Abstract
Perianal localization of Crohn’s disease involves significant morbidity, affects quality of life and results in an increased use of healthcare resources. Medical and surgical therapies contribute to its management. The objective of this review is to address the current understanding in the management of perianal Crohn’s disease, with the main focus in reviewing pharmacological therapies, including stem cells. In complex fistulas, once local sepsis has been controlled by surgical drainage and/or antibiotics, anti-TNF drugs (infliximab, adalimumab) are the first-line therapy, with or without associated immunomodulators. Combining surgery and anti-TNF therapy has additional benefits for healing. However, response is inadequate in up to half of cases. A possible role of new biological drugs in this context (vedolizumab, ustekinumab) is an area of ongoing investigation, as is the local application of autologous or allogeneic mesenchymal stem cells. These are non-hematopoietic multipotent cells with anti-inflammatory and immunomodulatory properties, the use of which may successfully treat refractory patients, and seem to be a promising and safe alternative to achieving fistula healing in Crohn’s disease, without known systemic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In complex Crohn’s disease fistulas, anti-TNF drugs are the first-line therapy following adequate surgical drainage, but half of patients do not respond. |
New biological drugs such as vedolizumab and ustekinumab may play a role in the treatment of fistulizing Crohn’s disease, and this potential indication is the subject of ongoing research. |
Local application of autologous and allogeneic mesenchymal stem cells may be a good option to treat perianal fistulas without having systemic complications. |
1 Introduction
In spite of its growing incidence and the amount of interest and resources dedicated to research, Crohn’s disease (CD) remains to some extent a poorly understood condition. It probably arises as the consequence of a faulty interaction between host defence mechanisms and environmental factors in susceptible subjects who have a genetic predisposition. However, it has been impossible to identify a precise aetiological trigger. Our knowledge has undoubtedly advanced in areas such as diagnosis, follow-up, attention to patient issues, use of telemedicine and, especially, therapy [1]. There is little doubt that the limits of treatment have moved forward during recent years. Some important events were the recognition of the deleterious effects of long-term steroids, the rationalizing of immunosuppression, the arrival of biological therapies, strategies to prevent and manage adverse events, and the development of surgical advances, notably bowel-sparing techniques, and laparoscopic surgery. However, complete control of the disease is still far away, and CD sometimes negatively surprises patients and clinicians with its aggressiveness. Although most individuals with CD lead what could be considered an unimpeded life, a few of them will see their personal quality of life (QoL) severely affected [2, 3]. Perianal CD (PCD) stands out as one of the situations in which this sad prediction can become reality. Lesions are painful, sometimes socially embarrassing and make their presence felt in everyday life. Their consequences can affect the patient’s QoL by inducing irreversible anatomical damage.
This review attempts to address the current understanding in the management of PCD, with special emphasis on complex situations and with the main focus of reviewing pharmacological therapies, including anti-TNF drugs, new biological drugs and local application therapies such as mesenchymal stem cells. We hope to contribute to a better understanding of this characteristic manifestation of CD.
2 Definition and Epidemiology
Perianal CD remains one of the main clinical challenges in the management of this condition [1]. Its prevalence varies between 21 and 54% of all CD patients, appearing more frequently in patients with isolated colonic involvement (41 vs. 12% in isolated ileitis). It is an indicator of poor prognosis and a determinant of increased healthcare costs [1,2,3]. Moreover, CD is a strong predictor of fistula formation after an episode of perianal sepsis [4].
Lesions commonly considered to represent true PCD are non-fistulizing lesions such as hypertrophic skin tags, fissures and ulcers, and fistulizing lesions including perianal fistulas, and perianal abscesses [1,2,3]. Hypertrophic skin tags are generally indolent. However, their size is sometimes significant, and interferes with perianal hygiene. Fissures and ulcers represent lesions of skin and anal canal mucosa, which result in a continuity defect. They are sometimes difficult to differentiate from a sporadic anal fissure, although the latter tend to affect the anterior or posterior commissures. CD-related fissures, and especially ulcers, are not as painless as described classically. They have to be treated, in spite of a relative lack of clinical experience [5].
Abscesses are collections of pus that form in the perianal area, originated by a fistula. These, in turn, are abnormal communications between the perianal skin and the proximal anal canal or rectum, sometimes extending into neighbouring structures. They must be differentiated from their sporadic counterparts, cryptoglandular fistulas. The main differences relate to their origin (anal glands vs. Crohn’s ulcers), and in the fact that CD-related fistulas are generally more complex and refractory. Simple clinical exploration cannot be trusted when assessing a perianal fistula in CD. Conversely, cases of suspected simple perianal fistulas that are refractory to usual management must raise the suspicion of CD.
The term complex perianal Crohn’s disease (CPCD) is generally reserved for fistulas and abscesses. Its definition can follow the simple criteria proposed by Sandborn [6]: “A complex fistula is high (high intersphincteric or high transsphincteric or extrasphincteric or suprasphincteric origin of the fistula tract), may have multiple external openings, may be associated with the presence of pain or fluctuation to suggest a perianal abscess, may be associated with the presence of a rectovaginal fistula, may be associated with the presence of an anorectal stricture, and may be associated with the presence of active rectal disease at endoscopy”.
According to its response to therapy, PCD is not homogeneous. Some cases bear no influence on the overall disease burden. Complex perianal fistulas are more difficult to treat, and carry a high risk of recurrence after the discontinuation of therapy. Studies from the prebiological era [7, 8] reveal that fistulas are generally complex, sometimes impossible to heal, necessitate proctectomy in a significant subset of patients, and tend to recur after initial control. Durable remission is probably possible in less than half of cases.
All this makes it easy to understand why CPCD results in increased use of healthcare resources. Other costs, often not considered in studies, such as indirect costs derived from work absenteeism and sick leave, should be added to these figures. A retrospective multicentre study from Spain [3] included 97 adult patients with CPCD. Immunomodulators were used in 20.5%, biologics in 20.3% and surgery (other than simple drainage) in 27%. These measures resulted in a mean annual global cost per patient of €8289, 75% of which was attributed to pharmacological treatments (mainly biologics), 12.4% to hospitalizations and surgery, and 7.7% to outpatient visits. Other studies addressing the direct costs of CD calculated an average of €2104–4464 per patient/year (€10.594 per patient/year if biologics were used) [9].
Although all age groups are affected, the morbidity of PCD in young patients is especially significant. Paediatric patients with PCD frequently have complex disease, are more likely to be treated surgically, have longer admissions and result in higher hospital charges, as compared with those without PCD [10].
There are few studies that have tried to quantify the alterations in QoL induced by PCD. In one study [11], patients who were treated surgically for PCD were invited to answer several questionnaires measuring male and female sexual function, and both general [36-Item Short Form Health Survey (SF-36)] and disease-related [Inflammatory Bowel Disease Questionnaire (IBDQ)] QoL. When compared to matched controls, they presented worse scores on the 12-Item Short Form Health Survey (SF-12; physical and mental health domains) and IBDQ. These differences did not affect sexual health.
3 Mechanisms and Classification of Perianal Crohn’s Disease (PCD)
The exact reasons why some CD patients go on to develop PCD are poorly understood. Colonic and rectal involvement is the main predictor. However, there are limited data on the possible identification of other disease traits that could identify a CD population that is more susceptible to PCD.
Classification of PCD is not just an intellectual exercise. The type and severity of the fistula are the main drivers of medical and surgical approaches. Many different systems have been proposed. PCD can be classified anatomically, according to the widespread Park’s rule, that divides perianal fistulas according to their relationship to the muscular apparatus of the anus into superficial, intersphincteric, trans-sphincteric, suprasphincteric, and extrasphincteric [1]. However, classification of PCD is best done following the functional system developed by the American Gastroenterological Association [6]; according to this system, fistulas are classified as either simple or complex. Simple fistulas are low lying, and involve only superficial tissues or the distal part of the sphincters (with intersphincteric or intrasphincteric trajects, but always distal to the dentate line). They have a single opening, and perianal complications are typically absent. Continence is generally preserved, and unaffected by therapy. In turn, complex fistulas are higher (high intersphincteric or trans-sphincteric, suprasphincteric and extrasphincteric), and may have multiple openings. They may have local complications (abscess, rectal stricture, extension to bladder or genital tract), or proctitis. The anatomical proximity of the female genital organs to the anterior aspect of anus and rectum justifies the inclusion of any anterior fistula in a woman as a possible complex case.
Fistulas can also be classified according to their clinical activity [12]. The Perianal Disease Activity Index (PDAI) has been validated in clinical trials. It evaluates several items, such as type of perianal disease, pain, discharge, induration and restriction of activity; an active fistula is defined by a PDAI score > 4 [13]. The earlier trials of infliximab in PCD came up with a simple definition, designed as ‘fistula drainage assessment’. This is a very primitive evaluation that relies on the examiner’s impression of the presence of fistula drainage: if this is observed spontaneously or can be elicited by finger compression, the fistula is classified as ‘open’.
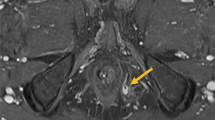
The description of fistula anatomy and activity can be further refined by the use of imaging procedures [13,14,15,16,17,18,19]. An imaging procedure must be considered in all cases with PCD. Endoanal ultrasound (EUS) is a good alternative, when available [14, 15]. It is not feasible in the presence of critical anal stenosis. Other than that, its main limitations are the high operator dependence, its low accuracy in delineating higher anatomical regions (ischiorectal fossa). Transperineal US [17] may be an alternative, if available. Perianal magnetic resonance imaging (MRI) is preferred in many institutions. It is radiation free and more accurate and less operator-dependent than EUS in the definition of fistula anatomy and the detection of complications [14]. A meta-analysis comparing MRI and EUS in perianal fistula assessment [17] showed comparable sensitivities for MRI [0.87, 95% confidence interval (CI) 0.63–0.96] and EUS (0.87, 95% CI 0.70–0.95), although the specificity for MRI (0.69, 95% CI 0.51–0.82) was higher than that for EUS (0.43, 95% CI 0.21–0.69). Closure of the external opening is not an adequate endpoint, and should not guide therapeutic decisions by itself. We struggle to define healing, and it is important to avoid subjective endpoints. EUS or, preferably, MRI should be used to monitor the results of therapy. CT scan, and especially fistulography, are almost never used in this setting [14, 18, 19].
