Abstract
Olaparib (Lynparza™) is an oral, small molecule, poly (ADP-ribose) polymerase inhibitor being developed by AstraZeneca for the treatment of solid tumours. The primary indication that olaparib is being developed for is BRCA mutation-positive ovarian cancer. A capsule formulation of the drug has received approval for use in this setting in the EU and USA, and a tablet formulation is in global phase III trials (including in the USA, EU, Australia, Brazil, Canada, China, Israel, Japan, Russia and South Korea). In addition, phase III trials in breast, gastric and pancreatic cancer are underway/planned, and phase I/II investigation is being conducted in other malignancies, including prostate cancer, non-small cell lung cancer, Ewing’s sarcoma and advanced cancer. This article summarizes the milestones in the development of olaparib leading to this first approval for ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Patients with certain cancers, including those of the ovaries and breast, can carry mutations in the BRCA1 and/or BRCA2 gene and consequently have tumours that lack the homologous recombination (HR) pathway involved in error-free repair of DNA double-strand breaks (DSBs) [1–3]. As poly (ADP ribose) polymerase (PARP) is involved in the repair of single-strand DNA breaks, inhibiting PARP can result in replication-associated DSBs that require repair by HR. In HR-deficient cells (such as those of BRCA1/2 tumours), these lesions can persist (which can be lethal) or are repaired via error-prone pathways (causing genetic instability) [3]. Thus, drugs that inhibit PARP are able to selectively kill BRCA-deficient tumour cells lacking HR and have consequently become a focus of therapy for such cancers [1, 3].
Olaparib is a PARP inhibitor being developed for the treatment of solid tumours, including BRCA mutation-positive ovarian cancer. In December 2014, the capsule formulation of olaparib, under the trade name Lynparza™, was approved in the EU [4] and USA [5] for the treatment of BRCA-mutated ovarian cancer (dosage: 400 mg twice daily). In the EU, there is a risk management plan for olaparib, involving a number of planned/ongoing studies, to ensure the drug is used as safely as possible [6]. Olaparib (tablet formulation) is also under phase III investigation in breast, gastric and pancreatic cancer and phase I/II investigation for other malignancies, including prostate cancer, non-small cell lung cancer (NSCLC), Ewing’s sarcoma and advanced cancer.
Features and properties of olaparib
Alternative names | AZD 2281; AZD-2281; AZD2281; KU-0059436; KU-59436; Lynparza™ |
Class | Amides, Cyclopropanes, Fluorobenzenes, Phthalazines, Piperazines, Small-molecules |
Mechanism of action | Poly (ADP-ribose) polymerase inhibitor |
Route of administration | Oral |
Pharmacodynamics | Displays anti-neoplastic activity in various cancer cell lines. Its benefits may be enhanced by other anti-cancer agents, according to preclinical studies |
Pharmacokinetics | Rapidly absorbed and eliminated |
Most frequent adverse events | Nausea, fatigue, vomiting, anaemia |
ATC codes | |
WHO | L01X-X (other antineoplastic agents) |
EphMRA | L1X (all other antineoplastics) |
Chemical name | 4- [3- [4-(Cyclopropylcarbonyl)piperazin-1-ylcarbonyl] -4-fluorobenzyl] phthalazin-1(2H)-one |
1.1 Company Agreements
In December 2005, AstraZeneca announced plans to acquire the UK biotechnology company KuDOS Pharmaceuticals Limited. KuDOS Pharmaceuticals had expertise in cancer therapies that exploit inhibition of DNA repair and had olaparib in phase I trials at the time of acquisition [7].
2 Scientific Summary
2.1 Pharmacodynamics
PARP inhibition may be high with olaparib in some instances. For example, PARP was inhibited >90 % with olaparib dosages of ≥60 mg twice daily in the mononuclear cells of patients with advanced solid tumours, including ovarian cancers, in a phase I trial [8]. Similarly, up to 80 % PARP inhibition was seen with olaparib 10–400 mg twice daily in tumour samples from breast cancer patients in another phase I study [9].
Olaparib displayed anti-neoplastic activity in various cancer cell lines, including ovarian [10], endometrial [11], gastric [12, 13], head and neck [14], colorectal (HR deficient due to MRE11 mutation or with microsatellite instability) [15], breast [including triple-negative cells; i.e. negative for estrogen and progesterone receptors and human epidermal growth factor receptor (HER) 2] [16] and mouse BRCA2-deficient mammary tumours [17]. Notably, sensitivity of gastric cancer cell lines to olaparib correlated with low-level expression of ataxia telangiectasia protein, which (like BRCA1/2) is involved in DSB repair [13].
Combining olaparib with agents that inhibit HR may enhance the effectiveness of olaparib in HR-proficient ovarian cancer cells according to in vitro and in vivo data [18, 19]. Further in vitro data suggest there may also be additional/synergistic anti-neoplastic activity in certain cancers when olaparib is used in combination with cyclin-dependent kinase or HER inhibition [16], standard chemotherapeutic agents [12, 17, 20–24] or irradiation [14]. Olaparib was radiosensitizing (independent of P53 status [25]) in colorectal cancer [25] and lung cancer [25, 26] cell lines, with stronger radiosensitization observed when in combination with the topoisomerase I inhibitor campothecin [25].

