Abstract
Secukinumab (Cosentyx™) is a fully human monoclonal antibody against interleukin-17A, formulated for intravenous and subcutaneous administration. It received its first global approval in Japan on 26 December 2014 for the treatment of psoriasis and psoriatic arthritis in adults who are not adequately responding to systemic therapies (except for biologic agents). In the USA and the EU, secukinumab was approved in early 2015 for the treatment of patients with moderate-to-severe plaque psoriasis. Secukinumab is also being investigated in patients with ankylosing spondylitis and rheumatoid arthritis. This article summarizes the milestones in the development of secukinumab leading to its first approval for the treatment of adult patients with psoriasis and psoriatic arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Psoriasis is an immune-mediated, chronic inflammatory disease occurring in 2–3 % of the population, which most commonly presents with local or widespread, reddened, scaly, thickened areas of skin (plaque psoriasis) that are painful and itchy [1]. The disease is also associated with arthritis (psoriatic arthritis), cardiovascular, psychiatric and other comorbidities and reduced quality of life. Psoriasis is most frequently mild, in which case it is well managed with topical medicines and phototherapy. However, in 20–30 % of patients, it is moderate-to-severe and these patients usually require systemic therapy. Conventional systemic therapies are not always successful, with patients experiencing an insufficient response, loss of efficacy or intolerable adverse effects of treatment [1]. Thus, new systemic treatments for psoriasis are required.
Interleukin 17 (IL-17) is a family of proinflammatory cytokines, with six ligand members (IL-17A to IL-17-F) produced mainly by T helper (Th) 17 cells, but also by other immune cells [1]. Genetic studies have linked several IL-17-related genes to psoriasis and IL-17A is also implicated in the aetiology of other autoimmune disorders, such as psoriatic arthritis, rheumatoid arthritis and ankylosing spondylitis [1, 2]. In psoriasis, it is thought that T cells are the major source of IL-17A in skin, as psoriatic skin lesions and psoriatic plaques are both abundant in IL-17A-producing γδ-T cells. Inhibition of IL-17A and of IL-17RA (a component of a heteromeric receptor complex) has been demonstrated to have clinically important therapeutic effects in psoriasis [1]. The dysregulation of Th17 cells is also presumed to be important in multiple sclerosis and Crohn’s disease, suggesting that IL-17 inhibitors could also have a role in the treatment of these diseases [2].
Biologic agents that target IL-17 and that have been evaluated in trials in patients with psoriasis include secukinumab (Cosentyx™) and ixekizumab (both targeting IL-17A) and brodalumab (targeting IL-17RA) [1]. Secukinumab, the subject of this article, is a fully human monoclonal antibody (mAb) that received its first global approval in Japan on 26 December 2014 for the treatment of psoriasis and psoriatic arthritis in adults who are not adequately responding to systemic therapies (except for biologic agents) [3]. Secukinumab was also approved in the EU [4] and USA [5] on 15 and 21 January, respectively, for the treatment of moderate-to-severe plaque psoriasis in patients who are candidates for systemic therapy. Novartis, the company developing the agent, is also expecting to file separate applications in 2015/2016 for approval of the use of secukinumab in psoriatic arthritis in the US and the EU, and in ankylosing spondylitis and rheumatoid arthritis.
Features and properties of secukinumab
Alternative names | AIN 457; AIN-457; AIN457; Cosentyx™; human anti-interleukin-17A monoclonal antibody; KB 03303A; KB-03303A; NVP AIN 457; NVP-AIN 457 |
Class | Monoclonal antibodies |
Mechanism of action | Interleukin-17A inhibitor |
Routes of administration | Intravenous and subcutaneous |
Pharmacodynamics | Inhibits the inflammatory response by selectively binding to cytokine interleukin-17A (IL-17A), thereby inhibiting IL-17A signalling |
Pharmacokinetics | After subcutaneous injection reaches maximum plasma concentrations in 5–6 days. The average bioavailability was 73 % and the mean elimination half-life was ~27 days |
Adverse events | |
Most frequent in psoriasis trials during weeks 1–12 | Nasopharyngitis, headache, diarrhoea, upper respiratory tract infection |
Of special interest | Mucosal/cutaneous candidiasis (responsive to standard treatment), neutropenia (infrequently ≥ grade 3) |
ATC codes | |
WHO ATC code | A10X (other drugs used in diabetes), L04A-C10 (secukinumab), M01A (anti-inflammatory and anti-rheumatic products, non-steroids), R03 (drugs for obstructive airway diseases), R07A-X (other respiratory system products), S01X-A (other ophthalmologicals) |
EphMRA ATC code | A10X (other drugs used in diabetes), A7E (intestinal anti-inflammatory agents), D5 (nonsteroidal products for inflammatory skin disorders), D5B (systemic anti-psoriasis products), L4X (other immunosuppressants), M1A (anti-rheumatics, non-steroidal), N7X (all other CNS drugs), R3 (anti-asthma and COPD products), R7X (all other respiratory system products), S1X1 (other ophthalmologicals, systemic) |
Molecular formula | C6584 H10134 N1754 02042 S44 |
2 Scientific Summary
2.1 Pharmacodynamics
Secukinumab is a first-in-class, fully human mAb that neutralizes the proinflammatory cytokine IL-17A by selectively binding to it, thus reducing the psoriatic inflammatory response [1, 2, 6]. Secukinumab binding to and capture of circulating IL-17A has been confirmed in studies that show that total serum IL-17A levels (consisting of free IL-17A and secukinumab-IL-17A complex) increase to a plateau during secukinumab treatment, then gradually decrease at the end of treatment as the complex is cleared from the body [2]. Clinical improvements in single-dose studies of intravenous secukinumab in patients with plaque psoriasis were paralleled by cellular and histological changes in psoriatic skin, including reductions in thickness and keratosis of the epithelium, decreases in keratinocyte proliferation and in CD3+ T cells, along with early decreases in other innate immune cell populations [2].