All guidelines consider examination under anaesthesia (EUA) to be the gold standard in the evaluation of PCD. It must not be delayed if an abscess is suspected. If an experienced colorectal surgeon is not available, a limited examination is preferable, allowing drainage of collections, and taking every measure to avoid sphincter damage. This procedure can be later completed by a more experienced colleague; in these circumstances, its accuracy may reach 90% [13]. An additional advantage of EUA is the possibility of therapeutic intervention (abscesses drainage, seton placement, or fistulotomy). The combination of EUA and one imaging technique improves the accuracy of this approach to reach 100% [13].
Proctosigmoidoscopy is mandatory in all patients. The presence of proctitis defines a complicated PCD, and compromises the results of any therapeutic approach [6]; additionally, endoscopy can rule out complications such as stricture or malignancy [13].
4 Clinical Presentation and Diagnosis
Typical symptoms of perianal fistulas are discomfort on defaecation, perianal itching, bleeding and discharge of purulent material, and can follow abscess drainage. The differential diagnosis for fistulizing PCD includes trauma, hidradenitis suppurativa, tuberculosis, HIV infection, lymphogranuloma venereum, perianal actinomycosis and a post-rectal dermoid inclusion cyst. The question of isolated PCD remains unanswered; old clinical series report this possibility, although this idea should probably be revisited with the current diagnostic tools. Anal abscesses may present with pain in the anal area associated in more severe cases with fever. They can be diagnosed by clinical and physical examination characterized by an erythematous, indurated and fluctuating zone [1].
As outlined above, clinical examination has to be complemented by EUA and, at least, one imaging procedure (MRI or EUS).
5 Treatment
In this section we review medical and surgical therapies used in PCD patients, either as single interventions or as part of a combined approach; in particular, we focus on the efficacy of anti-TNF drugs. In Fig. 1 we present a flow diagram of management for PCD.
5.1 Surgery
The management of fistulizing CD patients should always be multidisciplinary, and involve colorectal surgeons and gastroenterologists, all experienced in the management of CD. A fundamental aspect in the surgical treatment of perianal fistula disease is that perianal sepsis, if not drained, is destructive to sphincters and therefore associated with a long-term severe impairment of anal function. We report a brief description of the surgical techniques in PCD since it is not the main focus of this review.
Surgical treatment with fistulotomy (or rarely fistulectomy) is sometimes necessary for symptomatic simple fistulas [20]. These techniques should be performed very selectively in non-complex fistulas, because of the risk of incontinence.
In early stages, surgery should be conservative in order to prevent the destruction of muscular tissue and to preserve anal function. Thus, the first step in the surgical management is always related to abscess drainage. After the abscess has been drained and the fistula anatomy has been delineated through pelvic MR or EUS, it is recommended to undergo EUA, with seton placement if necessary, by a more experienced surgeon [21]. ECCO consensus recommend seton placement after surgical treatment of sepsis for complex fistulas [20]. Setons will allow a continuous drainage of the fistula tract, avoiding the accumulation of pus and debris that would result in recurrent destructive abscesses, and permit preservation of the sphincter and good postoperative function [22]. Setons can be left in place for months, even years in selected cases, and provide a tranquil situation that allows medical therapy to work as desired. The exact timing for seton removal is a matter of controversy, and depends on subsequent therapy. This decision has to be taken individually during the follow-up surgical visits [23]. Some authors have suggested that seton removal after the fifth dose of infliximab may result in lower recurrence rates [24]. As reviewed later in this article, in complex fistulous PCD, the combination of seton placement and medication achieve better results than seton placement alone [25].
In a few patients with more complex perianal disease, a temporary ileostomy will be the best option to control and prevent sphincter damage. Diversion of the faecal stream from an inflamed bowel combined with intensive medical treatment may allow PCD to be controlled and avoid the need for major surgery including proctectomy [26, 27]. In a systematic review with meta-analysis, temporary faecal diversion may improve symptoms in two-thirds of patients with refractory PCD, but bowel restoration was successful in only a few patients (17%) [28]. Some patients refractory to medical therapy may be managed by an experienced surgeon with other techniques, such as advancement flap. Definitive faecal diversion with colostomy or ileostomy is the last resort for ultra-refractory patients [26]. The details and complications of the surgical techniques are outside the scope of this review.
5.2 Antibiotics
Medical treatment of perianal fistulas also includes the use of antibiotics. Indeed, antibiotics are part of the first-line management of PCD. ECCO guidelines suggest that symptomatic simple perianal fistulas should be treated with seton placement in combination with antibiotics (metronidazole and/or ciprofloxacin). In complex fistulas, antibiotics improve short-term outcomes of anti-TNF drugs, the first-line therapy in these cases [20].
The evidence on use of antibiotics is of poor quality: short series, ill-defined patient populations and varying drug doses. In general, it has to be stressed that response to antibiotics is at best short-lived, and treatment of complex PCD with these drugs alone should not be attempted. They can be used as part of a general therapeutic plan. Although many practitioners choose amoxicillin-clavulanic to control soft tissue infection, as recommended in spontaneous anal fistulas, metronidazole and ciprofloxacin have been studied with more rigour in the setting of Crohn’s disease. The first studies with metronidazole in PCD were carried out in the 1980s [29,30,31]. Bernstein et al. found in a small open-label study that the improvement in perianal disease with metronidazole occurred within 6–8 weeks in 83% of patients, although treatment discontinuation was associated with a very frequent recurrence of fistulas (78%) [30]. Another very short non-controlled series found that metronidazole reduced the number of draining fistulas and halved the number of detectable fistula openings, resulting in complete symptom control in four out of eight patients [31]. In general, high metronidazole doses are typically recommended (20 mg/kg/day, or 750–1000 mg/day divided into three or four doses), although patients frequently complain of dyspepsia and a metallic taste. Regarding treatment duration, most studies indicate 8–12 weeks [23]. In addition to short-term intolerance, long-term use of metronidazole has been associated with significant peripheral neuropathy [32]. Another alternative would be the use of topical metronidazole ointment or cream [33, 34], although a blinded, randomized, placebo-controlled study including 74 patients failed to show any effect in the reduction of PDAI score compared to the placebo group (however, perianal discharge was reduced, p = 0.012) [33].
Ciprofloxacin has been also studied in PCD. Two small case series demonstrated an improvement in the 80–100% of patients with perianal disease [35, 36]. The doses recommended are 1000–1500 mg/day. Adverse events are uncommon but can include headache, nausea, diarrhoea, rash and tendon rupture [35].
These drugs can also be used in combination. Solomon et al. found that 60% of patients with severe complex fistulas included in their study had clinical response and 20% showed closure of fistula within 12 weeks of treatment with ciprofloxacin and metronidazole in combination [37].
Despite the wide use of both antibiotics there is only one comparative controlled trial. This study included 25 patients with active perianal disease and assigned, in a double-blind manner, therapy for 10 weeks with ciprofloxacin, metronidazole or placebo. Remission and response occurred more often in patients treated with ciprofloxacin, but the differences were not significant [38]. An important limitation of this study was the small number of patients evaluated. A systematic review by Khan et al. including three trials evaluating antibiotic treatment of PCD fistula in 123 patients, using either ciprofloxacin or metronidazole, shows a significant effect of either drug in reducing fistula drainage (relative risk = 0.8; 95% CI 0.66–0.98) with a number needed-to-treat of five [39].
The use of antibiotics in association with thiopurines and anti-TNF agents has also been analysed. A prospective, open-label trial studied the use of metronidazole and/or ciprofloxacin during 8 weeks as a bridge to therapy with azathioprine. At week 20, patients who received azathioprine were more likely to achieve a response than those without immunosuppression (48 vs. 15%, p = 0.03) [40].
West et al. evaluated in a double-blind placebo-controlled study the effect of combined ciprofloxacin and infliximab in PCD. Patients who received a combination of ciprofloxacin and infliximab were more likely to have a fistula response at week 28 compared to patients on treatment with infliximab alone (73 vs. 39%: p = 0.12) [41]. In addition, a randomized, double-blind, placebo-controlled trial showed that adalimumab combined with ciprofloxacin was superior to adalimumab monotherapy in perianal fistula closure in CD patients. However, after discontinuation of antibiotic therapy, the beneficial effect of initial co-administration was not maintained and no differences were observed in the clinical response at week 24 in both groups [42].
5.3 Immunomodulators
5.3.1 Thiopurines
No randomized controlled trials (RCTs) have evaluated the effectiveness of thiopurines on the closure of perianal fistulas as the primary end-point. In a meta-analysis, five RCTs assessing perianal fistula closure with thiopurines as a secondary endpoint in patients with CD were included [43]. Twenty-two of 41 patients (54%; 95% CI 37–69) treated with azathioprine or mercaptopurine responded with complete healing or decreased discharge of fistula, compared with six of 29 patients (21%; 95% CI 8–40) treated with placebo (odds ratio (OR) 4.4; 95% CI 1.5–13.2). Current ECCO guidelines recommend anti-TNF drugs as first-line therapy in complex fistulizing disease following surgical drainage, and suggest combination therapy with thiopurines a viable option to enhance the effect [20].
