Abstract
Delamanid (Deltyba®), a nitroimidazo-oxazole derivative, is a new anti-tuberculosis (TB) drug which exhibits potent in vitro and in vivo antitubercular activity against drug-susceptible and -resistant strains of Mycobacterium tuberculosis. It is approved in several countries, including Japan and those of the EU, for use as part of an appropriate combination regimen in adults with multidrug-resistant tuberculosis (MDR-TB) when an effective treatment regimen cannot otherwise be composed due to resistance or tolerability. In a robust phase II trial in adult patients with MDR-TB, oral delamanid 100 mg twice daily for 2 months plus an optimized background regimen improved sputum culture conversion rates to a significantly greater extent than placebo. In a 6-month extension study, long-term (≤8 months) treatment with delamanid was associated with a higher incidence of favourable outcomes (i.e. cured or completed all treatment) than short-term (≤2 months) treatment, with an accompanying reduction inunfavourable outcomes as defined by the WHO (i.e. pre-specified proportion of TB-positive sputum cultures, death or treatment discontinuation for ≥2 months without medical approval). Delamanid was not associated with clinically relevant drug-drug interactions, including with antiretroviral drugs and those commonly used in treating TB. Delamanid was generally well tolerated in patients with MDR-TB, with gastrointestinal adverse events and insomnia reported most commonly. Although the incidence of QT interval prolongation was higher with delamanid-based therapy, it was not associated with clinical symptoms such as syncope and arrhythmia. In conclusion, delamanid is a useful addition to the treatment options currently available for patients with MDR-TB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Potent in vitro and in vivo antitubercular activity against Mycobacterium tuberculosis |
Higher sputum culture conversion rates than placebo in patients with multidrug-resistant tuberculosis |
Lack of drug-drug interactions when co-administered with antiretroviral agents |
Generally well tolerated, with gastrointestinal adverse events and insomnia reported most commonly |
Increased risk of QT interval prolongation |
1 Introduction
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis that primarily affects the lungs [1]. It is one of the ten most common causes of death worldwide, with an estimated 8.6 million incident (i.e. new and relapse) cases and 1.3 million deaths reported in 2012, and is a major cause of morbidity [1, 2]. Currently available treatment regimens, including the standard WHO-recommended first-line regimen of rifampicin, isoniazid, ethambutol and pyrazinamide, are effective against most drug-susceptible strains of TB [1]. Indeed, treatment success rates of ≥85 % for new cases of drug-susceptible TB are reported to the WHO on a regular basis [1]. However, the improper use of effective TB therapy and other errors have led to the emergence of multidrug-resistant TB (MDR-TB) [1–3].
MDR-TB is characterized by resistance to at least rifampicin and isoniazid, and is particularly difficult to treat [1]. The increasingly widespread incidence of MDR-TB has made the goal of eliminating TB in the 21st century more challenging [2]. Approximately 4 % of new TB cases and 20 % of retreated cases involve MDR-TB [1], although substantially higher figures have been reported in some countries [4]. Annual mortality from MDR-TB was estimated to be 170,000 in 2012, a comparatively high number when compared with the estimated 450,000 incident cases [1]. Treatment recommendations for MDR-TB are based on direct susceptibility testing for selection of active agents [5]. The current WHO-recommended regimen consists of daily administration of injectable agents for ≥8 months, with a total treatment duration of ≥20 months [4]. Overall MDR-TB treatment success rates are low, and less than 20 % of patients are currently receiving adequate treatment [2, 4]. The low rates of treatment success among patients with MDR-TB are attributed to long, complex and expensive courses of therapy with poorly tolerated drugs, inadequate patient adherence and the use of less effective and more toxic second- and third-line drugs [2–4].
Extensively drug-resistant TB (XDR-TB) is defined as MDR-TB that is also resistant to any fluoroquinolone and ≥1 injectable second-line drug (e.g. amikacin, capreomycin, kanamycin) [5, 6]. By the end of 2012, XDR-TB had been reported by 92 countries, including 13 countries and territories with more than ten XDR-TB cases in a single year [1]. According to recent data from surveys or continuous surveillance, XDR-TB represents almost 10 % of MDR-TB isolates [1]. Treatment options for XDR-TB are decidedly limited [5] and some cases are essentially untreatable [6], thereby emphasizing the need for new anti-TB drugs.