Chemical structure of olaparib
Olaparib also demonstrated anti-tumour activity when used alone [27] or in combination with chemotherapeutic agents [27, 28] or radiotherapy [26] in preclinical models of cancer, including murine xenografts of patient-derived BRCA2-mutated ovarian cancer tissue [27] or lung cancer cells [26] and a murine model of hereditary BRCA1-deficient breast cancer [28]. Olaparib also displayed vasodilatory properties ex vivo and increased tumour perfusion [26].
In the EU, olaparib is not recommended for use in combination with other anti-cancer agents (as myelosuppressive activity may be potentiated/prolonged) and requires caution if coadministered with immunosuppressants or vaccines (as the potential pharmacodynamic interactions have not been assessed) [4].
Cancer cells deficient in RAD51C were highly sensitive to olaparib in vitro, indicating that this protein (which is essential for HR) may be a useful biomarker for olaparib benefit [29]. A seven-gene signature has been identified as a potential predictor of olaparib outcome, with some genes being associated with sensitivity (CHEK2, MK2) and others with resistance (BRCA1, MRE11A, NBS1, TDG, XPA) [30]. Additional mechanisms of olaparib resistance may involve drug efflux pump expression/upregulation [10, 28], although may be reversible with combined use of a p-glycoprotein inhibitor [28].
2.2 Pharmacokinetics
Absorption of olaparib is rapid after oral administration of the capsule formulation, with maximal plasma concentrations being achieved within 1–3 h post-dose in patients with cancer [5, 31]. In adults with advanced solid tumours, taking olaparib capsules with food reduced the rate at which the drug was absorbed relative to fasting administration, with the time taken to reach maximal concentrations delayed by ≈2 h [32]. In the EU, olaparib should be administered ≥1 h after food; patients should not eat for up to 2 h after taking the drug [4].
Olaparib had a mean apparent volume of distribution of 167 L after administration of a 400 mg capsule dose in patients with cancer [33], and the concentration of olaparib in breast tumours was ≈41 % that detected in plasma [9]. Olaparib-related material may bind to blood components, as the drug’s concentration in blood versus plasma was 0.8 after a single 100 mg dose in patients with advanced solid tumours [31].
Olaparib is metabolized primarily via dehydrogenation and oxidation, as well as glucuronide and sulphide conjugation [34]. The cytochrome P450 enzyme CYP3A4 is predominant in the metabolism of olaparib in vitro [4, 5]. Excretion of the drug occurs largely via the faeces (42 %) and urine (44 %) [31], with unchanged drug being the main excreta component (accounting for ≈21 % of the dose) [34]. Plasma concentrations of olaparib were undetectable 16–24 h after administration of a single dose [31]. The drug had a mean terminal elimination half-life of 11.9 h and a mean apparent clearance of 8.64 L/h following a 400 mg capsule dose in patients with cancer [33].
Preliminary data suggest that mild renal impairment [creatinine clearance (CLCR) 50–80 mL/min] may increase olaparib exposure versus normal renal function (mean 1.5-fold increase; however, the drug can be used in these patients in the USA [5] and EU [4], without adjustment of the starting dosage [5]. There are limited pharmacokinetic data for olaparib in patients with moderate/severe renal impairment (CLCR < 50 mL/min) [4] and none in patients undergoing dialysis [5] or with hepatic impairment [4, 5]. Use of olaparib is not recommended in patients with hepatic impairment (serum bilirubin >1.5 times the upper limit of normal) or moderate/severe renal impairment in the EU [4].
2.2.1 Potential Drug Interactions
As olaparib is metabolized predominantly via CYP3A, drugs that inhibit or induce these enzymes may increase or decrease exposure to olaparib, respectively. Consequently, coadministration of olaparib with strong [4, 5] or moderate [5] CYP3A inhibitors/inducers is not recommended.
In vitro, olaparib inhibited CYP3A4 and induced CYP2B6 when used at concentrations higher than those achieved clinically; there was little, if any, inhibition of other CYP enzymes [5]. In the EU, caution is advised if administering olaparib in combination with CYP3A4 substrates, particularly those with a narrow therapeutic index [4].
Further in vitro data indicate that olaparib also inhibits breast cancer resistance protein [4, 5], organic anion-transporting polypeptide 1B1 [4, 5], organic cation transporters 1 and 2 [4, 5], organic anion transporter 3 [5], and multidrug and toxin extrusion proteins 1 and 2K [5], and is a substrate [4, 5] and inhibitor [4] of p-glycoprotein. It is not yet known if these findings are of clinical relevance [5], although caution is recommended in the EU if coadministering olaparib with a statin [4].
2.3 Therapeutic Trials
2.3.1 Ovarian Cancer
2.3.1.1 Monotherapy
Maintenance treatment with oral olaparib 400 mg twice daily administered as capsules (n = 136) significantly prolonged median progression-free survival (PFS) (primary endpoint) relative to placebo (n = 129) in women with relapsed, platinum-sensitive ovarian cancer in a phase II trial (NCT00753545) (8.4 vs. 4.8 months; HR 0.35, 95 % CI 0.25–0.49; p < 0.001) [35], although no overall survival (OS) benefit was observed at the latest interim analysis (median OS was 29.8 vs. 27.8 months with placebo) [36]. This double-blind trial, known as Study 19, enrolled women with high-grade serous cancers (recurrent ovarian or fallopian tube cancer or primary peritoneal cancer) with or without germline BRCA1/2 mutations who had already received ≥2 platinum-based chemotherapy regimens and had responded to the last.