Some of the evidence regarding thiopurine use in PCD deserves further comment. As explained above, one experience in PCD patients treated with antibiotics for 8 weeks showed that administration of azathioprine increases the percentage of response obtained when compared with patients without immunosuppressive therapy [40]. In the RAPID study, early azathioprine therapy was compared with conventional step-up treatment strategy in 147 patients within 6 months of diagnosis of CD with risk of disabling disease [44]. As a secondary end-point, patients in the early-azathioprine group had less active perianal lesions than the conventional strategy group (14 vs. 27%, p = 0.049), and a higher cumulative proportion of patients were free of perianal surgery in the early-azathioprine group during the 3-year follow-up (96 vs. 82%, p = 0.04). In another cohort of 1035 CD patients, the risk of perianal surgery was lower in those patients with immunosuppressive therapy (thiopurines and/or methotrexate) within 3 years of CD diagnosis and adherence of at least 6 months than in patients without early immunosuppressive therapy (hazard rate 0.30; 95% CI 0.16–0.56; p < 0.01) [45].
5.3.2 Calcineurin Inhibitors
5.3.2.1 Tacrolimus
A randomized placebo-controlled trial evaluated the efficacy of oral tacrolimus 0.2 mg/day for 10 weeks in CD patients with draining perianal or enterocutaneous fistulas. Response was defined as closure of ≥ 50% of fistulas that were draining at baseline, maintained for at least 4 weeks. Nine of 21 (43%) patients treated with tacrolimus and two of 25 (8%) patients receiving placebo achieved response (p = 0.01). Fistula remission rate was similar in both groups (10 vs. 8%, p = 0.86). Side effects were more frequent in the tacrolimus group (4.2 vs. 2.3%, p = 0.01), including creatinine level increase, headache, insomnia, paresthesias and tremor, which were managed by dose reduction. Therapeutic drug monitoring is recommended to minimize tacrolimus toxicity [12].
A randomized controlled trial including 12 patients with fistulizing PCD failed to show any advantage of topical tacrolimus (1 mg/g ointment applied twice a day) on placebo after 12 weeks of therapy [46].
5.3.2.2 Cyclosporine
There is a lack of data about the effectiveness of cyclosporine in CD fistulas treatment. Although in several uncontrolled case series cyclosporine infusion followed by oral administration achieved an initial response, it was usually lost on drug withdrawal [47,48,49].
5.3.3 Other Immunomodulatory Therapies
In a review based on four studies including 40 PCD patients treated with oral thalidomide for induction of remission, 25% of patients achieved remission and 27.5% had partial response to thalidomide treatment (50–300 mg/day) [50]. Case reports and uncontrolled case series have reported benefit from other therapies as mycophenolate mofetil, methotrexate and granulocyte colony-stimulating factor, but they are not recommended for clinical practice.
5.4 Anti-TNF Drugs
Anti-TNFs were the first biologics available in inflammatory bowel disease, and evidence and clinical experience with these drugs are abundant. Treatment with infliximab, the first anti-TNF used in perianal disease, determined a significant change in the prognosis of this condition, achieving remissions of complex PCD fistula never seen before with any other form of therapy. Current ECCO consensus in PCD recommends infliximab, or adalimumab, as first-line therapy for perianal disease, following adequate surgical drainage if indicated [20].
Infliximab is the only biological treatment shown to be effective in PCD in a specifically designed randomized controlled trial, both for inducing perianal fistula closure and for maintaining this response [51, 52]. Induction therapy at 5 mg/kg, with infusions at weeks 0, 2 and 6, induced complete closure in 55% of cases, versus 13% with placebo. The ACCENT II trial, designed to study the efficacy of infliximab in the long term, showed fistula remission to persist in 36% of the infliximab group at week 52, compared to 19% on placebo; additionally, maintenance infliximab reduced hospitalizations and surgery [53]. These effects have been confirmed in clinical practice by several case series [54, 55]. A retrospective study on 156 patients evaluated long-term outcome after infliximab treatment for fistulizing PCD. After a median follow-up of 250 weeks, about two-thirds of patients achieved fistula closure, whereas one-third developed fistula recurrence [56].
The effect of adalimumab on perianal fistulas was evaluated, albeit only as a secondary endpoint, in three randomized controlled trials (CLASSIC, CHARM and GAIN studies). There were no detectable differences between adalimumab and placebo in studies with shorter follow-up (CLASSIC-1 and GAIN), although it has to be noted, that they respectively included only 32 (anti-TNF-naïve) and 45 (infliximab failures) patients. The CHARM trial described the evolution of 117 patients with perianal fistulas, 30% of which attained complete closure at week 26 (vs. 13% on placebo), and 33 versus 13% at week 56 (p < 0.02) [57, 58].
Several open-label trials have evaluated adalimumab for fistula healing, including a Spanish trial with 23% fistula remission at 4 weeks [59]. The influence of previous exposure to infliximab was evaluated in a study with 68 patients; at week 24, fistula healing rates were higher for anti-TNF-naive patients (60%) versus infliximab-experienced patients (28%; p < 0.01) [60]. In the CHOICE trial, performed on a population of refractory patients, who failed to respond or had lost response to infliximab, complete fistula healing was achieved by 34/88 (39%) patients with baseline fistulas. This open-label trial further supports the effectiveness of adalimumab in patients who failed infliximab therapy, including primary non-responders [61].
A recent systematic review and meta-analysis by de Groof describes 34 cohorts and case-series totalling 1449 patients treated with anti-TNF (22 studies used infliximab, eight adalimumab and four both drugs). Complete closure rates varied between 16.7 and 93%, with similar figures for partial closure, and recurrence rates oscillated between 8 and 40% [22]. These data have several limitations, mainly the small sample size of some studies, and the premature assessment of endpoints (8 weeks). This paper includes four randomized controlled trials (previously described), totalling 179 CD patients with active fistula treated with anti-TNF, and 109 with placebo. The authors described results of 30% complete response and 44% partial response with anti-TNF (vs. 12 and 24% with placebo, respectively). Overall, they found no differences between anti-TNF drugs and placebo, probably due to the heterogeneity of the studies. However, when only studies with follow-up longer than 4 weeks were selected, those differences became apparent (fistula healing 46% infliximab, 30% adalimumab and 13% placebo) [25]. Furthermore, these same authors analysed the four cohort studies comparing combined therapy with anti-TNF and seton placement, to either treatment used separately, with a follow-up between 15 weeks and 30 months. Percentages of fistula closure with combined therapy varied widely (0–100%), but combined therapy achieved better results, both compared to seton placement without anti-TNF (45 vs. 17%; p = 0.001) and to anti-TNF therapy without seton placement (100 vs. 82%; p = 0.014) [62, 63]. A systematic review addressing the results of combined surgical and medical therapy, included eight studies, with 797 patients. In the group treated only surgically or medically, complete remission was observed in 191/448 (43%), whereas combining medical and surgical management attained fistula healing in 180/349 (52%). The authors conclude that combined surgical and medical (anti-TNF, with or without immunomodulators) may have additional beneficial effects on perianal fistula healing in patients with Crohn’s disease, compared with surgery or medical therapy alone. These data all favour combined therapy, but a well-designed Crohn’s perianal fistula clinical trial in a multidisciplinary medical and surgical setting is still lacking [64]. In this regard, the PISA study is an ongoing multicentre, randomized controlled trial on patients with Crohn’s disease and high perianal fistulas, which will compare three possible treatment strategies after initial seton placement (Nederlands Trial Register identifier: NTR4137). One group will be treated with chronic seton drainage (with oral 6-mercaptopurine for 1 year), a second group with seton removal and anti-TNF medication (with 6-mercaptopurine for 1 year), and a third group with advancement flap after 8 weeks of seton drainage (under 4 months of anti-TNF and 6-mercaptopurine for 1 year).
Regarding the third anti-TNF drug use in CD, certolizumab pegol, pivotal studies (PRECISE) were not designed to assess the efficacy on perianal fistula; results were not encouraging [65, 66], with similar percentages of fistula remission in certolizumab and placebo arms, both in PRECISE 1 (30 and 31%, respectively) and PRECISE 2 trials (54 and 43%, respectively). However, a sub-analysis was done in PRECISE 2, considering only patients with a response to open-label induction, who were then randomized at week 6 to certolizumab 400 mg (n = 28) or placebo (n = 30) every 4 weeks. In these groups, more patients had complete fistula closure at week 24 with certolizumab (36%) than with placebo (17%) [67]. On the other hand, data from a retrospective medical record-based review suggest that certolizumab may be altogether more effective in Crohn’s patients without perianal fistula.
Another question is the value of adding antibiotics to anti-TNF therapy. As previously described, two series showed that combination therapy with ciprofloxacin and anti-TNFs is more effective than anti-TNF monotherapy to achieve fistula closure in CD at week 12 [41, 42]. Therefore, prescription of ciprofloxacin in patients who initiate induction therapy with an anti-TNF may be an option to potentiate the effects of the latter.
Primary lack of response and secondary loss of response to anti-TNF are common. Escalation of anti-TNF treatment (increasing the dosage and/or shorting the interval) or switching to another anti-TNF drug is useful. Serum trough levels and antibody concentrations could guide us in this choice. In case the patient has low levels of drug in the absence of antibodies, we will consider it a bioavailability problem and recommend increasing the dose of the same drug. In case the levels are low but the patient has antibodies against the drug we will consider that it is an immunogenicity problem and recommend switching from anti-TNF. If the patient has adequate levels, it is recommended to change the therapeutic target, although the experience in PCD with biologicals different to the anti-TNF is scarce [68]. Recent studies indicate that higher infliximab trough levels (> 9 to 10 µg/mL) are associated with fistula healing in PCD patients, and those cases with anti-infliximab antibodies had a lower chance of achieving fistula healing [69, 70].