The WHO’s post-2015 global TB strategy includes the proposed goal of ending the global TB epidemic by 2035 [1]. Intensified research and development is one of the three pillars of this strategy [1]. For the first time in several decades, multiple new anti-TB drugs are being developed [1–4, 7, 8]. As well as re-engineering of existing antibacterial drugs and repurposing of old drugs, several new compounds have been discovered [3]. One such compound is delamanid (Deltyba®), a non-mutagenic nitroimidazo-oxazole (or nitroimidazopyran) [9, 10] with early bactericidal activity (EBA) [2, 3] (Fig. 1). Delamanid has been approved in the EU and Japan for the treatment of MDR-TB, when administered in combination with an optimized background regimen (OBR). This article reviews the pharmacological properties of delamanid and its clinical efficacy and tolerability in patients with MDR-TB, with discussion focusing on the approved dosage of 100 mg twice daily.
2 Pharmacodynamic Properties
2.1 Mechanism of Action
Delamanid (formerly OPC-67683) is a nitroimidazo-oxazole derivative that acts as a mycolic acid biosynthesis inhibitor [5, 11], thereby disrupting metabolism of the cell wall and facilitating better drug penetration into mycobacteria [10]. Delamanid is a pro-drug that requires bioreduction of its nitro group by M. tuberculosis to produce a reactive species with antimycobacterial efficacy [5, 12–14]. This activation is dependent on the actions of the reduced deazaflavin cofactor F420, its reductively activating enzyme F420-dependent glucose-6-phosphate dehydrogenase and the nitroreductase gene product of Rv3547 [13]. Delamanid interferes with mycolic acid production by inhibiting the synthesis of ketomycolic and methoxymycolic, but not alphamycolic, acids [9–12, 15]. An in vitro study demonstrated that the concentrations of delamanid required to inhibit ketomycolic and methoxymycolic activity by 50 % (IC50) were 0.021 and 0.036 µg/mL, which were lower than those of isoniazid [14].
2.2 Antitubercular Activity
The antibacterial activity of delamanid is specific for Mycobacteria [5]. The minimum inhibitory concentrations (MICs) of delamanid against standard drug-susceptible and -resistant strains of M. tuberculosis ranged from 0.006 to 0.012 µg/mL, and were between 2 and 512 times lower than those of the established anti-TB drugs rifampicin, isoniazid, ethambutol and streptomycin [14]. Against clinically isolated M. tuberculosis strains, the minimum concentrations of delamanid required to inhibit 90 % of isolates (MIC90) ranged from 0.006 to 0.024 µg/mL, and were between 8 and 303 times lower than those of rifampicin, isoniazid, ethambutol and streptomycin [14]. A clinical breakpoint for delamanid has not yet been determined [11].
Delamanid did not demonstrate antagonism with rifampicin, isoniazid, ethambutol or streptomycin against clinical isolates in in vitro assays using checkerboard methods [14]. The respective fractional inhibitory concentration index for each of these drugs was classified as synergistic or partially synergistic for 92.6, 44.4, 88.9 and 25.9 % of all tested strains [14].
Delamanid was shown to have dose-dependent activity against intracellular M. tuberculosis in human macrophages after 4-h pulsed exposure [14]. At a concentration of 0.1 µg/mL, the intracellular activity of delamanid was similar to that of rifampicin 3 µg/mL (0.41 vs. 0.43 log reduction; values estimated from graph) [14].