In a predefined Study 19 subgroup analysis, a notable PFS benefit was observed with olaparib versus placebo in women with known BRCA1/2 germline mutations (HR 0.11; 95 % CI 0.04–0.27; values estimated from a figure) [35]. These findings were supported by a subsequent PFS analysis of total BRCA1/2 status, in which the corresponding HR was 0.18 (95 % CI 0.10–0.31; p < 0.0001) in patients with a germline and/or tumor BRCA1/2 mutation [37]. Significant (p < 0.05) benefit over placebo was also seen with olaparib for other clinical endpoints (including time to first or second subsequent therapy or death); as with PFS, the benefit was more pronounced in women with BRCA mutations [36].
In the phase II ICEBERG 3 trial in women with BRCA1/2-mutated advanced ovarian cancer (NCT00628251), oral olaparib (200 or 400 mg twice daily) did not differ significantly from intravenous pegylated liposomal doxorubicin (PLD) (the current standard of care) in terms of median PFS (primary endpoint; 6.5 and 8.8 vs. 7.1 months) or overall response rate (25 and 31 vs. 18 %) [38]. Patients in this randomized, open-label study had epithelial ovarian, primary peritoneal or fallopian tube carcinoma recurrence within 1 year of platinum-based chemotherapy and received olaparib 200 or 400 mg twice daily continuously as capsules (n = 32 in each group) or PLD 50 mg/m2 via infusion every 28 days (n = 33).
An earlier phase II study (ICEBERG 2; NCT00494442) established the effectiveness of olaparib in women with BRCA-mutated recurrent ovarian cancer, with dosages of 400 and 100 mg twice daily being associated with objective response rates (primary endpoint) of 33 % (11 of 33 recipients) and 13 % (3 of 24 recipients) [39]. Clinical benefit was also seen with olaparib in ovarian cancer in other studies, including a non-randomized phase II trial (NCT00679783) that evaluated 400 mg twice daily (capsule formulation) in women with high-grade serous/poorly differentiated ovarian carcinoma (n = 65) or triple-negative breast cancer (n = 26) with/without BRCA1/2 mutations [40], and the dose-escalation (40 mg daily for 2–3 weeks to 600 mg twice daily) and dose-expansion (200 mg twice daily) cohorts of a phase I trial in women with BRCA1/2-mutated ovarian cancer (n = 50) [41]. Another phase I trial (NCT00777582) in 77 ovarian or breast cancer patients with BRCA1/2 mutations suggested the new tablet formulation of olaparib may have dose-dependent efficacy at 200–400 mg twice daily [42]. In an expansion of this trial (n = 62) [43], olaparib 300 mg twice daily was considered the best dosage for phase III trials.
2.3.1.2 Combination Therapy
Administering olaparib capsules in combination with tablets of the anti-angiogenic agent cediranib (an inhibitor of vascular endothelial growth factor receptor kinases) showed promise as a treatment for recurrent ovarian cancer in a phase I dose-escalation study (NCT0116648), with an overall response rate of 44 % among the 18 women evaluable [44]. Women with metastatic triple-negative breast cancer were also included in the study, although none of the seven evaluable achieved a clinical response [44]. On the basis of this trial, olaparib 200 mg twice daily plus cediranib 30 mg daily was taken into the phase II open-label portion of the study and compared with olaparib alone in women with recurrent platinum-sensitive ovarian cancer [45]. In this trial, the dual regimen (n = 44) was associated with a significantly longer median PFS (17.7 vs. 9.0 months; p = 0.005) and a significantly greater objective response (79.6 vs. 47.8 % of patients; p = 0.002) than olaparib 400 mg twice daily as monotherapy (n = 46).
Olaparib (capsules [46] or tablets [47]) may also be effective in ovarian cancer when used in combination with either the phosphoinositide 3 kinase inhibitor buparlisib (NCT01623349; n = 25 treated to date) [47] or the alkylating agent carboplatin (NCT01445418; n = 37) [46], according to dose-escalating phase I/Ib trials (which also included patients with breast cancer; n = 9 [47] or 8 [46]).
Triple combination therapy with olaparib, carboplatin and paclitaxel is also under investigation for ovarian cancer in phase Ib/II trials. In the largest of these studies (NCT01081951; n = 162 randomized), olaparib capsules 200 mg twice daily plus carboplatin and paclitaxel, followed by olaparib maintenance monotherapy (400 mg twice daily) significantly prolonged PFS compared with carboplatin plus paclitaxel followed by no further treatment in women with platinum-sensitive recurrent serous ovarian cancer (12.2 vs. 9.6 months; hazard ratio 0.51, 95 % CI 0.34–0.77; p = 0.0012), with the benefit of triple versus dual therapy being greater in those with mutated BRCA 1/2 (hazard ratio 0.21, 95 % CI 0.08–0.55; p = 0.0015) [48]. OS did not significantly differ between regimens in the overall population or the BRCA mutated subgroup at final analysis [48]. In another study in 14 women with advanced relapsed ovarian cancer (NCT01650376), half achieved a complete or partial response with olaparib tablets plus carboplatin and paclitaxel (four and three patients, respectively) and the rest had stable disease, progressive disease or were not evaluable (three, two and two patients) [49].