2.2 Pharmacokinetics
In pharmacokinetic studies in healthy adults and patients with psoriasis, secukinumab was slowly absorbed, with systemic exposure that was dose-proportional over a dose range of 0.3–10 mg/kg when administered intravenously, and of 25–300 mg with subcutaneous administration [2]. Following a single subcutaneous injection of 150 or 300 mg, the maximum concentration (Cmax) was reached after 5–6 days, while following weekly administration at these dosages it was reached after 31–34 days. Steady-state concentrations were two-fold higher than those after a single dose, and were reached after 20 weekly administrations, with a time to Cmax at steady state of 4–8 days. Following subcutaneous administration, the mean bioavailability was 73 %. The terminal phase volume of distribution after intravenous administration ranged from 7–9 L, consistent with low distribution to peripheral compartments [2]. In healthy adults and patients with moderate-to-severe plaque psoriasis, the antibody was found to distribute to dermal interstitial fluid following a single subcutaneous injection of secukinumab 300 mg [7].
It is thought that secukinumab is metabolized by the same intracellular catabolic pathways as endogenous immunoglobulin [2]. Clearance of secukinumab is dose- and time-independent, with slow clearance from the systemic circulation. The secukinumab elimination half-life was approximately 27 days.
Given its metabolism via intracellular processes, secukinumab has low potential for drug interactions [2]. Secukinumab pharmacokinetics were not affected by coadministration with methotrexate [2], and coadministration of secukinumab with inactivated influenza vaccine or meningococcal vaccine had no impact on antibody responses to the vaccines [8].
Based on population studies, the pharmacokinetics of secukinumab in patients aged ≥65 years are similar to those in patients aged <65 years [2]. Due to its large molecular size, and metabolic pathways involved in its degradation, renal and hepatic impairment are unlikely to affect its overall pharmacokinetic profile.
2.3 Therapeutic Trials
The efficacy of secukinumab has been evaluated in phase II and phase III studies across a variety of conditions, as discussed below. The study programme for the indications of plaque psoriasis, ankylosing spondylitis and psoriatic arthritis are most advanced, with support for efficacy in these conditions from large, randomized, double-blind, placebo-controlled, multicentre, phase III trials. In these trials, secukinumab was administered with initial intravenous or subcutaneous loading doses, generally at baseline, weeks 1, 2, 3 and 4, followed by subcutaneous secukinumab administered every 4 weeks.
2.3.1 Plaque Psoriasis
The US FDA and European Medicines Agency advisory committees have recommended the approval of secukinumab therapy in patients with moderate-to-severe plaque psoriasis, based chiefly on data from phase II, dose-ranging studies [9, 10] (not discussed further), and four key phase III studies.
The phase III studies were similarly-designed, randomized, double-blind, placebo-controlled trials (one also included an etanercept active comparator group) [11–13]. Included patents were adults with moderate-to-severe plaque psoriasis that was poorly controlled with other therapies, who scored or ≥12 on the Psoriasis Area and Severity Index (PASI), had scores of 3 or 4 on the modified Investigator’s Global Assessment (IGA) [a scale of 0–4, with higher scores indicating more severe disease] [14] and who had involvement of ≥10 % of body-surface area. Patients received subcutaneous secukinumab 150, 300 mg or placebo or etanercept (one trial), weekly for 4 weeks and then every 4 weeks until study end (placebo non-responders generally crossed over to secukinumab after 12 weeks). In two trials, subcutaneous secukinumab was administered as lyophilisate in a vial for reconstitution (ERASURE, NCT01365455; FIXTURE, NCT01358578 [11]), in one trial via a prefilled syringe (FEATURE, NCT01555125 [12]) and in one trial via an autoinjector pen (JUNCTURE, NCT01636687 [13]). Across trials, the coprimary endpoints were the response rates at week-12 based on the PASI 75 (proportions of patients with ≥75 % improvement from baseline in PASI score) and IGA (proportion of patients with a score of 0–1 plus a reduction of ≥2 points from baseline).
Secukinumab administered as lyophilisate in a vial was an efficacious therapy for plaque psoriasis [11]. In the ERASURE trial (n = 738), both secukinumab dosage groups had a significantly (p < 0.001) higher response rate than the placebo group [11]. The PASI 75 response rates were 72, 82 and 5 % in the secukinumab 150, 300 mg and placebo groups, respectively; in the corresponding groups, the IGA response rates were 51, 65 and 2 %, respectively. In the FIXTURE trial (n = 1,306), in the secukinumab 150, 300 mg and etanercept groups, 67, 77 and 44 % of patients had a PASI 75 response (both secukinumab groups p < 0.001 vs. etanercept), compared with 5 % of patients in the placebo group (both secukinumab groups p < 0.001 vs. placebo) [11]. The IGA response rates in the corresponding active treatment groups were 51, 63 and 27 %, vs. 3 % in the placebo group (both secukinumab groups p < 0.001 vs. etanercept and p < 0.001 vs. placebo). In these efficacy analyses, missing values were imputed as nonresponses, which is likely to provide a conservative estimate not reflective of real clinical practice [15]. In a secondary analysis evaluating response over time, PASI and IGA response rates increased from week 12 to 16 and then remained stable to week 52 [11].
