1433–1438
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Berkey CS, Hoaglin DC, Mosteller F, Colditz GA (1995) A random-effects regression model for meta-analysis. Stat Med 14:395–411
Bolea-Alamañac B, Nutt DJ, Adamou M, Bazire S, Coghill D, Heal D, Müller U, Nash J, Santosh P, Sayal K, Sonuga-Barke E, Young SJ, British Association for Psychopharmacology (2014) Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the British Association for Psychopharmacology. J Psychopharmacol 28:179–203. doi:10.1177/0269881113519509
Burcu M, Zito JM, Metcalfe L, Underwood H, Safer DJ (2016) Trends in stimulant medication use in commercially insured youths and adults, 2010-2014. JAMA Psychiatry 73:992–993. doi:10.1001/jamapsychiatry.2016.1182
Bushe CJ, Savill NC (2013) Suicide related events and attention deficit hyperactivity disorder treatments in children and adolescents: a meta-analysis of atomoxetine and methylphenidate comparator clinical trials. Child Adolesc Psychiatry Ment Health 19:19. doi:10.1186/1753-2000-7-19
Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA) (2011) Canadian ADHD practice guidelines, Third edn. CADDRA, Toronto
Charach A, Yeung E, Climans T, Lillie E (2011) Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry 50:9–21. doi:10.1016/j.jaac.2010.09.019
Cochran WC (1954) The combination of estimates from different experiments. Biometrics 10:110–129
Coghill DR, Seth S, Pedroso S, Usala T, Currie J, Gagliano A (2014) Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol Psychiatry 76:603–615. doi:10.1016/j.biopsych.2013.10.005
Coughlin CG, Cohen SC, Mulqueen JM, Ferracioli-Oda E, Stuckelman ZD, Bloch MH (2015) Meta-analysis: reduced risk of anxiety with psychostimulant treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 25:611–617. doi:10.1089/cap.2015.0075
Cunill R, Castells X, Tobias A, Capellà D (2015) Pharmacological treatment of attention deficit hyperactivity disorder with co-morbid drug dependence. J Psychopharmacol 29:15–23. doi:10.1177/0269881114544777
Cunill R, Castells X, Tobias A, Capellà D (2016) Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology 233:187–197. doi:10.1007/s00213-015-4099-3
Daley D, Birchwood J (2010) ADHD and academic performance: why does ADHD impact on academic performance and what can be done to support ADHD children in the classroom? Child Care Health Dev 36:455–464. doi:10.1111/j.1365-2214.2009.01046.x
Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG (2015) Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet 385:2190–2196. doi:10.1016/S0140-6736(14)61684-6
Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A (2002) Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 31:140–149
Faraone SV, Glatt SJ (2010) A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry 71:754–763. doi:10.4088/JCP.08m04902pur
Feldman HM, Reiff MI (2014) Clinical practice. Attention deficit-hyperactivity disorder in children and adolescents. N Engl J Med 370:838–846. doi:10.1056/NEJMcp1307215
Graham J, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, Döpfner M, Hamilton R, Hollis C, Holtmann M, Hulpke-Wette M, Lecendreux M, Rosenthal E, Rothenberger A, Santosh P, Sergeant J, Simonoff E, Sonuga-Barke E, Wong IC, Zuddas A, Steinhausen HC, Taylor E, European Guidelines Group (2011) European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry 20:17–37. doi:10.1007/s00787-010-0140-6
Harpin VA (2005) The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch Dis Child 90(Suppl 1):i2–i7. doi:10.1136/adc.2004.059006
Higgins JPT, Green S (2011a) The Cochrane Collaboration tool for assessing risk of bias. In Cochrane handbook for systematic reviews of interventions version 5.1.0. [updated March 2011]. The Cochrane Collaboration Available from www. cochrane-handbook.org
Higgins JPT, Green S (2011b) How to include multiple groups from one study. In Higgins JPT, Green S (eds), Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration Available from www. cochrane-handbook.org
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. doi:10.1136/bmj.327.7414.557
Hirota T, Schwartz S, Correll CU (2014) Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 53:153–173. doi:10.1016/j.jaac.2013.11.009
Hoza B (2007) Peer functioning in children with ADHD. J Pediatr Psychol 32:655–663. doi:10.1093/jpepsy/jsm024
Jensen CM, Steinhausen HC (2015) Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Atten Defic Hyperact Disord 7:27–38. doi:10.1007/s12402-014-0142-1
Jerome L, Segal A, Habinski L (2006) What we know about ADHD and driving risk: a literature review, meta-analysis and critique. J Can Acad Child Adolesc Psychiatry 15:105–125
Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE (2000) A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med 343:1290–1297. doi:10.1056/NEJM200011023431802
Langley K, Fowler T, Ford T, Thapar AK, van den Bree M, Harold G, Owen MJ, O'Donovan MC, Thapar A (2010) Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. Br J Psychiatry 196:235–240. doi:10.1192/bjp.bp.109.066274
Larson K, Russ SA, Kahn RS, Halfon N (2011) Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics 127:462–470. doi:10.1542/peds.2010-0165
Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, Davis JM (2012) Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379:2063–2071. doi:10.1016/S0140-6736(12)60239-6
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. doi:10.1056/NEJMoa051688
Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L (2017) Industry sponsorship and research outcome. Cochrane Database Syst Rev 2 doi: 10.1002/14651858.MR000033.pub3.