2.3.2 Other Malignancies
A noncomparative open-label phase II trial (NCT01078662) indicates that monotherapy with olaparib capsules 400 mg twice daily may have benefit in various advanced solid tumours (including ovarian, breast, pancreatic and prostate) with germline BRCA1/2 mutations that are refractory to standard chemotherapy [50]. Among the patients in this study (n = 298), 26 % achieved a partial or complete response and 42 % had stable disease for ≥8 weeks. However, in a similarly designed phase II trial in 12 patients with advanced Ewing sarcoma refractory to standard chemotherapy (NCT01583543), there were no objective tumour responses and only four patients achieved stable disease after treatment with olaparib tablets 400 mg twice daily for a median 5.7 weeks [51].
Several phase I/Ib trials also provide preliminary evidence of clinical benefit with olaparib (capsules [52–55] or tablets [56], where reported) used alone [8, 52] or in combination with conventional chemotherapy agents (including PLD [53], cisplatin [54] and carboplatin and/or paclitaxel [55, 56]), in patients with advanced solid tumours, some of which included only patients with BRCA1/2 mutations [8]. Another phase I advanced solid tumour study (NCT00710268) showed the potential for olaparib capsules to be used in combination with bevacizumab [57], whereas similar studies assessing olaparib (capsules where specified [58]) in combination with cisplatin/gemcitabine [59], dacarbazine [58] or topotecan [60] in patients with advanced solid tumours were limited by haematological toxicities.
Additional phase I/Ib [61–65] or II [66, 67] trials have evaluated olaparib-based therapy specifically in patients with advanced breast cancer (BRCA1/2-mutated [67] or triple-negative [64, 65] disease), locally advanced head/neck cancer [61], relapsed glioblastoma [62], recurrent/metastatic gastric cancer [66] or epidermal growth factor receptor-positive advanced NSCLC [63]. Where specified, these studies used olaparib capsules [64, 65] or tablets [66].
Key clinical trials of olaparib
Drugs(s) | Indication | Phase | Status | Location(s) | Identifier | Sponsor |
|---|---|---|---|---|---|---|
Olaparib + AZD2014 or AZD5363 | Ovarian cancer | I/II | Recruiting | USA | NCT02208375 | M.D. Anderson Cancer Center |
Olaparib + cediranib | Ovarian or breast cancer | I/II | Recruiting | USA | NCT01116648 | National Cancer Institute |
Olaparib + carboplatin + paclitaxel | Ovarian or uterine cancer | I/II | Recruiting | USA | NCT01650376 | Swedish Medical Center |
Olaparib | Ovarian or breast cancer | II | Ongoing | Canada | NCT00679783 | AstraZeneca |
Olaparib + carboplatin + paclitaxel | Ovarian cancer | II | Ongoing | Multinational | NCT01081951 | AstraZeneca |
Olaparib | Ovarian cancer | II | Withdrawn | USA | NCT01661868 | Dane-Faber Cancer Institute |
Olaparib | Ovarian cancer | II | Ongoing | Multinational | NCT00628251 (ICEBERG 3) | AstraZeneca |
Olaparib | Ovarian cancer | II | Ongoing | Multinational | NCT00494442 (ICEBERG 2) | AstraZeneca |
Olaparib | Ovarian cancer | II | Completed | Multinational | NCT00753545 (Study 19) | AstraZeneca |
Olaparib | Ovarian cancer | III | Recruiting | Multinational | NCT01844986 (SOLO 1; OSTRIA1) | AstraZeneca |
Olaparib | Ovarian cancer | III | Ongoing | Multinational | NCT01874353 (SOLO2; OSTRIA2) | AstraZeneca |
Olaparib | Ovarian cancer | III | Planned | Multicentre | NCT02282020 (SOLO 3) | AstraZeneca |
Olaparib + paclitaxel | Breast cancer | I/II | Ongoing | Multinational | NCT00707707 | AstraZeneca |
Olaparib | Breast cancer | II | Ongoing | Multinational | NCT00494234 (ICEBERG 1) | AstraZeneca |
Olaparib | Breast cancer | III | Recruiting | USA, Europe | NCT02000622 | AstraZeneca |
Olaparib | Breast cancer | III | Recruiting | Multinational | NCT02032823 | AstraZeneca |
Olaparib + abiraterone | Prostate cancer | II | Recruiting | Multinational | NCT01972217 (EudraCT2013-003520-37) | AstraZeneca |
Olaparib | Prostate cancer | II | Recruiting | UK | NCT01682772 (TOPARP) | Institute of Cancer Research, UK |
Irinotecan + cisplatin + mitomycin C +/− olaparib | Pancreatic cancer | I/II | Ongoing | USA | NCT01296763 | Sidney Kimmel Comprehensive Cancer Center |
Olaparib | Pancreatic cancer | III | Planned | USA | NCT02184195 | AstraZeneca |
Olaparib + paclitaxel | Gastric cancer | II | Completed | Republic of Korea | NCT01063577 | AstraZeneca |
Olaparib + paclitaxel | Gastric cancer | III | Recruiting | Taiwan, Japan, China, Republic of Korea | NCT01924533 | AstraZeneca |