In an experimental murine model of chronic TB, delamanid was associated with a dose-dependent reduction in the viable mycobacterial count [14]. The dose of delamanid required to reduce colony-forming units (CFU) by ≥95 % was 0.625 mg/kg, compared with 3.5, 5, >160, 40 and 160 mg/kg for rifampicin, isoniazid, ethambutol, streptomycin and pyrazinamide, respectively [14]. The sterilizing activity of delamanid in an in vitro model of drug-tolerant M. tuberculosis was consistent with its activity in mice [16]. In culture, delamanid demonstrated dose-dependent killing of clinical isolates of M. tuberculosis and, at a concentration of 1.0 µg/mL, killing was superior to that of isoniazid (p < 0.001) and equal to that of rifampicin [16]. Delamanid was also associated with a dose-dependent reduction in pulmonary CFU counts in immunocompromised mice, with activity equivalent to that in immunocompetent mice [14]. In the mouse model, a regimen containing delamanid 2.5 mg/kg, rifampicin 5 mg/kg and pyrazinamide 100 mg/kg achieved faster eradication of bacteria than the global standard regimen of rifampicin, isoniazid, ethambutol and pyrazinamide, with no mycobacterial colonies detected after 4 months of treatment with the delamanid-containing regimen, whereas colonies were detected in four out of five mice after 6 months of the standard regimen [14].
Delamanid demonstrated monophasic EBA in patients with newly diagnosed smear-positive pulmonary TB [17]. In this phase IIa study, 54 patients were randomized to receive delamanid 100, 200, 300 or 400 mg once daily or standard anti-TB therapy with isoniazid, rifampicin, pyrazinamide and ethambutol for 14 days. All delamanid recipients experienced a steady decline in CFU count during the study. The proportion of delamanid recipients with a strong treatment response (≥0.9 log10 CFU/mL sputum decline over 14 days) was 45, 70, 80 and 27 %, respectively, in the 100, 200, 300 and 400 mg/day groups. The average EBA of all delamanid dosages combined was 0.040 log10 CFU/mL per day over days 0–14, and was significant from day 3 of treatment onwards. On day 14, there was a moderate but significant correlation between the peak plasma concentration (Cmax) and EBA (r = 0.298; p = 0.044) of delamanid [17].
2.3 Potential Mechanism of Resistance
Mutation in one of the five coenzyme F420 genes (fgd, Rv3547, fbiA, fbiB and fbiC) has been proposed as the mechanism for resistance against delamanid in mycobacteria [5, 11]. The definition of resistance to delamanid in clinical trials is any growth in the presence of a delamanid concentration of 0.2 µg/mL that is >1 % of that in drug-free control cultures [11]. In vitro, the development of resistance to delamanid in clinical isolates of M. tuberculosis was due to lack of conversion of delamanid to the desnitro-imidazo-oxazole form by the bacteria [5]. Resistance to delamanid has been observed during treatment [11]. The risk of selecting for resistance to delamanid appears to be higher when the drug is used with few agents predicted to be active and/or when these additional agents are not among those considered to be most effective against M. tuberculosis [11]. Cross-resistance between delamanid and rifampicin, isoniazid, ethambutol or streptomycin has not been observed [14].
3 Pharmacokinetic Properties
3.1 Absorption and Distribution
Delamanid plasma exposure increased in a less than dose-proportional manner in patients with smear-positive pulmonary TB who received delamanid 100, 200, 300 or 400 mg once daily for 14 days [17] and in patients with MDR-TB who received delamanid 100 or 200 mg twice daily for 2 months [18]. In single-dose studies in healthy volunteers, exposure to delamanid was markedly increased in the fed state (approximate threefold increase after a 200 or 400 mg dose) and after a high-fat meal (threefold to more than fourfold increase after a 400 mg dose) compared with the fasted state [5, 11], while oral bioavailability of the drug increased ≈2.7-fold in the fed state (Sect. 6) [11]. Although the absolute oral bioavailability of delamanid in humans is yet to be established, evidence suggests that 25–47 % of the delamanid dose is absorbed following oral administration in the fed state [5].
In trial 204 (Sect. 4.1) in patients with MDR-TB receiving delamanid 100 or 200 mg twice daily with standard meals, time to reach Cmax (tmax) occurred approximately 4 h after each dose [5]. Delamanid plasma concentrations reached 90 % (steady state) of the day 56 values by day 14. In the delamanid 100 mg twice-daily group, Cmax values following the first and second daily doses on day 14 were 369 and 361 ng/mL. The area under the plasma concentration-time curve (AUC) from time zero to 24 h on day 14 was 7,234 h·ng/mL. Exposure to delamanid was not influenced by drug-resistance status, age, mild renal impairment, OBR or other concomitant medications [5].