Similar positive results favouring secukinumab were obtained from trials in which it was administered via a prefilled syringe [12] and via an autoinjector pen [13]. In the FEATURE trial (n = 177), in the secukinumab 150, 300 mg and placebo groups, the PASI 75 response rates were 70, 76 and 0 %, respectively (both secukinumab groups p < 0.001 vs. placebo); in the corresponding groups, 53, 69 and 0 % of patients, respectively, had an IGA response (both secukinumab groups, p < 0.0001 vs. placebo) [3]. In the JUNCTURE trial (n = 182), PASI 75 response rates were 72, 87 and 3 % in the secukinumab 150, 300 mg and placebo groups, respectively (both secukinumab groups p < 0.0001 vs. placebo); in the corresponding groups, 53, 73 and 0 % had an IGA response (p < 0.0001 vs. placebo) [13]. Based on the Self-Injection Assessment Questionnaire scores, self-administration of secukinumab was acceptable to patients at baseline, with numerical increases (improvements) from baseline to week 12 in self-confidence and satisfaction using a prefilled syringe [12, 16] or an autoinjector pen [13, 17].
Analyses of other secondary endpoints and subgroup analyses in one or more of the key efficacy trials, or in pooled analyses, were generally supportive of findings on primary endpoints. For instance, benefits with secukinumab were observed in terms of patient-reported pain, itching and scaling [11, 13], PASI 90 response rates (improvement in PASI score of ≥90 %) [11–13], and health-related quality of life (HR-QoL) [11]. Benefits to secukinumab therapy occurred irrespective of the presence or not of psoriatic arthritis in addition to plaque psoriasis [18, 19], previous exposure to biological therapy and inadequate response to prior biological therapy [20], bodyweight stratum [21], age <65, =65 and =75 years [22], or Japanese ethnicity [23].
Across trials, secukinumab maintenance therapy was continued from week 12 to week 52 [11–13]. Where reported [11], secukinumab recipients in these trials continued to have sustained responses over the duration of the studies. For instance, in the ERASURE trial, the respective PASI 75 and IGA response rates to week 52 were 72 and 59 % for secukinumab 150 mg groups, and 81 and 74 % for the secukinumab 300 mg group. In the FIXTURE trial, the PASI 75 and IGA response rates at 52 weeks were 82 and 68 % for the secukinumab 150 mg group, 84 and 80 % for the secukinumab 300 mg group, and 73 and 57 % for the etanercept group [11].
The phase III STATURE study (NCT01412944) [n = 43] included secukinumab recipients from the SCULPTURE trial who were partial responders (achieved 50–74 % improvement in the PASI score) [24]. Patients were randomized to secukinumab 10 mg/kg administered intravenously or 300 mg administered subcutaneously for 8 weeks, followed by a 32-week, open-label maintenance period, during which all patients received subcutaneous secukinumab 300 mg. At week 8, the PASI 75 response rates were 91 % with intravenous versus 67 % with subcutaneous administration; in the corresponding groups, the IGA response rates were 67 vs. 33 % (p = 0.03) and the PASI 90 response rates were 62 and 10 %. Small sample size could account for failure to reach statistical significance for differences on the PASI 75 coprimary endpoint.
The SCULPTURE trial (NCT01406938) [n = 966] evaluated a secukinumab retreatment-as-needed regimen [25]. Patients with plaque psoriasis were initially randomized to secukinumab 150 or 300 mg dose regimens, following which PASI 75 responders at 12-weeks were re-randomized within dosage groups to fixed-interval maintenance secukinumab (every 4 weeks) or secukinumab re-treatment as needed (defined as a loss of ≥20 % of maximum PASI improvement vs. baseline, plus loss of PASI 75 response); both strategies were continued from week 12 to week 48. The retreatment-as-needed strategy failed to meet a noninferiority criterion for the PASI 75 response rate (52 and 68 % of patients in the 150 and 300 mg dosage groups responded at week 40 or 52) when compared with the fixed dose maintenance strategy (62 and 78 % of patients for the 150 and 300 mg dosage groups).
2.3.2 Psoriatic Arthritis
Results from the randomized, placebo-controlled, phase III FUTURE 1 and 2 trials (NCT01392326 and NCT01752634) indicate that secukinumab is an efficacious treatment for psoriatic arthritis [26, 27].
In both trials, patients had active disease despite treatment with non-steroidal anti-inflammatory drugs (NSAIDs); a proportion of patients had also previously received anti-tumour necrosis factor (TNF) agents and/or disease modifying anti-rheumatic drugs (DMARDs). In the FUTURE 1 trial, patients (n = 606) were randomized to secukinumab 10 mg/kg intravenously at weeks 0, 2 and 4 followed by 75 or 150 mg subcutaneously every 4 weeks, or placebo on the same schedule [26]. Response rates at week 24 according to the American College of Rheumatology 20 % improvement criterion (ACR20) [primary endpoint] were 51, 50 and 17 % in the secukinumab 75, 150 mg and placebo groups, respectively (both secukinumab groups p < 0.0001 vs. placebo). Both dosages of secukinumab were also significantly more effective than placebo with regard to secondary endpoints, including, but not limited to, dactylitis (p < 0.0001), ACR 70 (p < 0.0001) and inhibition of radiographic structural joint damage (p-value not reported). Improvements in primary and secondary endpoints were maintained throughout 52 weeks’ treatment [26].