Olaparib | Colorectal cancer | II | Completed | USA | NCT00912743 | AstraZeneca |
Olaparib + gefitinib | Non-small cell lung cancer | I/II | Recruiting | Spain | NCT01513174 | Spanish Lung Cancer Group |
Olaparib | Non-small cell lung cancer | II | Recruiting | UK | NCT01788332 | AstraZeneca |
Olaparib | Ewing’s sarcoma | II | Ongoing | USA | NCT01583543 | Massachusetts General Hospital |
Olaparib | Advanced cancer | II | Ongoing | Multinational | NCT01078662 | AstraZeneca |
2.4 Adverse Events
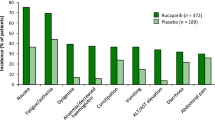
Oral olaparib 400 mg twice daily had acceptable tolerability when administered as maintenance monotherapy in women with relapsed ovarian cancer (with or without BRCA1/2 mutations) responding to platinum-based therapy in the pivotal phase II trial (Study 19) [35]. Adverse events (AEs) reported in 10 % more olaparib than placebo recipients included nausea (68.4 vs. 35.2 %), fatigue (48.5 vs. 37.5 %), vomiting (31.6 vs. 14.1 %) and anaemia (16.9 vs. 4.7 %). AEs were generally mild or moderate in severity; however, olaparib was associated with a 1.7-fold higher incidence of grade 3 or 4 AEs than placebo (35.3 vs. 20.3 %), with the most common being fatigue (6.6 vs. 3.1 %) and anaemia (5.1 vs. 0.8 %). More olaparib than placebo recipients had dose interruptions (27.9 vs. 8.6 %) or reductions (22.8 vs. 4.7 %) because of AEs (most frequently vomiting, nausea and fatigue) [35].
Similarly, in the phase II ICEBERG 3 trial in women with BRCA1/2-mutated recurrent ovarian cancer, the most frequent AE was grade ≤2 nausea with oral olaparib 400 or 200 mg twice daily (72 and 56 vs. 50 % with PLD) and grade ≤2 stomatitis with PLD (53 vs. 0 % in each olaparib group) [38]. Two deaths occurred in the olaparib 200 mg twice daily group (as a result of cerebrovascular accident or myelodysplastic syndrome); both were considered possibly treatment related [38]. A similar adverse event profile was seen with olaparib (400 or 100 mg twice daily) in other phase II monotherapy studies in ovarian cancer [39, 40].
Use of olaparib plus cediranib in women with recurrent ovarian or triple-negative breast cancer in phase I [44] or II [45] studies was most commonly associated with fatigue, diarrhoea, nausea and hypertension, with these AEs occurring more frequently than with olaparib alone in the comparative trial [45]. However, when olaparib was used in combination with carboplatin and/or paclitaxel in phase Ib/II ovarian cancer trials [46, 49], the most common adverse events were consistent with those typical of chemotherapy (e.g. neutropenia, anaemia).
2.5 Companion Diagnostic
Myriad Genetics have developed BRCA gene testing for use as a companion diagnostic (BRACAnalysis CDx™) with PARP inhibitors, such as olaparib; all four modules of the premarket approval application for the test have been submitted to the US FDA [68]. The next-generation companion diagnostic, Tumour BRACAnalysis CDx™, detects both germline and somatic BRCA mutations (and thus identifies up to 50 % more BRCA mutation carriers than conventional germline testing alone) and is launched in Europe [68]. Myriad Genetics have made an agreement with AstraZeneca to provide companion diagnostic BRCA testing for the olaparib phase III trial programme [69].
2.6 Ongoing Clinical Trials
AstraZeneca has initiated the phase III SOLO programme of olaparib maintenance monotherapy in patients with BRCA-mutated ovarian cancer with complete or partial response to first-line platinum-based chemotherapy [SOLO 1 (OSTRIA 1); NCT01844986] or after completing ≥2 lines of platinum-based chemotherapy [SOLO 2 (OSTRIA 2); NCT01874353] [70]. Each trial is double-blind, placebo-controlled and uses the tablet formulation of olaparib. Enrollment began in September 2013; by January 2014, 10 % of the target patient population of SOLO 1 and 2 had been recruited (≈344 and ≈264 patients randomized) [70]. AstraZeneca is planning to initiate SOLO 3, a randomized, open-label study comparing olaparib monotherapy with single-agent chemotherapy in patients with platinum-sensitive, BRCA1/2 mutation-positive, relapsed ovarian cancer (NCT02282020). This study will include ≈411 patients who have received ≥2 lines of platinum-based chemotherapy. In addition, two phase III trials are planned to evaluate olaparib plus cediranib for the treatment of ovarian cancer [71], and a phase I/II trial (NCT02208375) designed to assess olaparib in combination with a mammalian target of rapamycin complex inhibitor or a kinase inhibitor is recruiting participants.