Delamanid is highly plasma protein bound (≥99.5 %), as are its metabolites DM-6704 (99.60 %), DM-6705 (99.70 %) and DM-6706 (99.21 %) [5, 11]. Delamanid has a large volume of distribution (V/F) that increases with dose in relation to the non-dose-proportional behaviour of the drug [5]. In healthy subjects, the V/F was 1,100 L after administration of delamanid 100 mg twice daily. Across several studies, the estimated apparent volume of distribution during the terminal phase (Vz/F) ranged from 936.5 to 16,100 L, and the bodyweight-adjusted Vz/F ranged from 15.48 to 163.2 L/kg [5].
3.2 Metabolism and Elimination
The complete metabolic profile of delamanid has not yet been determined [11]. The drug is predominantly metabolized in plasma by albumin [11, 19]. Delamanid is rapidly metabolized to DM-6705 due to a reaction between the amino acid groups in albumin and the 5-C of 6-nitro-2,3-dihydroimidazo[2,1-b]oxazole moiety of delamanid [5]. Minimal metabolism of delamanid also occurs in human liver microsomes by cytochrome P450 (CYP) 3A4 [5, 11, 19]. The delamanid metabolite DM-6705 is thought to be metabolized via hydrolysis to DM-6704 and DM-6706, and via CYP3A4 oxidation to DM-6720 [5]. CYP3A4 also facilitates further biotransformation of DM-6704 and DM-6720 to finally form DM-6717 and DM-6718, respectively [5].
The plasma half-life (t1/2) of delamanid is 30–38 h [5, 11], and the t1/2 of the metabolites ranges from 122 to 322 h [5]. The presence of hypoalbuminaemia was associated with higher delamanid clearance, which is likely related to confounding pre-existing physiological conditions in this patient population. Elimination of delamanid following administration of radiolabelled drug was mainly via the faeces [5, 19]. Delamanid is not excreted in urine [11, 19].
3.3 Potential Drug Interactions
In transporter studies, delamanid was shown not to be a substrate for P-glycoprotein transport [5]. In vitro studies demonstrated that at concentrations of ≈5- to 20-fold greater than the Cmax at steady state, delamanid and its metabolites had no effect on transporters such as multidrug resistance protein 1, breast cancer resistance protein, organic anion-transporting polypeptide (OATP)1, OATP3, OATP1B1, OATP1B3, organic cation transporter (OCT)1, OCT2 and bile salt export pump [11, 19]. In vitro, delamanid did not inhibit, induce or stimulate CYP enzyme activity at concentrations up to 100 µM, suggesting that therapeutic concentrations of delamanid are unlikely to cause clinically significant interactions with other drugs undergoing CYP-mediated metabolism [14].
A lack of clinically relevant drug-drug interactions was seen when delamanid 100 mg twice daily was administered in combination with lopinavir/ritonavir 400/100 mg daily or tenofovir 300 mg daily for 14 days [11, 20] and in combination with efavirenz 600 mg daily for 10 days [21] in healthy subjects (available as abstracts). Delamanid exposure was not affected by the CYP1A2 inhibitor tenofovir or the weak CYP3A4 inducer efavirenz, but was 20 % higher when co-administered with the strong CYP3A inhibitor lopinavir/ritonavir [11, 19–21]. Delamanid did not affect tenofovir, lopinavir, ritonavir or efavirenz exposure [19–21].
In a 15-day study in healthy subjects, co-administration of delamanid 200 mg daily and rifampicin/isoniazid/pyrazinamide 300/720/1,800 mg daily reduced plasma exposure to delamanid by 45 % [11, 19]. Delamanid did not affect exposure of rifampicin, isoniazid or pyrazinamide. Co-administration of delamanid 200 mg daily and ethambutol 1,100 mg daily increased steady-state plasma concentrations of ethambutol by ≈25 %[11, 19].