In addition to simple synergy, combination of anti-TNF treatment with thiopurines may be useful to prevent the development of antibodies against the drug. In a recent study, Yarur et al. suggest that infliximab levels needed for fistula healing are higher than those generally targeted for mucosal healing. In their experience, patients with fistula healing had higher median serum infliximab levels than those with active fistulas (15.8 vs. 4.4 μg/mL, respectively; p < 0.0001) [71]; more specifically, infliximab levels ≥ 10.1 µg/mL may improve the rate of fistula healing.
Small series suggest that local injection of infliximab or adalimumab close to the fistula tract may be useful in some refractory patients, in which insufficient local drug delivery could be the reason for the lack of response [72,73,74,75]. A recent review in local injection of anti-TNF for PCD, a safe therapy but without long-term data on its efficacy, suggests a potential role in those patients in whom systemic treatment is contraindicated [76]. Perhaps in non-responder patients the drug does not reach the tissue in which the fistula arises and local anti-TNFs could offer a solution in this scenario.
PCD is a chronic disease, and long-term follow-up is essential. Therefore, duration of an effective therapy constitutes a subject for debate. Lichtenstein et al. have described a 50% reopening of the fistulas after discontinuing anti-TNF, suggesting the persistence of deep fistula tracts despite superficial healing. Persistence of the internal tract detected by MRI or ultrasound studies is a condition associated with a higher risk of fistula recurrence [77]. Many of the patients with complex fistulas will need a longer duration of therapy, or even continuous maintenance to keep their perianal disease under control. If discontinuation is contemplated, clinical examination alone is not sufficient, and the use of image studies is mandatory [78].
6 Emerging Treatment Options
New biologic drugs with therapeutic targets other than TNF-alpha and with an indication for treatment in CD, as well as local application therapies such as stem cells are reviewed in this section.
6.1 Vedolizumab
Vedolizumab is a humanized monoclonal antibody α4β7 integrin antagonist indicated for the treatment of adult patients with moderately to severely active ulcerative colitis or CD [79]. The pivotal GEMINI 2 study on vedolizumab for CD was not specifically designed to analyse results on perianal disease; however, 12% of the study population was affected by fistulas at the study onset [80]. By week 14, 28% of patients with fistulas treated with vedolizumab (every 8 and every 4 week schedules combined) had achieved remission, compared to 11% with of those receiving placebo. By week 52, 41% of patients treated with vedolizumab every 8 weeks (n = 17) and 22.7% with vedolizumab every 4 weeks (n = 22) had achieved fistula closure (vs. 11% with placebo, n = 18). Kaplan–Meier probabilities of fistula closure with vedolizumab were 29 and 33% at 6 and 12 months, respectively [81]. These preliminary findings support a possible role of vedolizumab in the treatment of fistulizing disease.
Some data are available from clinical practice studies that include patients with perianal disease. In a post-marketing experience in adults, five of 10 (50%) patients with active perianal disease followed for at least 12 weeks demonstrated fistula improvement [82]. However, as indicated with certolizumab, results from the US VICTORY Consortium indicate that active perianal disease is one of the factors associated with a reduction in treatment efficacy [83]. In a post-hoc analysis of the OBSERV-IBD cohort from GETAID, 35 (20.2%) patients presented with active perianal disease at baseline. At week 14, complete remission of PCD was observed in 15 (42.9%) of patients. At week 54, vedolizumab was effective for perianal disease in one-third of patients [84]. All these data, however, are insufficient to draw definitive conclusions. A clinical trial of vedolizumab in fistulizing CD is currently underway [vedolizumab 300 mg intravenously in the Treatment of Fistulizing CD (ENTERPRISE. ClinicalTrials.gov Identifier: NCT02630966)] [85].
6.2 Ustekinumab
Ustekinumab is a monoclonal antibody to the shared p40 subunit of the proinflammatory interleukin (IL)-12 and IL-23 cytokines, indicated for treatment of patients with moderately to severely active Crohn’s disease [86]. Several small retrospective observational studies include CD patients treated with ustekinumab. These patients had previously failed at least one anti-TNF agent, and two or more in most cases. Table 1 shows the most prevalent induction and maintenance regimens used in these studies and treatment efficacy, mostly based on physician judgement, with percentages between 48 and 69% of response and 26–33% of remission [87,88,89,90].
Sands et al. have collected results regarding fistula healing in the subset of patients with active perineal fistulas included in pivotal studies [91]. In the UNITI-1, UNITI-2 and CERTIFI studies, almost 40% of patients had a history of fistulizing CD, but only 10.8–15.5% of patients across the studies had active perineal fistulas at baseline [92, 93]. Complete fistula closure was achieved in 24.7% of patients with ustekinumab (vs. 14.1% with placebo; p = 0.073) at week 8. In the CERTIFI maintenance phase fistula response occurred in 9/19 (47%) ustekinumab group and 6/20 (30%) of placebo patients at week 22. In IM-UNITI, at week 44, a higher proportion of patients had response with ustekinumab (80%; 12/15) compared with placebo (45.5%; 5/11; p = 0.64). The authors suggest efficacy of ustekinumab in fistula healing. A treatment effect (delta of 10–13) was seen during the induction therapy and a possible increase of efficacy with a longer duration of ustekinumab treatment was detected. However, data are scarce and new trials designed specifically to demonstrate the utility of ustekinumab in complex fistula are warranted. Table 2 summarizes the efficacy of biological therapy for CPCD compared with placebo in the main pivotal trials with these drugs.
6.3 Mesenchymal Stem Cell Therapy
Pharmacological treatments achieve durable fistula closure in only a half of patients with CPCD. The alternative in these cases is to resort to repeat surgery with risk of eventual proctectomy and permanent stoma [94]. Stem cell therapy is a promising alternative to achieve fistula healing in CD. Mesenchymal stem cells (MSCs) are non-haematopoietic multipotent cells that have the ability to differentiate into several different cell types, and possess anti-inflammatory and immunomodulatory properties [95]. The exact mechanism by which MSCs cure perianal fistulas remains undefined. MSCs are able to exert different effects on different cell types. In some cases (monocytes, CD4+, CD8+ T-cells), their action is functionally inhibitory, whereas it results in cell function modulation in other cases (increase of both circulating and mucosal T-regulatory cells) [96, 97]. Recent research describes a complex paracrine and cell-cell contact-mediated action, inducing T-cell apoptosis and inhibiting the production of proinflammatory cytokines (TNF-alpha, IFN-gamma, IL-17 and IL-21) [98]. Furthermore, MSCs can migrate to sites of inflammation or injury, and promote tissue repair [99]. Several inflammatory conditions, in different immune-mediated disorders, may result in different responses to MSC treatment. Most patients treated with MSC therapy concurrently receive immunosuppressants or biologic drugs that alter the inflammatory profile in the cell environment. The influence of these drugs in the functional plasticity of MSC in CD patients remains a potential area of research [100].
Several clinical trials performed in CD patients, both with autologous and with allogeneic stem cells, and concurrent immunosuppression, show significant results in fistula healing. Most of the patients included were affected by disease refractory to conventional treatment (Table 3) [101]. Although therapy involving local injections of MSC in PCD is a field in continuous investigation, no head-to-head study comparing this approach with biological drug therapy has been carried out so far.
6.3.1 Technical Aspects
With regard to the origin of the cells, both adipose tissue-derived MSC and bone marrow-derived MSC have been used in trials on perianal fistulas. The immunomodulatory capacities of both cell types were similar, according to a study performed on age-matched donors, but the immunomodulatory effects of bone marrow-derived cells seem to be more potent, due to differences in cytokine secretion [102]. Conversely, an advantage of adipose tissue-derived MSC is the ability to obtain more cells with a simple procedure like liposuction [103], resulting in similar immunomodulatory capacities. A phase III trial using adipose tissue-derived MSC [104] showed similar results to previous phase II trials with bone marrow-derived mesenchymal stem cells, suggesting that the origin of the cells does not affect their efficacy in the context of perianal fistula therapy.
Cells can be derived from different donor sources. Both autologous and allogeneic MSC have been used to treat patients with complex fistulas. Autologous cells are obtained from the patient, and are logically less immunogenic. However, there is a logistic problem: the steps involved in obtaining MSC from our own adipose tissue or bone marrow make it impossible to complete cell harvesting and injection in a single procedure. Isolation and expansion of autologous MSC to sufficient numbers of cells is a time-consuming process that extends over weeks. Allogeneic MSC preparations, in contrast, are ready to use and allow the prompt treatment of patients, avoiding both the necessary steps that require obtaining autologous MSC and the possible disease-related effects of this type of MSC. This results in the possibility of treating a higher number of patients, which adds interest to the use of readily available allogeneic MSC preparations [105].
Two important questions still remain unanswered, namely the dosage of cell products, and the necessary number of intrafistula injections. Clinical trials in CPCD have used different MSC doses, with a general tendency to use increasing amounts of cells in an attempt to improve outcomes [106]. In some cases, the administration involved a fixed number of MSCs, whereas in others the number of injected MSCs depended on the fistula depth and length [107]. In the study performed by Molendijk et al., patients were randomized to treatment with a single injection of one of three doses: 1 × 107 cells, 3 × 107 cells, or 9 × 107 cells; the best results were achieved with a dose of 3 × 107 cells [108]. Ciccocioppo et al. used repeat injections of 2 × 107 cells (a median number of three administrations) [97]. The number of MSCs injected in the phase III trial by Panés et al. was fixed at of 12 × 107; in some patients, the cells were split between two fistula tracts, without differences in efficacy between patients with one or two treated tracts [104]. Additionally, the survival kinetics of these cells after injection is not well known. Thus, the optimal number of MSCs should be explored in ongoing and future trials. Closure of the internal opening is essential, because this is the origin of the fistula, and its sealing avoids faecal contamination of the fistula tract [108].