In the FUTURE 2 trial, patients (n = 397) were randomized to subcutaneous secukinumab 75, 150 or 300 mg or placebo administered at weekly intervals for 5 weeks and then every 4 weeks [27]. The ACR20 response rates at week 24 (primary endpoint) were 29, 51 and 54 % in the secukinumab 75, 150 and 300 mg groups, respectively, versus 15 % in the placebo group (p < 0.05 vs. placebo for the 75 mg group, and p < 0.0001 vs. placebo for the 150 and 300 mg groups).
2.3.3 Ankylosing Spondylitis
Secukinumab is an efficacious therapy for ankylosing spondylitis, based on findings from a phase II study (NCT00809159) [28] and two randomized, placebo-controlled, phase III trials.
In the phase III trials, all patients had active disease despite treatment with NSAIDs; a proportion of patients had also previously received anti-TNF agents and/or DMARDs. Patients received secukinumab loading doses administered intravenously (MEASURE 1; NCT01358175) [29] or subcutaneously (MEASURE 2; NCT01649375), followed by subcutaneous secukinumab maintenance [30]. In both trials, the primary endpoint was the proportion of patients with a ≥20 % improvement in the Assessment of Spondyloarthritis International Society response criteria (ASAS20) at week 16. In the MEASURE 1 trial, patients (n = 371) were randomized to intravenous secukinumab 10 mg/kg (week 0, 2 and 4) followed by subcutaneous secukinumab 75 or 150 mg every 4 weeks, or placebo on the same schedule [29]. At week 16, the ASAS20 response rates were 60, 61 and 29 % in the secukinumab 75, 150 mg and placebo groups, respectively (p < 0.01 vs. placebo both doses). Both dosages of secukinumab were also significantly more effective than placebo with regard to secondary endpoints including, but not limited to, ASAS40 (p < 0.01), and improvements in primary and secondary endpoints were maintained throughout 52 weeks’ treatment [29].
In the MEASURE 2 trial, patients (n = 219) were randomized to receive subcutaneous secukinumab 75, 150 mg or placebo weekly for 4 weeks, followed by secukinumab or placebo at 4-week intervals [30]. Secukinumab was efficacious at the 150 mg dose, as the ASAS20 response rate at week 16 in patients receiving this dose was 61 % (vs. 27 % with placebo; p < 0.001).
2.3.4 Rheumatoid Arthritis
Phase II studies provide support for the efficacy of secukinumab in patients with rheumatoid arthritis; secukinumab therapy is now being further evaluated in phase III trials in this disease.
In a randomized, phase II, dose-finding study (NCT00928512) conducted in patients without stable disease despite methotrexate therapy (n = 237), the ACR20 response rates at week 16 (primary endpoint) were 34, 47, 47 and 54 % with secukinumab 25, 75, 150 and 300 mg regimens, respectively, versus 36 % with placebo, with non-significant between-group differences [31]. Nevertheless, there were sustained improvements in terms of ACR20 response rates to week 52 and on secondary endpoints, particularly for the patients who remained on secukinumab 150 mg through to week 52 [32]. In analyses that combined secukinumab dosage groups, significant (p < 0.05) benefits over placebo were observed in terms of improvements in HR-QoL, fatigue and physical function [33]. In a second phase II trial in patients predominantly from Eastern Europe who had active disease despite methotrexate therapy (n = 221), the ACR20 response rate at week 12 was 49 % with secukinumab and 41 % with placebo [34]. In a phase II biomarker study, patients with rheumatoid arthritis evaluable for candidate biomarkers (n = 96) were randomized 2:1 to intravenous secukinumab 10 mg/kg or placebo every other week for six doses [35]. The ACR20 response rates at week 12 were 88 and 25 % in the secukinumab and placebo groups, respectively. The biomarker HLA-DRB1*DE was associated with secukinumab response, but this association was driven by lack of response to placebo. Further biomarker studies are needed to identify markers of treatment response.
2.3.5 Non-Infectious Uveitis
Three randomized, placebo-controlled trials have evaluated the efficacy of secukinumab in patients with non-infectious uveitis: SHIELD (NCT00995709) in patients with Behçet’s uveitis (n = 118); INSURE (NCT01095250) in patients with active non-Behçet’s uveitis (n = 31); and ENDURE (NCT01032915) in quiescent non-Behçet’s uveitis (n = 125) [36]. The INSURE and ENDURE trials were both terminated based on a lack of positive findings in the SHIELD trial. Across studies, there was no significant between-group differences in vitreous haze scores (primary endpoint).
2.3.6 Relapsing–Remitting Multiple Sclerosis
A phase II study (NCT01051817) suggests that secukinumab is a promising therapy for relapsing-remitting multiple sclerosis (RRMS) [37]. In this study, patients with RRMS (n = 73) were randomized to secukinumab or placebo. Compared with placebo, secukinumab was associated with a significant (p = 0.003) 67 % reduction in new gadolinium-enhancing lesions, which are a marker of multiple sclerosis disease activity.
2.3.7 Other Conditions
The efficacy of secukinumab in several other conditions remains uncertain, largely because of small numbers in studies, or equivocal or negative results. Small (≤71 patients) proof-of-concept or phase II studies have been conducted in Crohn’s disease (phase II study NCT00584740 with extension NCT01009281) [38], polymyalgia rheumatic (proof-of-concept study) [39] and dry eye (NCT01250171) [40], while a phase II trial in patients with new onset type 1 diabetes mellitus was terminated (NCT02044848).