4 Therapeutic Efficacy
The efficacy of oral delamanid in adult patients (aged 18–64 years) with sputum culture-positive MDR-TB was evaluated in the pivotal, multinational phase II trial (trial 204; n = 481 randomized) (Sect. 4.1) [18]. This was followed by a non-controlled, open-label extension (trial 208) [22] which, in turn, was followed by a 24-month observational study of patients who were randomized in trial 204 (study 216) [22] (Sect. 4.2). Some data were derived from the European Medicine Agency’s assessment report [5]. This section focuses on data relevant to the approved dosage of delamanid (100 mg twice daily; Sect. 6).
4.1 In the Pivotal Trial
In the pivotal trial, patients received an OBR in combination with delamanid 100 or 200 mg twice daily or placebo for 2 months [18]. In addition to positive sputum cultures for MDR-TB at day −1 and day 1, the other key inclusion criterion was an abnormal chest radiograph consistent with TB. Key exclusion criteria included a Karnofsky score <50 %, HIV-positive with a CD4 cell count <350 cells/mm3 or receiving antiretroviral therapy and the presence of significant relevant disease such as cardiovascular disease. The use of moxifloxacin was not permitted. Approximately 25 % of patients were from the Americas and just over half of all patients were from Asia. Most patients (≈70 %) had lung cavitation, which was bilateral in ≈25 % of patients. Overall, 91 % of patients had received previous anti-TB therapy ≥30 days prior to randomization, about half of whom had received a first-line regimen only [18].
Treatment was administered with food as directly-observed therapy (DOT), and patients were hospitalized throughout the treatment period [18]. OBR was defined according to WHO guidelines for treating MDR-TB, and could be adjusted as needed by the site investigators (see Table 1 for OBR regimens). The primary endpoint was the proportion of patients with sputum culture conversion in liquid broth medium at 2 months [assessed using an automated mycobacterial growth indicator tube (MGIT) system]. Sputum culture conversion was defined as a series of ≥5 consecutive cultures that were negative for growth of M. tuberculosis, without subsequent positive cultures. Efficacy analyses were conducted in the modified intent-to-treat population (i.e. all patients with a positive sputum culture for MDR-TB at baseline) [18].
In patients with MDR-TB, treatment with delamanid 100 mg twice daily for 2 months was associated with a significantly higher rate of sputum culture conversion than placebo, as assessed using the MGIT system (Table 2) [18]. Similar results were seen for assessment of sputum culture conversion in solid mycobacteriologic culture medium (Table 2) [18].
There was a significant (p = 0.001) difference between the delamanid 100 mg twice-daily and placebo groups regarding the time to MGIT sputum culture conversion [18]; a similar trend was observed with the use of solid culture medium. According to a Kaplan–Meier analysis of time to sputum culture conversion using MGIT, there was a 10 % separation between delamanid and placebo by day 36. The hazard ratio (HR) for increased time to sputum culture negativity in the delamanid 100 mg twice-daily group was 0.58 (95 % CI 0.39–0.89) with the use of MGIT and 0.54 (95 % CI 0.36–0.81) with the use of solid medium based on a Cox regression analysis of sputum culture conversion (included study drug assignment and the presence or absence of cavitation) [18].
A subgroup analysis of 38 Chinese patients enrolled in trial 204 demonstrated that the results in this patient subgroup were similar to those seen in the overall study population [23].
4.2 In Long-Term Studies
Patients who completed trial 204 were eligible to enter trial 208, during which they received delamanid 100 and/or 200 mg twice daily in combination with an OBR for a further 6 months [22]. All patients who completed trial 204 were invited to participate in study 116, regardless of whether or not they had participated in trial 208. Study 116 evaluated long-term outcomes until 24 months after randomization to trial 204 or until the end of treatment, whichever came first. Patients continued to receive a simplified OBR throughout study 116 [22].
A total of 421 of 481 (87.5 %) patients from trial 204 consented to participate in study 116 and were included in the analysis population, comprising 192 of 213 patients who entered trial 208 and 229 of 268 patients who did not [5]. Between trials 204 and 208, there was a ≥4-week break in delamanid treatment, with more than half of the patients resuming delamanid within 2 months [22]. Patients entering trial 208 initiated delamanid as DOT at a dosage of 100 mg twice daily, with the option of increasing the dosage to 200 mg twice daily after 2 weeks. All patients continued to receive an OBR throughout the 6-month study period [22].