The number of injections is another matter of debate. Many of the trials used a single local injection of MSC in the wall of the fistula. There may be an advantage to repeating an injection if closure is not achieved with the first injection, as shown by Ciccocioppo et al. [97]. In addition, some trials used fibrin glue to deliver MSCs [103], without an evident advantage, and without completely ruling out a potential effect of glue on cell viability. All these points are issues that should be addressed in future trials.
6.3.2 Clinical Experience with Autologous Stem Cells
A number of trials used MSCs derived from autologous adipose tissue. García-Olmo et al. reported the first case of rectovaginal fistula treatment with MSCs [109]. The same authors conducted a phase I trial in refractory CD complex fistula, with a single injection of MSCs. They observed fistula closure in three of four patients at week 8 [110]. A subsequent phase IIb study was performed, randomizing patients to receive fibrin glue alone versus fibrin glue supplemented with one injection of MSCs, and a second injection if fistula healing was not complete at week 8. CD complex fistula healed at 8 weeks in five out of seven patients (71%) of the MSC group versus one of seven patients (14%) in the control group [103]. Two of the five patients (40%) with fistula healing maintained fistula closure after 3 years of follow-up [111].
Another group conducted a phase I and phase II trials, adjusting doses of MSC to the fistula size [107, 112]. The phase I study was performed in ten patients, showing complete healing at week 8 in 30% of them, and partial closure in the remaining [112]. The phase II study involved a first MSC injection followed by a second one if fistula healing was not complete at week 8. It involved 42 patients, describing complete healing at week 8 in 82% of them [107]. The fistula tract was first injected with MSCs, and then filled up with a mixture of MSCs in combination with fibrin glue. Long-term healing was observed in 88% of patients at 1 year, and in 75% at 2 years [113].
A recent phase I trial with 12 patients employed autologous adipose tissue MSCs suspended in a bio-absorbable matrix (STem cells On Matrix Plugs; STOMP); at 6 months, 83% of patients achieved complete clinical healing, with radiographic markers of response. The operation involved removal of a previously placed seton, curettage of the fistula tract, and placement of the MSC-MATRIX fistula plug, which was passed through the tract. The average dose was 2 × 107 cells per plug [114]. Choi et al. treated 15 patients with an injection of autologous adipose tissue MSC (with a second injection if fistula healing was not complete at week 8); 13 patients completed the study, of which 69.2% achieved complete healing at 8 weeks [115].
One single trial used autologous bone marrow MSCs, administered to ten patients in 2–5 injections, and achieving fistula healing in 67% of cases at 8 weeks [97]. A long-term follow-up of the patients treated in this study of Ciccocioppo et al. reported that 50% and 37% of them maintained healing at 2 and 4 years [116], respectively. These results support the long-term efficacy of this therapy. More trials are currently studying the efficacy of bone marrow MSCs.
6.3.3 Clinical Experience with Allogenic Stem Cells
A phase I/IIa trial in 24 patients used a single injection of adipose tissue MSC, followed by a second injection if fistula healing was not complete at week 12. It achieved success in 38% of patients at week 12, and 56% at week 24 [117]. A smaller study described sustained fistula closure in six patients, prolonged to month 8 in half of them [118]. The largest patient series (n = 212) was studied in a phase III randomized controlled trial, by Panés et al. comparing a single injection of adipose tissue MSCs with a single injection of saline solution (placebo); this experience has provided strong evidence of the benefit of MSC in complex CD fistula. The primary endpoint in this study included not only the clinical assessment of fistula closure, but also the absence of activity by MRI, according to guidelines [20]. Fifty percent of patients achieved fistula closure with MSC at week 24. All patents underwent fistula curettage, surgical drainage and closure of the internal fistula orifice, which may explain the relatively high placebo response rate (34%). This trial allowed patients to continue concomitant immunomodulator and anti-TNF drug, which surely increased the efficacy of MSC therapy. Finally, time to clinical remission was rapid, and twice as fast in the MSC group (6.7 weeks) than in the placebo arm.
Another placebo-controlled trial, by Molendijk et al., included 21 patients, randomized to receive a single intralesional injection of allogenic bone marrow of different doses of MSCs (1, 3 or 9 × 107 MSC) or placebo. Efficacy evaluation involved physical exam and MRI at week 12. Mean fistula duration prior to inclusion into the trial was 5.5 years. An injection of 3 × 107 MSC resulted in accelerated fistula healing and remission in up 85% of fistulas (vs. 33% with placebo). Intermediate doses of MSC attained the highest efficacy, suggesting a dose-dependent response, and maybe a different immunogenic performance of different doses. Other authors have used intravenous injection of allogenic bone marrow MSC to treat active Crohn’s disease, with fistula healing planned as a secondary outcome [119].
All these trials showed higher efficacy of MSCs at 1 year than that observed with biological drugs. The proportion of patients experiencing fistula relapse in the follow-up period increased with time [111, 116], and the long-term efficacy of this therapy should be evaluated in future studies. Qiu et al. performed a systematic review and meta-analysis on the efficacy of stem cell therapy for CD, totalling 251 patients; it showed that 57% (95% CI 44–69, n = 251) of cases achieved fistula healing with MSC. Three trials involved a control arm, and in these, the OR of fistula healing with stem cells was 3.83 (95% CI 1.06–13.86, p = 0.04) versus control [120].
The absence of systemic complications related to MSC in clinical trials is of high relevance. The most commonly reported adverse events were pain at the injection site, related to the surgical manipulation of this sensitive zone, and the development of anal abscess, probably due to the contamination of the fistula tract with bacteria. Concerns about a possible malignant transformation of the administered cells seem unlikely, due to the limited lifespan in vivo of the MSCs [108].
In summary, MSC injections seem to be a safe therapy that rescues refractory patients and restores responsiveness to previously failing drugs. The main patient that can benefit of MSC therapy is the one with an inadequate response to usual medical treatment (immunosuppressants and/or anti-TNF drugs). Future studies should assess the efficacy and the safety of repeated injections of MSC, their usefulness in complex fistulas with multiple tracts, and combined therapy with MSC and biologics. Perhaps other potential candidates for this therapy would be patients in earlier stages of perianal disease, and those in whom perianal disease is the main manifestation of CD. It is important to remember that the alternative therapy in these settings involves repeated surgeries, with a significant risk of faecal incontinence due to sphincter damage, and eventual proctectomy in some patients. For the time being, patients access MSC therapy only as part of clinical trials or compassionate programs, following the regulation of drug agencies. Several trials are now recruiting PCD patients, most of them using MSC therapy (https://clinicaltrials.gov). NCT02403232 Phase II; NCT03279081 Phase III; NCT01915927 Phase 1; NCT03056664 Phase II-III). Future research should address the mechanisms by which MSCs display their therapeutic effects in CPCD fistula patients.
6.4 Other Therapies
Hyperbaric oxygen increases oxygenation of tissues like perianal fistulas. A systematic review including 40 patients with PCD describes complete healing in 45% of cases, but heterogeneity in the procedures and the evaluation of response limit its applicability in clinical practice [121].
The injection of fibrin glue to seal the fistula tract is a mechanical therapy used in perianal fistula. This therapy was used in 14 patients and performed under continuous endosonographic monitoring. The drainage had ceased in ten patients (71%) at three months and in eight patients (57%) at nearly 2 years [122]. In a multicentre, open-label, randomized controlled trial, clinical remission was observed in 13 of the 34 patients (38%) of the fibrin glue group compared with 16% in the observation group, with an OR of 3.2 (95% CI 1.1–9.8; p = 0.04), with lesser results in complex fistulas [123].
The use of absorbable anal fistula plugs (Surgisis™ and GORE BIO-A™ plugs) is another procedure used to treat fistula with little evidence in CD patients. In a systematic review in 84 patients, closure of the fistula tract was achieved in 58.3% of cases. The results of this review are limited by multiple confounding factors. A recent open-label randomized trial in 106 CD patients does not demonstrate [124] more percentage of fistula closure with Surgisis™ plug than with seton removal alone (controls) [125].
Finally, laser techniques that act on the fistula epithelium and obliterate the tract of the fistula have been used in small series with carbon diode laser in PCD [126] or diode laser (FiLaC™) (with only two CD cases in this 45-patient series) [127]. We need more data with these types of therapies to be included in our daily clinical practice.
7 Conclusions
PCD is a complication that influences the QoL of many patients with CD, and is a determinant of poor prognosis and increased healthcare costs. The treatment of simple perianal fistulas includes sepsis control (surgical drainage, and seton placement in combination with antibiotics, such as metronidazole and/or ciprofloxacin), with fistulotomy being reserved for simple superficial fistulas that do not affect the muscular apparatus of the anal sphincters. In complex fistulas, antibiotics improve short-term outcomes of anti-TNF drugs, which should be the first-line therapy following adequate surgical drainage. Combined surgical treatment and anti-TNF may have additional beneficial effects on perianal fistula healing in CD patients. Preliminary findings with new biologic drugs like vedolizumab and ustekinumab suggest their possible role in the treatment of fistulizing CD. Trials designed specifically to demonstrate their utility in complex fistula are warranted. A new area of investigation is local application therapies such as MSC, above all in cases with an inadequate response to usual medical treatment, half of patients with CPCD. Both autologous and allogeneic MSC have achieved significant results in healing complex perianal fistula without systemic complications. Some aspects like the dosage of cell products and the necessary number of intrafistula injections will be the subject of study in future research.
References
Gomollon F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohn’s Colitis. 2017;11(1):3–25. https://doi.org/10.1093/ecco-jcc/jjw168.
Wright EK, Kamm MA. Impact of drug therapy and surgery on quality of life in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2015;21(5):1187–94. https://doi.org/10.1097/MIB.0000000000000271.
Chaparro M, Zanotti C, Burgueno P, Vera I, Bermejo F, Marin-Jimenez I, et al. Health care costs of complex perianal fistula in Crohn’s disease. Dig Dis Sci. 2013;58(12):3400–6. https://doi.org/10.1007/s10620-013-2830-7.