Phase III acute treatment trials of secukinumab (Novartis Pharma, Bristol-Myers Squibb, Alcon Division)
Indication | Study type (comparators) | Status | Location(s) | Identifier |
|---|---|---|---|---|
Plaque psoriasis | ES (PL) | Completed | Multinational | NCT01365455 (ERASURE) |
Plaque psoriasis | ES (PL, etanercept) | Completed | Multinational | NCT01358578 (FIXTURE) |
Plaque psoriasis | MvR (NC) | Completed | Multinational | NCT01406938 (SCULPTURE) |
Plaque psoriasis | ES SEC autoinjector pen (PL) | Completed | Multinational | NCT01636687 (JUNCTURE) |
Plaque psoriasis | ES SEC pre-filled syringe (PL) | Completed | Multinational | NCT01555125 (FEATURE) |
Plaque psoriasis | Uptitration in SEC partial responders (SEC sc vs. iv) | Completed | Multinational | NCT01412944 (STATURE) |
Plaque psoriasis | ES (ustekinumab) | Results pending | Multinational | NCT02074982 (CLEAR) |
Plaque psoriasis (non-responders to TNFαIs) | ES (NC) | Recruiting | UK | NCT01961609 (SIGNATURE) |
Palmoplantar plaque psoriasis | ES (PL) | Enrolment complete | Multinational | NCT01806597 (GESTURE) |
Nail psoriasis | ES (PL) | Enrolment complete | Multinational | NCT01807520 (TRANSFIGURE) |
Plaque psoriasis | CVS (PL) | Recruiting | Germany | CAIN457ADE02 |
Palmoplantar pustular psoriasis | ES (PL) | Recruiting | Multinational | NCT02008890 |
Generalized pustular psoriasis | ES SEC pre-filled syringe (PL) | Enrolment complete | Japan | NCT01952015 |
Scalp psoriasis | ES (PL) | Recruiting | USA | NCT02267135 ES(SCALP) |
Psoriatic arthritis | ES (PL) | Completed | Multinational | NCT01392326 (FUTURE 1) |
Psoriatic arthritis | ES SEC pre-filled syringe (PL) | Completed | Multinational | NCT01752634 (FUTURE 2) |
Psoriatic arthritis | ES SEC autoinjector pen (PL) | Recruiting | Multinational | NCT01989468 |
Psoriatic arthritis | ES SEC pre-filled syringe (PL) | Initiated | USA | NCT02294227 (FUTURE 4) |
Ankylosing spondylitis | ES (PL) | Completed | Multinational | NCT01358175 (MEASURE 1) |
Ankylosing spondylitis | ES (PL) | Completed | Multinational | NCT01649375 (MEASURE 2) |
Ankylosing spondylitis | ES (PL) | Recruiting | Multinational | NCT02008916 (MEASURE 3) |
Ankylosing spondylitis | ES (PL) | Planned | USA | NCT02159053 (MEASURE 4) |
Rheumatoid arthritis | ES (PL, abatacept) | Enrolment complete | Multinational | NCT01350804 (NURTURE 1) |
Rheumatoid arthritis | ES (PL) | Recruiting | Multinational | NCT01377012 (REASSURE 1) |
Rheumatoid arthritis | ES (PL) | Recruiting | Multinational | NCT01770379 (REASSURE 2) |
Behçet’s uveitis | ES (PL) | Completed | Multinational | NCT00995709 (SHIELD) |
Non-infectious, active non-Behçet’s uveitis | ES (PL) | Terminated | Multinational | NCT01095250 (INSURE) |
Non-infectious, quiescent non-Behçet’s uveitis | ES (PL) | Terminated | Multinational | NCT01032915 (ENDURE) |
2.4 Adverse Events
Across clinical trials, patients were monitored for adverse events and clinical and laboratory disturbances. The discussion in this section focusses chiefly on fully published data for adverse events during randomized treatment in key phase III trials and during follow-up for up to 52 weeks.
2.4.1 Plaque Psoriasis
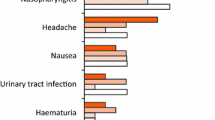
In the ERASURE trial, adverse events during weeks 1–12 occurred in 60, 55 and 47 % of patients in the secukinumab 150, 300 mg and placebo groups, respectively; in the corresponding groups, 27, 29 and 16 % of patients had infections and infestations [11]. The most common adverse events occurring in secukinumab recipients were nasopharyngitis, headache and upper respiratory tract infections (URTIs). During the full 52-week treatment period, there were no deaths and the incidence of serious adverse events (SAEs) was 6.4, 6.3 and 7.4 per 100 patient-years in the secukinumab 150, 300 mg and placebo groups, respectively. Similar adverse event rates were observed in a subanalysis in Japanese patients in this trial [23].
In the FIXTURE trial during weeks 1–12, 58, 56, and 58 % of secukinumab 150, 300 mg and etanercept recipients had adverse events, compared with 50 % of placebo recipients [11]. In the corresponding groups, the incidence of infections and infestations were 31, 27 and 25 % vs. 19 % with placebo. The most common adverse events in secukinumab recipients were nasopharyngitis, headache and diarrhoea. During the 52-week study period, incidence rates for SAEs were 6, 7 and 7 per 100 patient-years in the secukinumab, 150, 300 mg and etanercept groups, respectively, versus 8 per 100 subject-years in the placebo group.