With regard to other indications, phase III BRCA1/2-mutated breast cancer trials are planning to compare olaparib with standard chemotherapy in metastatic disease (NCT02000622) and assess adjuvant olaparib in high-risk, HER2-negative primary disease following local treatment and adjuvant/neoadjuvant chemotherapy (NCT02032823). In addition, a phase III trial (NCT01924533) comparing olaparib plus paclitaxel with paclitaxel alone as second-line therapy in advanced gastric cancer has been initiated, and another phase III study (NCT02184195) is planned to evaluate olaparib monotherapy in patients with BRCA-mutated metastatic pancreatic cancer without progression on first-line platinum-based chemotherapy. Olaparib is also being assessed in combination with gemcitabine (NCT00515866) or irinotecan, cisplatin plus mitomycin C (NCT01296763) in advanced pancreatic cancer and in combination with radiotherapy in oesophageal cancer (NCT01460888) in phase I or I/II trials.
There are also phase II trials assessing olaparib in other indications, including advanced castration-resistant prostate cancer [as monotherapy (NCT01682772; TOPARP) or in combination with abiraterone (NCT01972217; EudraCT2013-003520-37] and advanced NSCLC [as monotherapy (NCT01788332) or in combination with gefitinib (NCT01513174)]. Other phase I/II trials are evaluating olaparib in combination with cisplatin-based chemoradiotherapy in advanced squamous cell carcinoma of the head and neck (ORCA; EudraCT2010-023599-24), olaparib plus radiotherapy (with or without cisplatin) in advanced NSCLC (NCT01562210) and olaparib plus radiotherapy in glioblastoma unsuitable for radical chemoradiation (PARADIGM; ISRCTN52658296). In addition, several phase I trials are investigating olaparib in patients with solid tumours [including those with renal (NCT01894256) or hepatic (NCT01894243) impairment].
3 Current Status
On the 18th December 2014, olaparib was approved in the EU for the maintenance treatment of adults with platinum-sensitive, relapsed, BRCA mutation-positive (germline and/or somatic), high-grade, serous epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy [4, 72]. On the 19th December, the drug received approval in the USA for use as monotherapy in patients with deleterious or suspected deleterious germline BRCA-mutated (as detected by an FDA-approved test) advanced ovarian cancer treated with three or more prior lines of chemotherapy [5, 73].
References
Girolimetti G, Perrone AM, Santini D, et al. BRCA-associated ovarian cancer: from molecular genetics to risk management. Biomed Res Int. 2014;2014:787143.
National Cancer Institute. BRCA1 and BRCA2: cancer risk and genetic testing. 2014. http://www.cancer.gov/cancertopics/factsheet/Risk/BRCA. Accessed 24 Nov 2014.
Javle M, Curtin NJ. The role of PARP in DNA repair and its therapeutic exploitation. Br J Cancer. 2011;105(8):1114–22.
AstraZeneca AB. Lynparza 50 mg hard capsules: EU summary of product characteristics. 2014. http://www.ema.europa.eu/ema. Accessed 12 Jan 2015.
AstraZeneca Pharmaceuticals LP. Lynparza™ (olaparib) capsules, for oral use: US prescribing information. 2014. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed 5 Jan 2015.
European Medicines Agency. Summary of the risk management plan (RMP) for Lynparza (olaparib). http://www.ema.europa.eu/ema. Accessed 12 Jan 2015.
AstraZeneca. Acquisition of KuDOS Pharmaceuticals will enhance AstraZeneca’s ability to generate novel cancer treatments [media release]. 23 December 2005. http://www.Media/Press-releases/Article/20051223--Acquisition-Of-KuDOS-Pharmaceuticals-Will-Enhance-Ast.
Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34.
Bundred N, Gardovskis J, Jaskiewicz J, et al. Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: a phase I multicentre trial in patients scheduled for elective breast cancer surgery. Invest New Drugs. 2013;31(4):949–58.
Dedes KJ, Wilkerson P, Wetterskog D, et al. PARP-inhibitor olaparib in the treatment of ovarian clear cell cancer: predictors of sensitivity and resistance [abstract no. 1113]. Lab Invest. 2012;92:265A.
Miyasaka A, Oda K, Ikeda Y, et al. Anti-tumor activity of olaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, in cultured endometrial carcinoma cells. BMC Cancer. 2014;14 (article no. 179). doi:10.1186/471-2407-14-179.
Im SA, Min A, Hur HS, et al. Growth inhibitory effect of PARP inhibitor olaparib in gastric cancer cells [abstract no. 1775]. In: 101st Annual Meeting of the AACR; 2010.
Hodgson D, Mason H, Oplustilova L, et al. Activity of the PARP inhibitor olaparib in ATM-deficient gastric cancer: from preclinical models to the clinic [abstract no. 2398]. In: AACR annual meeting; 2014.
Tinhofer I, Mariya BF, Niehr F, et al. The PARP inhibitor olaparib (AZD2281) as potent radiosensitizer of head and neck cancer cells [abstract no. e16018]. J Clin Oncol. 2012;30(15 Suppl 1).
Knights C, Chresta C, Riches L, et al. Preclinical evaluation of the PARP inhibitor olaparib in homologous recombination deficient (HRD) MRE11 mutant microsatellite instable (MSI) colorectal cancer [abstract no. A114]. In: 21st AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; 2009.