Sahnan K, Askari A, Adegbola SO, Tozer PJ, Phillips RKS, Hart A, et al. Natural history of anorectal sepsis. Br J Surg. 2017. https://doi.org/10.1002/bjs.10614.
Bouguen G, Siproudhis L, Bretagne JF, Bigard MA, Peyrin-Biroulet L. Nonfistulizing perianal Crohn’s disease: clinical features, epidemiology, and treatment. Inflamm Bowel Dis. 2010;16(8):1431–42. https://doi.org/10.1002/ibd.21261.
Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB, American Gastroenterological Association Clinical Practice C. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125(5):1508–30.
Bell SJ, Williams AB, Wiesel P, Wilkinson K, Cohen RC, Kamm MA. The clinical course of fistulating Crohn’s disease. Aliment Pharmacol Ther. 2003;17(9):1145–51.
Molendijk I, Nuij VJ, van der Meulen-de Jong AE, van der Woude CJ. Disappointing durable remission rates in complex Crohn’s disease fistula. Inflamm Bowel Dis. 2014;20(11):2022–8. https://doi.org/10.1097/MIB.0000000000000148.
Saro C, da la Coba C, Casado MA, Morales JM, Otero B. Resource use in patients with Crohn’s disease treated with infliximab. Aliment Pharmacol Ther. 2007;26(10):1313–23. https://doi.org/10.1111/j.1365-2036.2007.03507.x.
Zwintscher NP, Shah PM, Argawal A, Chesley PM, Johnson EK, Newton CR, et al. The impact of perianal disease in young patients with inflammatory bowel disease. Int J Colorectal Dis. 2015;30(9):1275–9. https://doi.org/10.1007/s00384-015-2251-5.
Riss S, Schwameis K, Mittlbock M, Pones M, Vogelsang H, Reinisch W, et al. Sexual function and quality of life after surgical treatment for anal fistulas in Crohn’s disease. Tech Coloproctol. 2013;17(1):89–94. https://doi.org/10.1007/s10151-012-0890-x.
Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63(9):1381–92. https://doi.org/10.1136/gutjnl-2013-306709.
Schwartz DA, Ghazi LJ, Regueiro M, Fichera A, Zoccali M, Ong EM, et al. Guidelines for the multidisciplinary management of Crohn’s perianal fistulas: summary statement. Inflamm Bowel Dis. 2015;21(4):723–30. https://doi.org/10.1097/MIB.0000000000000315.
Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohn’s Colitis. 2013;7(7):556–85. https://doi.org/10.1016/j.crohns.2013.02.020.
Zawadzki A, Starck M, Bohe M, Thorlacius H. A unique 3D endoanal ultrasound feature of perianal Crohn’s fistula: the ‘Crohn ultrasound fistula sign’. Colorectal Dis. 2012;14(9):e608–11. https://doi.org/10.1111/j.1463-1318.2012.03047.x.
Maconi G, Ardizzone S, Greco S, Radice E, Bezzio C, Bianchi Porro G. Transperineal ultrasound in the detection of perianal and rectovaginal fistulae in Crohn’s disease. Am J Gastroenterol. 2007;102(10):2214–9. https://doi.org/10.1111/j.1572-0241.2007.01441.x.
Siddiqui MR, Ashrafian H, Tozer P, Daulatzai N, Burling D, Hart A, et al. A diagnostic accuracy meta-analysis of endoanal ultrasound and MRI for perianal fistula assessment. Dis Colon Rectum. 2012;55(5):576–85. https://doi.org/10.1097/DCR.0b013e318249d26c.
Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s perianal fistulas. Gastroenterology. 2001;121(5):1064–72.
Sahni VA, Ahmad R, Burling D. Which method is best for imaging of perianal fistula? Abdom Imaging. 2008;33(1):26–30. https://doi.org/10.1007/s00261-007-9309-y.
Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohn’s Colitis. 2017;11(2):135–49. https://doi.org/10.1093/ecco-jcc/jjw169.
Makowiec F, Jehle EC, Becker HD, Starlinger M. Perianal abscess in Crohn’s disease. Dis Colon Rectum. 1997;40(4):443–50.
Sangwan YP, Schoetz DJ Jr, Murray JJ, Roberts PL, Coller JA. Perianal Crohn’s disease. Results of local surgical treatment. Dis Colon Rectum. 1996;39(5):529–35.
Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Rodriguez-de-Santiago E, Lopez-Sanroman A. Management of complex perianal Crohn’s disease. Ann Gastroenterol. 2017;30(1):33–44. https://doi.org/10.20524/aog.2016.0099.
Marzo M, Felice C, Pugliese D, Andrisani G, Mocci G, Armuzzi A, et al. Management of perianal fistulas in Crohn’s disease: an up-to-date review. World J Gastroenterol. 2015;21(5):1394–403. https://doi.org/10.3748/wjg.v21.i5.1394.
de Groof EJ, Sahami S, Lucas C, Ponsioen CY, Bemelman WA, Buskens CJ. Treatment of perianal fistula in Crohn’s disease: a systematic review and meta-analysis comparing seton drainage and anti-tumour necrosis factor treatment. Colorectal Dis. 2016;18(7):667–75. https://doi.org/10.1111/codi.13311.
Hong MK, Craig Lynch A, Bell S, Woods RJ, Keck JO, Johnston MJ, et al. Faecal diversion in the management of perianal Crohn’s disease. Colorectal Dis. 2011;13(2):171–6. https://doi.org/10.1111/j.1463-1318.2009.02092.x.
Edwards CM, George BD, Jewell DP, Warren BF, Mortensen NJ, Kettlewell MG. Role of a defunctioning stoma in the management of large bowel Crohn’s disease. Br J Surg. 2000;87(8):1063–6. https://doi.org/10.1046/j.1365-2168.2000.01467.x.
Singh S, Ding NS, Mathis KL, Dulai PS, Farrell AM, Pemberton JH, et al. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn’s disease. Aliment Pharmacol Ther. 2015;42(7):783–92. https://doi.org/10.1111/apt.13356.
Schneider MU, Laudage G, Guggenmoos-Holzmann I, Riemann JF. Metronidazole in the treatment of Crohn disease. Results of a controlled randomized prospective study. Dtsch Med Wochenschr. 1985;110(45):1724–30. https://doi.org/10.1055/s-2008-1069077.
Bernstein LH, Frank MS, Brandt LJ, Boley SJ. Healing of perineal Crohn’s disease with metronidazole. Gastroenterology. 1980;79(3):599.
Jakobovits J, Schuster MM. Metronidazole therapy for Crohn’s disease and associated fistulae. Am J Gastroenterol. 1984;79(7):533–40.
Brandt LJ, Bernstein LH, Boley SJ, Frank MS. Metronidazole therapy for perineal Crohn’s disease: a follow-up study. Gastroenterology. 1982;83(2):383–7.
Maeda Y, Ng SC, Durdey P, Burt C, Torkington J, Rao PK, et al. Randomized clinical trial of metronidazole ointment versus placebo in perianal Crohn’s disease. Br J Surg. 2010;97(9):1340–7. https://doi.org/10.1002/bjs.7121.
Stringer EE, Nicholson TJ, Armstrong D. Efficacy of topical metronidazole (10 percent) in the treatment of anorectal Crohn’s disease. Dis Colon Rectum. 2005;48(5):970–4. https://doi.org/10.1007/s10350-004-0873-8.
Turunen UM, Farkkila MA, Hakala K, Seppala K, Sivonen A, Ogren M, et al. Long-term treatment of ulcerative colitis with ciprofloxacin: a prospective, double-blind, placebo-controlled study. Gastroenterology. 1998;115(5):1072–8.
Wolf J. Ciprofloxacin may be useful in Crohn’s disease. Gastroenterology. 1990;98:A212 (Abstract).
Solomon MJMR, Oconnor BI. Combination ciprofloxacin and metronidazole in severe perianal. Crohns Dis. 1993;7:571–3.
Thia KT, Mahadevan U, Feagan BG, Wong C, Cockeram A, Bitton A, et al. Ciprofloxacin or metronidazole for the treatment of perianal fistulas in patients with Crohn’s disease: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis. 2009;15(1):17–24. https://doi.org/10.1002/ibd.20608.
Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):661–73. https://doi.org/10.1038/ajg.2011.72.
Dejaco C, Harrer M, Waldhoer T, Miehsler W, Vogelsang H, Reinisch W. Antibiotics and azathioprine for the treatment of perianal fistulas in Crohn’s disease. Aliment Pharmacol Ther. 2003;18(11–12):1113–20.
West RL, van der Woude CJ, Hansen BE, Felt-Bersma RJ, van Tilburg AJ, Drapers JA, et al. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn’s disease with infliximab: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2004;20(11–12):1329–36. https://doi.org/10.1111/j.1365-2036.2004.02247.x.
Dewint P, Hansen BE, Verhey E, Oldenburg B, Hommes DW, Pierik M, et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn’s disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut. 2014;63(2):292–9. https://doi.org/10.1136/gutjnl-2013-304488.
Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123(2):132–42.
Cosnes J, Bourrier A, Laharie D, Nahon S, Bouhnik Y, Carbonnel F, et al. Early administration of azathioprine vs conventional management of Crohn’s Disease: a randomized controlled trial. Gastroenterology. 2013;145(4):758–65 e2. https://doi.org/10.1053/j.gastro.2013.04.048 (quiz e14-5).
Kariyawasam VC, Selinger CP, Katelaris PH, Jones DB, McDonald C, Barr G, et al. Early use of thiopurines or methotrexate reduces major abdominal and perianal surgery in Crohn’s disease. Inflamm Bowel Dis. 2014;20(8):1382–90. https://doi.org/10.1097/MIB.0000000000000119.