In the study 116 analysis population, 126 patients had been treated with delamanid for a total of 8 months, and 66 had initially received placebo for 2 months during trial 204 followed by delamanid for 6 months during trial 208 [22]. Patients from these groups were combined into a single long-term treatment group (n = 192). The remaining patients who had received either delamanid (n = 156) or placebo (n = 73) for 2 months as part of trial 204 and who did not participate in trial 208 were combined into a single short-term treatment group (n = 229). MDR-TB treatment outcomes were categorized as favourable (i.e. cured or completed all treatment) or unfavourable [i.e. died, failed (pre-specified proportion of TB-positive sputum cultures) or defaulted (treatment discontinuation for ≥2 months without medical approval)] [22].
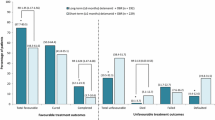
At 24 months in the overall population, significantly more patients in the long-term treatment group had favourable outcomes than in the short-term treatment group, although there was no significant between-group difference in cure rates (Fig. 2) [22]. Unfavourable outcomes and patient mortality were also significantly reduced following long-term treatment with delamanid (Fig. 2) [22].
Outcomes in a 2-year observational study in patients with multidrug-resistant tuberculosis [22]. Patients had received oral delamanid plus an optimized background regimen for ≤2 months (short-term) or 6–8 months (long-term) in trials 204 and/or 208. Numbers in brackets are 95 % CIs. RR risk ratio, *p < 0.001 vs. short-term group
In a subset of patients with XDR-TB (n = 56), favourable outcomes were seen in 61.4 % of patients in the long-term treatment group and 50.0 % of those in the short-term treatment group [22]. There was a significant difference in the mortality rate between the long- and short-term groups (0 vs. 25.0 %; p < 0.001), but no significant between-group differences were seen for other outcomes [22].
5 Tolerability
5.1 General Tolerability Profile
Treatment with oral delamanid was generally well tolerated when used in combination with OBR in patients with MDR-TB participating in trial 204 and the open-label extension (trial 208) discussed in Sect. 4 [5, 18]. Although the majority (≥89 %) of patients experienced at least one treatment-emergent adverse event in trial 204, few (3 %) patients discontinued treatment because of these adverse events. Most adverse events were of mild to moderate severity [18]. Across both trials, the most frequently reported adverse events in patients receiving delamanid included nausea, vomiting, insomnia and upper abdominal pain (Fig. 3). With the exception of QT interval prolongation (Sect. 5.2), there were no significant between-group differences in the incidence of individual treatment-emergent adverse events [18]. Of note, no new clinically important adverse events were observed in trial 208 (≤8 months of treatment) relative to those reported in trial 204 [5].
Tolerability of oral delamanid plus an optimized background regimen in patients with multidrug-resistant tuberculosis participating in trial 204 [18]. Treatment-emergent adverse events occurring in ≥15 % of patients in any treatment group and with a numerically higher incidence in either delamanid group than in the placebo group. bid twice daily
In trial 204, 38.5 % of patients receiving delamanid 100 mg twice daily and 35.6 % of placebo recipients experienced an adverse event that was considered by the investigator to be potentially causally related to the investigational medicinal product [18]. These included insomnia, vomiting, corrected QT (QTc) interval prolongation, somnolence, nausea and hypokalaemia.
The incidence of serious adverse events in trial 204 was 9.9 % in the delamanid 100 mg twice-daily group and 8.8 % in the placebo group [18]. In trial 208, serious adverse events occurred in 11.7 % of delamanid-treated patients [5]. The most commonly occurring serious adverse events were QTc interval prolongation (Sect. 5.2), haemoptysis, anaemia and psychotic disorder. Overall, 74 patients in trials 204 and 208 experienced severe adverse events, the majority of which occurred ≥4 months after starting delamanid [5].