In both of these trials over full treatment periods, there were no apparent clinically-important differences across groups in the incidence of serious infections, malignant or unspecified tumours and major cardiovascular events [11].
There were also no signals indicating new or unexpected adverse effects when secukinumab was administered via a prefilled syringe (FEATURE trial) [12] or by autoinjection (JUNCTURE) [13]. In a phase II, placebo-controlled, dose-ranging study in patients with plaque psoriasis (n = 404), there was no indication of secukinumab-associated changes in lipid profiles [41].
In a pooled analysis of 10 studies conducted in patients with plaque psoriasis (n ≥ 3,000), although infection rates were numerically higher in secukinumab than placebo recipients during weeks 0–12, serious infections occurred in just 0.2, 0.1 and 0.3 % of secukinumab 150, 300 mg and etanercept recipients, respectively, and in 0 % of placebo recipients [42]. Over 52 weeks, there were no clinically important differences between secukinumab and placebo recipients in infection rates, serious infections or serious opportunistic infections. The secukinumab 300 mg group had a higher exposure-adjusted incidence of Candida infections of 3.6 per 100 patient-years compared with 1.9, 1.4 and 1.0 per 100 patient-years in secukinumab 150 mg, etanercept and placebo groups, respectively; all such infections were non-serious and resolved fully, either alone or with antifungal treatment.
2.4.2 Other Indications
In clinical trials in patients with psoriatic arthritis (FUTURE 1 [26]) and ankylosing spondylitis (MEASURE 1 [29]), secukinumab was generally well tolerated during up to 52 weeks’ treatment exposure, with adverse events consistent with those observed in the plaque psoriasis trials. In the SHIELD trial (n = 117) in patients with noninfectious uveitis, non-ocular and ocular adverse events, but not ocular SAEs, appeared to be slightly higher in the secukinumab groups than the placebo group [36]. Adverse events occurred in 80, 82 and 69 % of secukinumab 300 mg every 4 weeks, 300 mg every 2 weeks and placebo recipients, respectively [36]. In the corresponding groups, 26, 41 and 18 % of patients had an ocular AE in the study eye and 28, 33 and 23 % in the fellow eye, whereas 5, 3 and 3 % had an ocular SAE in the study eye and 5, 5 an 8 % in the fellow eye.
2.4.3 Immunogenicity
The formation of treatment-emergent anti-drug antibodies (ADAs) was low in patients (n = 2,842) with plaque psoriasis who received any dose of secukinumab in six phase III trials, four of which included exposure over 52 weeks [43], Treatment-emergent ADAs were detected in ten patients, and there was no correlation with secukinumab dose, frequency or mode of administration. The emergence of ADAs or neutralizing antibodies was not associated with a loss of secukinumab efficacy.
2.5 Ongoing Clinical Trials
2.5.1 Plaque Psoriasis
A further randomized, placebo- and ustekinumab-controlled phase III efficacy trial (the CLEAR study; NCT02074982) is underway in patients with plaque psoriasis. A preliminary report of week 16 data indicated that secukinumab is superior to ustekinumab with regard to the primary endpoint of PASI 90 response rates [44]. In addition, randomized placebo-controlled phase III trials are ongoing in patients with nail psoriasis (a multinational study; NCT01807520) palmoplantar psoriasis (a multinational study; NCT02008890), generalized pustular psoriasis (a Japanese multicentre study; NCT01952015) and chronic scalp psoriasis (a US multicentre study; NCT02267135). A noncomparative, phase III study of two secukinumab dosage regimens is ongoing in patients with plaque psoriasis who are poorly controlled with TNFα inhibitors (a UK multicentre study; NCT01961609). A randomized, placebo-controlled study to evaluate the cardiovascular safety of secukinumab in patients with plaque psoriasis is also underway (a German multicentre study; CAIN457ADE02), while pooled analyses of ten phase II/III studies are planned to evaluate risk of neutropenia [45] and exacerbation of concomitant Crohn’s disease [46].
2.5.2 Psoriatic Arthritis, Ankylosing Spondylitis and Rheumatoid Arthritis
The FUTURE 1 (NCT01392326) and 2 (NCT01752634) trials in psoriatic arthritis are ongoing, with additional efficacy and safety analyses planned up to 2 and 5 years, respectively. Further randomized, placebo-controlled, phase III trials are also underway in patients with psoriatic arthritis to evaluate secukinumab administered via an autoinjector pen (a multinational study; NCT01989468) and via a prefilled syringe (a US multicentre study; NCT02294227).
The MEASURE 1 (NCT01358175) and 2 (NCT01649375) trials in ankylosing spondylitis are ongoing, with additional efficacy and safety analyses planned up to 2 and 5 years, respectively. There are two further ongoing, randomized, phase III trials in patients with ankylosing spondylitis (a multinational study; NCT02008916 study, and a US multicentre study; NCT02159053).
Based on positive findings from phase II studies, the efficacy of secukinumab is now also being evaluated in three phase III multinational trials in patients with rheumatoid arthritis with an inadequate response to TNF α inhibitors (the placebo-controlled NCT01377012 and NCT01770379 studies, and the placebo- and abatacept-controlled NCT01350804 study).
2.5.3 Other
Extension studies, generally for up to 3 years, are being conducted in patients from the key phase III studies in order to evaluate the longer-term efficacy, safety and tolerability of secukinumab in patients with plaque psoriasis, ankylosing spondylitis and psoriatic arthritis.