Hart AL, Plamondon S, Kamm MA. Topical tacrolimus in the treatment of perianal Crohn’s disease: exploratory randomized controlled trial. Inflamm Bowel Dis. 2007;13(3):245–53. https://doi.org/10.1002/ibd.20073.
Cat H, Sophani I, Lemann M, Modiglani R, Solue JC. Cyclosporin treatment of anal and perianal lesions associated with Crohn’s disease. Turk J Gastroenterol. 2003;14(2):121–7.
Hanauer SB, Smith MB. Rapid closure of Crohn’s disease fistulas with continuous intravenous cyclosporin A. Am J Gastroenterol. 1993;88(5):646–9.
Present DH, Lichtiger S. Efficacy of cyclosporine in treatment of fistula of Crohn’s disease. Dig Dis Sci. 1994;39(2):374–80.
Yang C, Singh P, Singh H, Le ML, El-Matary W. Systematic review: thalidomide and thalidomide analogues for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41(11):1079–93. https://doi.org/10.1111/apt.13181.
Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340(18):1398–405. https://doi.org/10.1056/NEJM199905063401804.
Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–85. https://doi.org/10.1056/NEJMoa030815.
Lichtenstein GR, Yan S, Bala M, Blank M, Sands BE. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology. 2005;128(4):862–9.
Farrell RJ, Shah SA, Lodhavia PJ, Alsahli M, Falchuk KR, Michetti P, et al. Clinical experience with infliximab therapy in 100 patients with Crohn’s disease. Am J Gastroenterol. 2000;95(12):3490–7. https://doi.org/10.1111/j.1572-0241.2000.03366.x.
Ricart E, Panaccione R, Loftus EV, Tremaine WJ, Sandborn WJ. Infliximab for Crohn’s disease in clinical practice at the Mayo Clinic: the first 100 patients. Am J Gastroenterol. 2001;96(3):722–9. https://doi.org/10.1111/j.1572-0241.2001.03612.x.
Bouguen G, Siproudhis L, Gizard E, Wallenhorst T, Billioud V, Bretagne JF, et al. Long-term outcome of perianal fistulizing Crohn’s disease treated with infliximab. Clin Gastroenterol Hepatol. 2013;11(8):975–81 e1–4. https://doi.org/10.1016/j.cgh.2012.12.042.
Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. https://doi.org/10.1053/j.gastro.2006.11.041.
Colombel JF, Schwartz DA, Sandborn WJ, Kamm MA, D’Haens G, Rutgeerts P, et al. Adalimumab for the treatment of fistulas in patients with Crohn’s disease. Gut. 2009;58(7):940–8. https://doi.org/10.1136/gut.2008.159251.
Hinojosa J, Gomollon F, Garcia S, Bastida G, Cabriada JL, Saro C, et al. Efficacy and safety of short-term adalimumab treatment in patients with active Crohn’s disease who lost response or showed intolerance to infliximab: a prospective, open-label, multicentre trial. Aliment Pharmacol Ther. 2007;25(4):409–18. https://doi.org/10.1111/j.1365-2036.2006.03232.x.
Panaccione R, Loftus EV Jr, Binion D, McHugh K, Alam S, Chen N, et al. Efficacy and safety of adalimumab in Canadian patients with moderate to severe Crohn’s disease: results of the Adalimumab in Canadian SubjeCts with ModErate to Severe Crohn’s DiseaSe (ACCESS) trial. Can J Gastroenterol. 2011;25(8):419–25.
Lichtiger S, Binion DG, Wolf DC, Present DH, Bensimon AG, Wu E, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32(10):1228–39. https://doi.org/10.1111/j.1365-2036.2010.04466.x.
Regueiro M, Mardini H. Treatment of perianal fistulizing Crohn’s disease with infliximab alone or as an adjunct to exam under anesthesia with seton placement. Inflamm Bowel Dis. 2003;9(2):98–103.
Gaertner WB, Decanini A, Mellgren A, Lowry AC, Goldberg SM, Madoff RD, et al. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis Colon Rectum. 2007;50(11):1754–60. https://doi.org/10.1007/s10350-007-9077-3.
Yassin NA, Askari A, Warusavitarne J, Faiz OD, Athanasiou T, Phillips RK, et al. Systematic review: the combined surgical and medical treatment of fistulising perianal Crohn’s disease. Aliment Pharmacol Ther. 2014;40(7):741–9. https://doi.org/10.1111/apt.12906.
Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OO, Hanauer SB, McColm J, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357(3):239–50. https://doi.org/10.1056/NEJMoa062897.
Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357(3):228–38. https://doi.org/10.1056/NEJMoa067594.
Schreiber S, Lawrance IC, Thomsen OO, Hanauer SB, Bloomfield R, Sandborn WJ. Randomised clinical trial: certolizumab pegol for fistulas in Crohn’s disease - subgroup results from a placebo-controlled study. Aliment Pharmacol Ther. 2011;33(2):185–93. https://doi.org/10.1111/j.1365-2036.2010.04509.x.
Guerra I, Bermejo F. Management of inflammatory bowel disease in poor responders to infliximab. Clin Exp Gastroenterol. 2014;7:359–67. https://doi.org/10.2147/CEG.S45297.
Yarur AJ, Kanagala V, Stein DJ, Czul F, Quintero MA, Agrawal D, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther. 2017;45(7):933–40. https://doi.org/10.1111/apt.13970.
Davidov Y, Ungar B, Bar-Yoseph H, Carter D, Haj-Natour O, Yavzori M, et al. Association of induction infliximab levels with clinical response in perianal Crohn’s disease. J Crohn’s Colitis. 2017;11(5):549–55. https://doi.org/10.1093/ecco-jcc/jjw182.
Yarur AJ, Kubiliun MJ, Czul F, Sussman DA, Quintero MA, Jain A, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13(6):1118–24 e3. https://doi.org/10.1016/j.cgh.2014.12.026.
Poggioli G, Laureti S, Pierangeli F, Rizzello F, Ugolini F, Gionchetti P, et al. Local injection of Infliximab for the treatment of perianal Crohn’s disease. Dis Colon Rectum. 2005;48(4):768–74. https://doi.org/10.1007/s10350-004-0832-4.
Asteria CR, Ficari F, Bagnoli S, Milla M, Tonelli F. Treatment of perianal fistulas in Crohn’s disease by local injection of antibody to TNF-alpha accounts for a favourable clinical response in selected cases: a pilot study. Scand J Gastroenterol. 2006;41(9):1064–72. https://doi.org/10.1080/00365520600609941.
Poggioli G, Laureti S, Pierangeli F, Bazzi P, Coscia M, Gentilini L, et al. Local injection of adalimumab for perianal Crohn’s disease: better than infliximab? Inflamm Bowel Dis. 2010;16(10):1631. https://doi.org/10.1002/ibd.21210.
Tonelli F, Giudici F, Asteria CR. Effectiveness and safety of local adalimumab injection in patients with fistulizing perianal Crohn’s disease: a pilot study. Dis Colon Rectum. 2012;55(8):870–5. https://doi.org/10.1097/DCR.0b013e31825af532.
Adegbola SO, Sahnan K, Tozer PJ, Phillips RK, Faiz OD, Warusavitarne J, et al. Review of local injection of anti-TNF for perianal fistulising Crohn’s disease. Int J Colorectal Dis. 2017. https://doi.org/10.1007/s00384-017-2899-0.
Ardizzone S, Maconi G, Colombo E, Manzionna G, Bollani S, Bianchi Porro G. Perianal fistulae following infliximab treatment: clinical and endosonographic outcome. Inflamm Bowel Dis. 2004;10(2):91–6.
Van Assche G, Vanbeckevoort D, Bielen D, Coremans G, Aerden I, Noman M, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol. 2003;98(2):332–9. https://doi.org/10.1111/j.1572-0241.2003.07241.x.
Poole RM. Vedolizumab: first global approval. Drugs. 2014;74(11):1293–303. https://doi.org/10.1007/s40265-014-0253-1.
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–21. https://doi.org/10.1056/NEJMoa1215739.
Feagan B, Schwartz D, Danese S, Rubin DT, Abhyankar B, Smyth M, Xu J, Lasch K. Vedolizumab for the treatment of fistulizing Crohn’s disease: an exploratory analysis of data from GEMINI 2. J Crohn’s Collitis. 2015;9(Suppl_1):S333.
Christensen B, Goeppinger S, Colman R, Siddiqui D, Yarur A, Bochenek AA, Wichmann A, Hirsch A, Kinnucan J, Sakuraba A, Cohen R, Rubin DT. Post-marketing experience of vedolizumab for IBD: the university of Chicago experience. J Crohn’s Colitis. 2015;9(Suppl_1):S388.
Dulai PS, Singh S, Jiang X, Peerani F, Narula N, Chaudrey K, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol. 2016;111(8):1147–55. https://doi.org/10.1038/ajg.2016.236.
Tadbiri S, Grimaud J, Peyrin-Biroulet L, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R, Marteau P, Vaysse T, Bourrier A, Nancey S, Laharie D, Allez M, Bouhnik Y, Amiot A. Efficacy of vedolizumab on extraintestinal manifestation in patients with inflammatory bowel disease: a posthoc analysis of the OBSERV-IBD cohort from the GETAID. J Crohn’s Collitis. 2017;11(Suppl_1):S42.
Vedolizumab IV 300 mg in the Treatment of Fistulizing Crohn’s Disease (ENTERPRISE). https://clinicaltrials.gov/ct2/show/NCT0280%630966.
Lamb YN, Duggan ST. Ustekinumab: a review in moderate to severe Crohn’s disease. Drugs. 2017;77(10):1105–14. https://doi.org/10.1007/s40265-017-0765-6.