Pierce A, McGowan PM, Cotter M, et al. Comparative antiproliferative effects of iniparib and olaparib on a panel of triple-negative and non-triple-negative breast cancer cell lines. Cancer Biol Ther. 2013;14(6):537–45.
Evers B, Drost R, Schut E, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14(12):3916–25.
Konstantinopoulos PA, Wilson AJ, Saskowski J, et al. Suberoylanilide hydroxamic acid (SAHA) enhances olaparib activity by targeting homologous recombination DNA repair in ovarian cancer. Gynecol Oncol. 2014;133(3):599–606.
Choi YE, Battelli C, Watson J, et al. Sublethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget. 2014;5(9):2678–87.
Dey N, Wu H, Sun Y, et al. Preclinical efficacy of the combination of olaparib plus carboplatin with vandetanib in triple-negative breast cancer [abstract no. e13579]. J Clin Oncol. 2012;30(15 Supp 1).
Hashimoto J, Kitamura Y, Takashima Y, et al. Synergistic interaction between olaparib, a parp inhibitor, and cytotoxic agent in triple negative breast cancer [abstract no. O3–049]. Ann Oncol. 2013;24(Suppl 9):ix59.
Minami D, Takigawa N, Takeda H, et al. Synergistic effect of olaparib with combination of cisplatin on PTEN-deficient lung cancer cells. Mol Cancer Res. 2013;11(2):140–8.
Musi E, Ambrosini G, Schwartz GK. The parp inhibitor olaparib (AZD2281) greatly enances the effect of temozolomide in soft tissue sarcoma cell lines [abstract no. 2073]. Cancer Res. 2013;73(8 Suppl 1).
Yamauchi T, Uzui K, Nishi R, et al. Gemtuzumab ozogamicin and olaparib exert synergistic cytotoxicity in CD33-positive HL-60 myeloid leukemia cells. Anticancer Res. 2014;34(10):5487–94.
Miura K, Sakata KI, Someya M, et al. The combination of olaparib and camptothecin for effective radiosensitization. Radiat Oncol. 2012;7 (article no. 62). doi:10.1186/748-717X-7-62.
Senra JM, Telfer BA, Cherry KE, et al. Inhibition of PARP-1 by olaparib (AZD2281) increases the radiosensitivity of a lung tumor xenograft. Mol Cancer Ther. 2011;10(10):1949–58.
Kortmann U, McAlpine JN, Xue H, et al. Tumor growth inhibition by olaparib in BRCA2 germline-mutated patient-derived ovarian cancer tissue xenografts. Clin Cancer Res. 2011;17(4):783–91.
Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105(44):17079–84.
Min A, Im S-A, Yoon Y-K, et al. RAD51C-deficient cancer cells are highly sensitive to the PARP inhibitor olaparib. Mol Cancer Ther. 2013;12(6):865–77.
Daemen A, Wolf DM, Korkola JE, et al. Cross-platform pathway-based analysis identifies markers of response to the PARP inhibitor olaparib. Breast Cancer Res Treat. 2012;135(2):505–17.
Ang JE, Clarkson-Jones JA, Swaisland H, et al. A mass balance study to investigate the metabolism, excretion and pharmacokinetics of [14C]-olaparib (AZD2281) in patients with advanced solid tumours refractory to standard treatments [abstract no. 405]. In: 22nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; 2010.
Vergote I, Rutten A, Rolfo CD, et al. Effect of food on the pharmacokinetics (PK) of olaparib after oral dosing of the capsule formulation [abstract no. 2599]. J Clin Oncol. 2014;32(15 Suppl 1).
AstraZeneca. ODAC Sponsor Briefing Book. 2014. http://www.fda.gov. Accessed 16 Dec 2014.
Clarkson-Jones J, Page C, Sarda S, et al. Human biotransformation of olaparib (AZD2281) an oral poly(ADP-ribose) polymerase (PARP) inhibitor [abstract no. 417]. In: 22nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; 2010.
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92.
Matulonis UA, Harter P, Gourley C, et al. Analysis of intermediate clinical endpoints from a phase II trial of olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer (PSR SOC) [abstract no. 132]. Gynecol Oncol. 2014;133(Suppl 1):54–5.
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–61.
Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2011;30(4):372–9.
Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–51.
Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61.
Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–9.
Molife LR, Mateo J, McGoldrick T, et al. Safety and efficacy results from two randomized expansions of a phase I study of a tablet formulation of the PARP inhibitor, olaparib, in ovarian and breast cancer patients with BRCA1/2 mutations [abstract no. 3048]. J Clin Oncol. 2012;30(15 Suppl 1).
Mateo J, Friedlander M, Sessa C, et al. Administration of continuous/intermittent olaparib in ovarian cancer patients with a germline BRCA1/2 mutation to determine an optimal dosing schedule for the tablet formulation [abstract no. 801]. In: European Cancer Congress; 2013.
Liu JF, Tolaney SM, Birrer M, et al. A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer. 2013;49(14):2972–8.
Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15(11):1207–14.
Lee J-M, Hays JL, Annunziata CM, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst. 2014;106(6):dju089.