Randomized, phase II studies are also being conducted to evaluate skin response and biomarkers in patients with plaque psoriasis (NCT01537432), and efficacy and safety in patients with RRMS (NCT01874340) and asthma inadequately controlled with corticosteroids and long-acting β-adrenergic receptor agonists (NCT01478360).
2.6 Current Status
On 26 December 2014, secukinumab was approved in Japan for the treatment of psoriasis and psoriatic arthritis in adults who are not adequately responding to systemic therapies (except for biologic agents) [3]. In January of this year, secukinumab was also approved in the USA [5] and EU [4] for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.
References
Lønnberg AS, Zachariae C, Skov L. Targeting of interleukin-17 in the treatment of psoriasis. Clin Cosmet Investig Dermatol. 2014;7:251–9.
Novartis Pharmaceuticals Corporation. Advisory Committee briefing materials: secukinumab (AIN457). 2014. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOphthalmicDrugsAdvisoryCommittee/UCM419023.pdf. Accessed 17 Dec 2014.
Novartis International AG. First in the world regulatory approval of Novartis’ Cosentyx™ in Japan for both psoriasis and psoriatic arthritis [media release]. 26 Dec 2014. http://www.novartis.com/newsroom/media-releases/en/2014/1883568.shtml.
European Commission. Commission implementing decision for Cosentyx™ (secukinumab). 15 Jan 2015. http://ec.europa.eu/health/documents/community…/dec_130444_en.pdf. Accessed 29 Jan 2015.
US Food and Drug Administration. FDA approves new psoriasis drug Cosentyx [media release]. 21 Jan 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm430969.htm.
Gan EY, Chong WS, Tey HL. Therapeutic strategies in psoriasis patients with psoriatic arthritis: focus on new agents. BioDrugs. 2013;27(4):359–73.
Bruin G, Dragatin C, Aigner B, et al. The anti IL-17A IgG1 antibody distributes in dermal interstitial fluid as demonstrated by using dermal open flow microperfusion after a single 300 mg subcutaneous administration; assessment in healthy volunteers and patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2014;134:S18.
Chioato A, Noseda E, Stevens M, et al. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19(10):1597–602.
Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402–11.
Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate to severe plaque psoriasis: a randomised, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2012;168(2):412–21.
Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis: results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety, and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2014. doi:10.1111/bjd.13348.
Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2014. doi:10.1111/jdv.12751.
Langley RGB, Feldman SR, Nyirady J, et al. The 5-point Investigator’s Global Assessment (IGA) scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2013. doi:10.3109/09546634.2013.865009.
Langley RG, Reich K, Papavassilis C, et al. Multiple imputation methodology is reflective of secukinumab efficacy in real clinical practice: data from the FIXTURE and ERASUE studies in moderate to severe plaque psoriasis [abstract plus poster P99]. In: Psoriasis from Gene to Clinic 7th International Congress. 2014.
Kingo K, Sofen H, Pathan R. Secukinumab prefilled syringes demonstrate patient satisfaction: analysis of the Self-Injection Assessment Questionnaire (SIAQ) in the FEATURE study [abstract no. P8137]. In: 72nd Annual Meeting of the American Academy of Dermatology. 2014.
Kreutzer K, Lacour JP, You R. Secukinumab autoinjectors demonstrate patient satisfaction: an analysis of the Self-Injection Assessment Questionnaire (SIAQ) in the JUNCTURE Study [abstract no. P8417]. In: 72nd Annual Meeting of the American Academy of Dermatology. 2014.
Blauvelt A, Gottlieb A, Sigurgeirsson B. Secukinumab efficacy in subjects with moderate-to-severe plaque psoriasis and concomitant psoriatic arthritis: a subanalysis of the ERASURE study [abstract no. P8033]. In: 72nd Annual Meeting of the American Academy of Dermatology. 2014.
Gottlieb A, Langley R, Philipp S, et al. Improvement in psoriasis symptoms and physical functioning with secukinumab compared with placebo and etanercept in subjects with moderate-to-severe plaque psoriasis and psoriatic arthritis: results of a subanalysis from the phase 3 fixture study [abstract no. L7]. Arthritis Rheum. 2013;65(12):3322.
Papp K, Karpov A, Papavassilis C. Secukinumab efficacy in relationship with response to previous biologic psoriasis therapy: a subanalysis from the ERASURE Study [abstract no. P8011]. In: 72nd Annual Meeting of the American Academy of Dermatology. 2014.
Rich P, Karpov A, Papavassilis C. Secukinumab efficacy stratified by body weight: a subanalysis from the ERASURE study [abstract no. P8013]. In: 72nd Annual Meeting of the American Academy of Dermatology. 2014.
Warren R, Guettner A, Morita A, et al. Secukinumab efficacy in subjects with moderate to severe plaque psoriasis: pooled subgroup analyses by patient age of 4 phase 3 clinical studies [abstract no. P7563]. J Am Acad Dermatol. 2014;70(5 Suppl 1):AB186.
Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41(12):1039–46.
Thaci D, Humeniuk J, Frambach Y, et al. Secukinumab in moderate-to-severe plaque psoriasis: results from the Secukinumab Trial Analyzing the potential of intravenous administration To Upgrade the REsponse in psoriasis (STATURE) [abstract]. J German Soc Dermatol. 2014;12(Suppl):13.