Mannuzza S, Klein RG, Moulton JL (2008) Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res 160:237–246. doi:10.1016/j.psychres.2007.11.003
McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA, Thase ME, Davis L, Biggs MM, Shores-Wilson K, Luther JF, Niederehe G, Warden D, Rush AJ (2006) Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry 163:1531–1541. doi:10.1176/ajp.2006.163.9.1531
Mick E, McManus DD, Goldberg RJ (2013) Meta-analysis of increased heart rate and blood pressure associated with CNS stimulant treatment of ADHD in adults. Eur Neuropsychopharmacol 23:534–541. doi:10.1016/j.euroneuro.2012.06.011
Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal T, Gibbons RD, Hur K, Houck PR, MTA Cooperative Group (2009) The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry 48:484–500. doi:10.1097/CHI.0b013e31819c23d0
MTA Cooperative Group (2004) National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics 113:762–769
National Institute for Health and Clinical Excellence (NICE) (2008) Attention deficit hyperactivitydisorder. Diagnosis and management of ADHD in children, young people and adults. Clinical guidelines CG72 (Last updated February 2016)
Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, Michelson D, Atomoxetine/Methylphenidate Comparative Study Group (2008) Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 165:721–730. doi:10.1176/appi.ajp.2007.05091676
Nigg JT (2013) Attention-deficit/hyperactivity disorder and adverse health outcomes. Clin Psychol Rev 33:215–228. doi:10.1016/j.cpr.2012.11.005
No authors (2015) Drugs for ADHD. Med Lett Drugs Ther 57:37–40
Nordic CT, Cochrane Centre TCC (2014) ReviewManager (RevMan). Copenhagen
Otasowie J, Castells X, Ehimare UP, Smith CH (2014) Tricyclic antidepressants for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 19:CD006997. doi:10.1002/14651858.CD006997.pub2
Oviedo-Joekes E, Brissette S, Marsh DC, Lauzon P, Guh D, Anis A, Schechter MT (2009) Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med 361:777–786. doi:10.1056/NEJMoa0810635
Perrin JM, Friedman RA, Knilans TK, Black Box Working Group, Section on Cardiology and Cardiac Surgery (2008) Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics 122:451–453. doi:10.1542/peds.2008-1573
Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, Vohra S (2016) Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2:CD009996. doi:10.1002/14651858.CD009996.pub2
Ruggiero S, Clavenna A, Reale L, Capuano A, Rossi F, Bonati M (2014) Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol 24:1578–1590. doi:10.1016/j.euroneuro.2014.08.001
Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA, CATIE-AD Study Group (2006) Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med 355:1525–1538. doi:10.1056/NEJMoa061240
Schwartz S, Correll CU (2014) Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry 53:174–187. doi:10.1016/j.jaac.2013.11.005
Seixas M, Weiss M, Müller U (2012) J Psychopharmacol 26:753–765. doi:10.1177/0269881111412095
StataCorp (2011) Stata statistical software: release 12 [computer program] (2011). StataCorp, College Station
Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, Rosendal S, Groth C, Magnusson FL, Moreira-Maia CR, Gillies D, Buch Rasmussen K, Gauci D, Zwi M, Kirubakaran R, Forsbøl B, Simonsen E, Gluud C (2015) Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev 11:CD009885. doi:10.1002/14651858.CD009885.pub2
Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA (2003) The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull 29:15–31. doi:10.1093/oxfordjournals.schbul.a006986
Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management, Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S (2011) ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022. doi:10.1542/peds.2011-2654
Swanson JM, Volkow ND (2008) Increasing use of stimulants warns of potential abuse. Nature 453:586. doi:10.1038/453586a
Tamminga HG, Reneman L, Huizenga HM, Geurts HM (2016) Effects of methylphenidate on executive functioning in attention-deficit/hyperactivity disorder across the lifespan: a meta-regression analysis. Psychol Med 46:1791–1807. doi:10.1017/S0033291716000350
Thomas R, Sanders S, Doust J, Beller E, Glasziou P (2015) Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135:994–1001. doi:10.1542/peds.2014-3482
Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, Blumberg SJ (2014) Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry 53:34–46. doi:10.1016/j.jaac.2013.09.001
Yildiz A, Vieta E, Leucht S, Baldessarini RJ (2011) Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology 36:375–389. doi:10.1038/npp.2010.192
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 855 kb).
Rights and permissions
About this article
Cite this article
Riera, M., Castells, X., Tobias, A. et al. Discontinuation of pharmacological treatment of children and adolescents with attention deficit hyperactivity disorder: meta-analysis of 63 studies enrolling 11,788 patients. Psychopharmacology 234, 2657–2671 (2017). https://doi.org/10.1007/s00213-017-4662-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4662-1