Kopylov U, Afif W, Cohen A, Bitton A, Wild G, Bessissow T, et al. Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn’s disease–the McGill experience. J Crohn’s Colitis. 2014;8(11):1516–22. https://doi.org/10.1016/j.crohns.2014.06.005.
Wils P, Bouhnik Y, Michetti P, Flourie B, Brixi H, Bourrier A, et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn’s disease refractory to anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol. 2016;14(2):242–50 e1–2. https://doi.org/10.1016/j.cgh.2015.09.018.
Khorrami S, Ginard D, Marin-Jimenez I, Chaparro M, Sierra M, Aguas M, et al. Ustekinumab for the treatment of refractory Crohn’s disease: the Spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis. 2016;22(7):1662–9. https://doi.org/10.1097/MIB.0000000000000842.
Ma C, Fedorak RN, Kaplan GG, Dieleman LA, Devlin SM, Stern N, et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther. 2017;45(9):1232–43. https://doi.org/10.1111/apt.14016.
Sands B, Gasink C, Jacobstein D, Gao L, Johanns J, Colombel JF, de Villiers WJ, Sandborn WJ. Fistula healing in pivotal studies of ustekinumab in Crohn’s disease. Gastroenterology. 2017;152(5):S185.
Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–60. https://doi.org/10.1056/NEJMoa1602773.
Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367(16):1519–28. https://doi.org/10.1056/NEJMoa1203572.
Geltzeiler CB, Wieghard N, Tsikitis VL. Recent developments in the surgical management of perianal fistula for Crohn’s disease. Ann Gastroenterol. 2014;27(4):320–30.
Garcia-Gomez I, Elvira G, Zapata AG, Lamana ML, Ramirez M, Castro JG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10(10):1453–68. https://doi.org/10.1517/14712598.2010.519333.
Kim N, Cho SG. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol. 2016;103(2):129–37. https://doi.org/10.1007/s12185-015-1918-6.
Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60(6):788–98. https://doi.org/10.1136/gut.2010.214841.
Ciccocioppo R, Cangemi GC, Kruzliak P, Gallia A, Betti E, Badulli C, et al. Ex vivo immunosuppressive effects of mesenchymal stem cells on Crohn’s disease mucosal T cells are largely dependent on indoleamine 2,3-dioxygenase activity and cell-cell contact. Stem Cell Res Ther. 2015;6:137. https://doi.org/10.1186/s13287-015-0122-1.
Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001. https://doi.org/10.1182/blood-2002-06-1830.
Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–16. https://doi.org/10.1038/ni.3002.
Garcia-Olmo D, Guadalajara H, Rubio-Perez I, Herreros MD, de-la-Quintana P, Garcia-Arranz M. Recurrent anal fistulae: limited surgery supported by stem cells. World J Gastroenterol. 2015;21(11):3330–6. https://doi.org/10.3748/wjg.v21.i11.3330.
Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2(6):455–63. https://doi.org/10.5966/sctm.2012-0184.
Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52(1):79–86. https://doi.org/10.1007/DCR.0b013e3181973487.
Panes J, Garcia-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388(10051):1281–90. https://doi.org/10.1016/S0140-6736(16)31203-X.
Lightner AL, Faubion WA. Mesenchymal stem cell injections for the treatment of perianal Crohn’s disease: what we’ve accomplished and what we still need to do. J Crohn’s Colitis. 2017. https://doi.org/10.1093/ecco-jcc/jjx046.
Garcia-Olmo D, Schwartz DA. Cumulative evidence that mesenchymal stem cells promote healing of perianal fistulas of patients with Crohn’s disease-going from bench to bedside. Gastroenterology. 2015;149(4):853–7. https://doi.org/10.1053/j.gastro.2015.08.038.
Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim DS, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells. 2013;31(11):2575–81. https://doi.org/10.1002/stem.1357.
Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, et al. Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2015;149(4):918–27 e6. https://doi.org/10.1053/j.gastro.2015.06.014.
Garcia-Olmo D, Garcia-Arranz M, Garcia LG, Cuellar ES, Blanco IF, Prianes LA, et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18(5):451–4. https://doi.org/10.1007/s00384-003-0490-3.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48(7):1416–23. https://doi.org/10.1007/s10350-005-0052-6.
Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27(5):595–600. https://doi.org/10.1007/s00384-011-1350-1.
Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22(2):279–85. https://doi.org/10.3727/096368912X656045.
Cho YB, Park KJ, Yoon SN, Song KH, Kim DS, Jung SH, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015;4(5):532–7. https://doi.org/10.5966/sctm.2014-0199.
Dietz AB, Dozois EJ, Fletcher JG, Butler GW, Radel D, Lightner AL, et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2017;153(1):59–62 e2. https://doi.org/10.1053/j.gastro.2017.04.001.
Choi S, Ryoo SB, Park KJ, Kim DS, Song KH, Kim KH, et al. Autologous adipose tissue-derived stem cells for the treatment of complex perianal fistulas not associated with Crohn’s disease: a phase II clinical trial for safety and efficacy. Tech Coloproctol. 2017;21(5):345–53. https://doi.org/10.1007/s10151-017-1630-z.
Ciccocioppo R, Gallia A, Sgarella A, Kruzliak P, Gobbi PG, Corazza GR. Long-term follow-up of crohn disease fistulas after local injections of bone marrow-derived mesenchymal stem cells. Mayo Clin Proc. 2015;90(6):747–55. https://doi.org/10.1016/j.mayocp.2015.03.023.
de la Portilla F, Alba F, Garcia-Olmo D, Herrerias JM, Gonzalez FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28(3):313–23. https://doi.org/10.1007/s00384-012-1581-9.
Park KJ, Ryoo SB, Kim JS, Kim TI, Baik SH, Kim HJ, et al. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: a pilot clinical trial. Colorectal Dis. 2016;18(5):468–76. https://doi.org/10.1111/codi.13223.
Mannon PJ. Remestemcel-L: human mesenchymal stem cells as an emerging therapy for Crohn’s disease. Expert Opin Biol Ther. 2011;11(9):1249–56. https://doi.org/10.1517/14712598.2011.602967.
Qiu Y, Li MY, Feng T, Feng R, Mao R, Chen BL, et al. Systematic review with meta-analysis: the efficacy and safety of stem cell therapy for Crohn’s disease. Stem Cell Res Ther. 2017;8(1):136. https://doi.org/10.1186/s13287-017-0570-x.
Dulai PS, Gleeson MW, Taylor D, Holubar SD, Buckey JC, Siegel CA. Systematic review: the safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39(11):1266–75. https://doi.org/10.1111/apt.12753.
Vitton V, Gasmi M, Barthet M, Desjeux A, Orsoni P, Grimaud JC. Long-term healing of Crohn’s anal fistulas with fibrin glue injection. Aliment Pharmacol Ther. 2005;21(12):1453–7. https://doi.org/10.1111/j.1365-2036.2005.02456.x.
Grimaud JC, Munoz-Bongrand N, Siproudhis L, Abramowitz L, Senejoux A, Vitton V, et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2010;138(7):2275–81, 81 e1. https://doi.org/10.1053/j.gastro.2010.02.013.
Nasseri Y, Cassella L, Berns M, Zaghiyan K, Cohen J. The anal fistula plug in Crohn’s disease patients with fistula-in-ano: a systematic review. Colorectal Dis. 2016;18(4):351–6. https://doi.org/10.1111/codi.13268.
Senejoux A, Siproudhis L, Abramowitz L, Munoz-Bongrand N, Desseaux K, Bouguen G, et al. Fistula plug in fistulising ano-perineal Crohn’s disease: a randomised controlled trial. J Crohn’s Colitis. 2016;10(2):141–8. https://doi.org/10.1093/ecco-jcc/jjv162.
Moy J, Bodzin J. Carbon dioxide laser ablation of perianal fistulas in patients with Crohn’s disease: experience with 27 patients. Am J Surg. 2006;191(3):424–7. https://doi.org/10.1016/j.amjsurg.2005.10.050.
Giamundo P, Esercizio L, Geraci M, Tibaldi L, Valente M. Fistula-tract Laser Closure (FiLaC): long-term results and new operative strategies. Tech Coloproctol. 2015;19(8):449–53. https://doi.org/10.1007/s10151-015-1282-9.
Ma C, Fedorak RN, Kaplan GG, Dieleman LA, Devlin SM, Stern N, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohn’s disease: real-world experience from a multicenter cohort study. Inflamm Bowel Dis. 2017;23(5):833–9. https://doi.org/10.1097/MIB.0000000000001074.
Battat R, Kopylov U, Bessissow T, Bitton A, Cohen A, Jain A, et al. Association among ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2017. https://doi.org/10.1016/j.cgh.2017.03.032.
Sands BE, Blank MA, Patel K, van Deventer SJ, Study AI. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol. 2004;2(10):912–20.
Colombel JF, Sandborn WJ, Rutgeerts P, Kamm MA, Yu AP, Wu EQ, et al. Comparison of two adalimumab treatment schedule strategies for moderate-to-severe Crohn’s disease: results from the CHARM trial. Am J Gastroenterol. 2009;104(5):1170–9. https://doi.org/10.1038/ajg.2009.59.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source of funding was used to support the writing of this review.
Conflict of interest
Fernando Bermejo has served as a speaker, a consultant and advisory member for MSD, Abbvie, Pfizer, Hospira, Takeda, Janssen and Gebro. Antonio López-Sanromán has served as a speaker, a consultant and advisory member for MSD, Abbvie, Pfizer, Takeda, Janssen, Ferring and Tigenix, and has received grants from MSD and Abbvie. Iván Guerra y Alicia Algaba has no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Bermejo, F., Guerra, I., Algaba, A. et al. Pharmacological Approach to the Management of Crohn’s Disease Patients with Perianal Disease. Drugs 78, 1–18 (2018). https://doi.org/10.1007/s40265-017-0842-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0842-x