Matulonis U, Wulf GM, Birrer MJ, et al. Phase I study of oral BKM120 and oral olaparib for high-grade serous ovarian cancer (HGSC) or triple-negative breast cancer (TNBC) [abstract no. 2510]. In: 50th Annual Meeting of ASCO; 2014.
Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2014. doi:10.1016/S1470-2045(14)71135-0.
Rivkin SE, Iriarte D, Sloan H, et al. Phase Ib/II with expansion of patients at the MTD study of olaparib plus weekly (metronomic) carboplatin and paclitaxel in relapsed ovarian cancer patients [abstract no. 5527]. J Clin Oncol. 2014;32(15 Suppl 1).
Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2014. doi: 10.1200/JCO.2014.56.2728.
Choy E, Butrynski JE, Harmon DC, et al. Phase II study of olaparib in patients with refractory Ewing sarcoma following failure of standard chemotherapy. BMC Cancer. 2014;14:813.
Yamamoto N, Nokihara H, Yamada Y, et al. A Phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors. Cancer Sci. 2012;103(3):504–9.
Del Conte G, Sessa C, von Moos R, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111(4):651–9.
Balmana J, Tung NM, Isakoff SJ, et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol. 2014;25(8):1656–63.
Van Der Noll R, Ang JE, Jager A, et al. Phase I study of olaparib in combination with carboplatin and/or paclitaxel in patients with advanced solid tumors [abstract no.2579]. J Clin Oncol. 2013;31(15 Suppl 1).
Van der Noll R, De Greve J, Jager A, et al. Safety results from a phase I study with a new tablet formulation of olaparib (O) in combination with carboplatin (C) and paclitaxel (Pa) [abstract no. 846]. In: European Cancer Congress; 2013.
Dean E, Middleton MR, Pwint T, et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012;106(3):468–74.
Khan OA, Gore M, Lorigan P, et al. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br J Cancer. 2011;104(5):750–5.
Rajan A, Carter CA, Kelly RJ, et al. A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors. Clin Cancer Res. 2012;18(8):2344–51.
Samol J, Ranson M, Scott E, et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Invest New Drugs. 2012;30(4):1493–500.
Waxweiler TV, Bowles D, Reddy K, et al. Safety and feasibility update of olaparib, an orally bioavailable PARP inhibitor, with concurrent cetuximab and radiation therapy in heavy smokers with stage III-IVB squamous cell carcinoma of the head/neck: a phase 1 trial [abstract no. 2884]. Int J Radiation Oncol Biol Phys. 2014;90(1 Suppl):S559.
Chalmers AJ, Jackson A, Swaisland H, et al. Results of stage 1 of the oparatic trial: a phase I study of olaparib in combination with temozolomide in patients with relapsed glioblastoma [abstract no. 2025]. J Clin Oncol. 2014; 32 (15 Suppl).
Garcia Campelo R, Felip E, Massuti T, et al. Phase IB study to evaluate the efficacy and tolerability of olaparib (AZD2281) plus gefitinib in patients with epidermal growth factor receptor (EGFR) mutation positive advanced non-small-cell lung cancer (NSCLC) patients (pts). (NCT = 1513174/GECP-GOAL) [abstract no. 3420]. Eur J Cancer. 2013;49:S802–3.
Chiou VL, Kohn EC, Annunziata CM, et al. Phase I/Ib study of the PARP inhibitor (PARPi) olaparib (O) with carboplatin (C) in triple negative breast cancer (TNBC) at low genetic risk (NCT00647062) [abstract no. CT337]. In: 105th Annual Meeting of the AACR; 2014.
Dent RA, Lindeman GJ, Clemons M, et al. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013;15(5):R88.
Bang YJ, Im SA, Lee KW, et al. Olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer: a randomized, double-blind phase II study [abstract no. 4013]. In: 49th Annual Meeting of ASCO; 2013.
Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–44.
Myriad Genetics. Myriad Genetics corporate presentation. 2014. https://myriad-web.s3.amazonaws.com/myriad.com/pdf/Myriad-Corporate-Presentation.pdf. Accessed 17 Dec 2014.
Myriad Genetics Inc. Myriad Genetics expands collaboration with AstraZeneca on olaparib phase 3 clinical trials [media release]. 4 Sept 2013. http://www.myriad.com.
Moore KN, Di Silvestro P, Lowe ES, et al. SOLO1 and SOLO2: Randomized phase III trials of olaparib in patients (pts) with ovarian cancer and a BRCA1/2 mutation (BRCAm) [abstract no. TPS5616]. J Clin Oncol. 2014;32(15 Suppl 1).
AstraZeneca. AstraZeneca welcomes positive data on the combination of olaparib and cediranib for the treatment of ovarian cancer patients [media release]. 31 May 2014. www.astrazeneca.com.
AstraZeneca Global. Lynparza™ approved in the European Union as first-in-class treatment for advanced BRCA-mutated ovarian cancer [media release]. 18 Dec 2014. http://astrazeneca.com.
US FDA. FDA approves Lynparza to treat advanced ovarian cancer [media release]. 19 Dec 2014. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm427554.htm.
Disclosure
The preparation of this report was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. E. D. Deeks is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Olaparib: First Global Approval. Drugs 75, 231–240 (2015). https://doi.org/10.1007/s40265-015-0345-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0345-6