Mrowietz U, Leonardi C, Girolomoni G, et al. Secukinumab ‘fixed-interval’ versus ‘retreatment-as-needed’ regimen for moderate-to-severe plaque psoriasis: results from the Study Comparing secukinumab Use in Long-term Psoriasis maintenance therapy: fixed regimen versus re-Treatment Upon start of Relapse (SCULPTURE) [abstract]. Aust J Dermatol. 2014;55(Suppl 1):42.
Mease P, McInnes IB, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, improves active psoriatic arthritis and inhibits radiographic progression: efficacy and safety data from a phase 3 randomized, multicenter, double-blind, placebo-controlled study [abstract no. 953]. Arthritis Rheum. 2014;66(Supp. 10):S423–S4.
McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, improves active psoriatic arthritis: 24-week efficacy and safety data from a phase 3 randomized, multicenter, double-blind, placebo-controlled study using subcutaneous dosing [abstract no. L1]. In: Annual Meeting of the American College of Rheumatology. 2014.
Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9906):1705–13.
Baeten DL, Braun J, Baraliakos X, et al. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a 52-week phase 3 randomized placebo-controlled trial with intravenous loading and subcutaneous maintenance dosing. [abstract no. 819]. Arthritis Rheum. 2014;66(Suppl 10):S360.
Sieper J, Braun J, Baraliakos X, et al. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a phase 3, randomized, placebo-controlled trial with subcutaneous loading and maintenance dosing. [abstract no. 536]. Arthritis Rheum. 2014;66(Supp. 10):S232.
Genovese MC, Durez P, Richards HB, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2012;72(6):863–9.
Durez P, Genovese MC, Richards HB, et al. Secukinumab treatment provides sustained response over one year in patients with rheumatoid arthritis [abstract no. O17]. Rheumatology. 2012;51(Suppl 3):329.
Strand V, Kosinski M, Gnanasakthy A, et al. Secukinumab treatment in rheumatoid arthritis is associated with incremental benefit in the clinical outcomes and HRQoL improvements that exceed minimally important thresholds. Health Qual Life Outcomes. 2014. doi:10.1186/1477-7.
Adami S, Beaulieu A, Rahman P, et al. A randomized placebo-controlled phase 2 study to evaluate efficacy, safety and tolerability of two secukinumab loading dose regimens in subjects with active rheumatoid arthritis despite treatment with methotrexate [abstract]. Arthritis Rheum. 2013;65(Suppl 10):S220.
Burmester GR, Durez P, Shestakova G, et al. Association of HLA-DRB1 alleles with clinical responses to the anti-interleukin-17A monoclonal antibody secukinumab in a cohort of patients with active rheumatoid arthritis: an exploratory phase 2 biomarker study [abstract no. 1737]. Arthritis Rheum. 2013;65(Suppl 10):1737.
Dick AD, Tugal-Tutkun I, Foster S, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120(4):777–87.
Havrdova E, Belova A, Goloborodko A, et al. Sensitivity analysis of a phase IIa study of secukinumab in relapsing remitting multiple sclerosis. Mult Scler. 2013;1:210–1.
Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–700.
Matteson EL, Dasgupta B, Schmidt WA, et al. A 2-week single-blind, randomized, 3-arm proof of concept study of the effects of secukinumab (anti-IL17 mAb), canakinumab (anti-IL-1b mAb), or corticosteroids on initial disease activity scores in patients with PMR, followed by an open-label extension to assess safety and effect duration [abstract no. 885]. Arthritis Rheum. 2014;66(Supp. 10):S391–S2.
US National Institutes of Health. The effects of a single intravenous administration of secukinumab (AIN457) or canakinumab (ACZ885) in dry eye patients. 2012. https://clinicaltrials.gov/ct2/show/results/NCT01250171?sect=X70156. Accessed 15 Dec 2014.
Philipp S, Escrig C, Papavassilis C, et al. Secukinumab treatment shows a neutral impact on the lipid profile of patients with moderate to severe plaque psoriasis in a dose-ranging study [abstract no. P6508]. In: 71st Annual Meeting of the American Academy of Dermatology. 2013.
Tsai T, Blauvelt A, Karpov A, et al. Evaluation of infections with secukinumab in a pooled analysis of 10 clinical studies of moderate-to-severe plaque psoriasis [abstract no. 193]. J Invest Dermatol. 2014;134:S33.
Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a novel anti-IL-17A antibody, exhibits low immunogenicity during long-term treatment in subjects with psoriasis [abstract plus poster P103]. In: Psoriasis from Gene to Clinic 7th International Congress. 2014.
Novartis. Head-to-head psoriasis study demonstrates superiority of Novartis Cosentyx™ to Stelara® in clearing skin [media release]. 12 Dec 2014. http://www.novartis.com. Accessed 6 Jan 2015.
Griffiths C, Guettner A, Prinz J, et al. Secukinumab safety in subjects with moderate to severe plaque psoriasis: a pooled analysis of neutropenia from 10 clinical studies [abstract no. P8266]. J Am Acad Dermatol. 2014;70(5 Suppl 1):AB188.
Ward N, Guettner A, Sands B, et al. Secukinumab safety and tolerability in subjects with moderate to severe plaque psoriasis: a pooled subgroup analysis of 10 clinical studies evaluating exacerbation of Crohn’s disease [abstract no. P8233]. J Am Acad Dermatol. 2014;70(5 Suppl 1):AB188.
Disclosure
The preparation of this report was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. M. Sanford and K. McKeage are salaried employees of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Sanford, M., McKeage, K. Secukinumab: First Global Approval. Drugs 75, 329–338 (2015). https://doi.org/10.1007/s40265-015-0359-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0359-0