The nature and frequency of biochemical abnormalities were generally similar in the combined delamanid group and the placebo group in trial 204 [5]. The only such event that was considered to be clinically significant that occurred with a numerically higher incidence in the delamanid group than in the placebo group was hyperuricaemia (26.1 vs. 22.9 %). Elevated serum cortisol levels (≥26 µg/dL) were seen in 36.0 % of patients receiving delamanid 100 mg twice daily and 29.4 % of placebo recipients; these elevations in cortisol level may be the result of disturbances in the peripheral metabolism of cortisol that are observed in progressive TB. The most frequently reported potentially drug-related chemistry abnormality was hypokalaemia, which occurred in 2.5 % of delamanid and 1.9 % of placebo recipients [5].
5.2 Cardiovascular Adverse Events
In trial 204, the frequency of ECG QT interval prolongation was significantly (p < 0.05) higher with delamanid 100 or 200 mg twice daily than with placebo (9.9 and 13.1 vs. 3.8 %, respectively) [18]. However, no episodes of QT interval prolongation were accompanied by clinical symptoms such as arrhythmias or syncope [18]. Evidence suggests that prolongation of the QTc interval is closely correlated with levels of the major delamanid metabolite DM-6705, the formation and metabolism of which is regulated by plasma albumin and CYP3A, respectively (Sect. 3.2) [11]. In trial 204, hypoalbuminaemia was associated with an increased risk of prolongation of the QTc interval (Sect. 6). Among patients receiving delamanid 100 mg twice daily, the mean Fredericia’s corrected QT interval (QTcF) increases from baseline were 7.6 ms at 1 month and 12.1 ms at 2 months. The proportion of patients who experienced a QTcF increase of ≥60 ms at some point during the study was 3 %; a QTc interval >500 ms was seen in one patient [11].
The only cardiac adverse events that occurred with an incidence of ≥2 % in the delamanid 100 mg, delamanid 200 mg and placebo groups in trial 204 were palpitations (8.1, 12.5 and 6.3 %, respectively) [18]. In trial 208, 13.1 and 0 % of patients in the delamanid 100 and 200 mg groups experienced palpitations, with no other cardiac adverse events reported in more than ≥2 % of patients [22]. The majority of these events were of mild severity [5]. In trial 204, most cardiac adverse events started between day 7 and 63, and lasted for 1–62 days. There was no consistent pattern in the time to onset or duration of palpitations. Most patients with palpitations had pre-existing conditions and/or were receiving concomitant medications that had a possible causative role [5].
6 Dosage and Administration
In the EU [11] and Japan [19], delamanid is indicated for use as part of an appropriate combination regimen in adult patients with pulmonary MDR-TB when an effective treatment regimen cannot otherwise be composed due to resistance or tolerability. The appropriate combination regimen should be continued after completion of delamanid treatment. The recommended dosage of delamanid in adults is 100 mg twice daily [11, 19]. The EU summary of product characteristics states that the recommended treatment period is 24 weeks [11]. Administration of delamanid for >6 consecutive months has not been evaluated; this should be noted when considering the risks and benefits of treatment [19]. Delamanid tablets should be taken orally with food [11, 19], and it is recommended that delamanid is administered as DOT [11].
No dosage adjustments are needed in patients with mild or moderate renal impairment, or in patients with mild hepatic impairment [11]. The use of delamanid is not recommended in patients with severe renal impairment or moderate to severe hepatic impairment. Delamanid is contraindicated in patients with serum albumin levels <2.8 g/dL and in patients receiving strong CYP3A inducers such as carbamazepine (Sect. 3.2). Patients with various cardiac risk factors including QTc interval prolongation should not receive delamanid unless the potential benefits of treatment are expected to outweigh the possible risks. For all patients, an ECG is recommended prior to starting delamanid, followed by monthly ECG monitoring during the full delamanid treatment period [11, 19]. Patients with serum albumin levels <3.4 g/dL, those receiving strong CYP3A inhibitors and patients with cardiac risk factors should receive very frequent ECG monitoring throughout the total period of delamanid treatment [11]. It is recommended that serum electrolyte abnormalities are checked prior to initiating treatment with delamanid [11, 19].
Local prescribing information should be consulted for further, detailed information regarding contraindications, warnings and potential drug interactions.
7 Place of Delamanid in the Treatment of Multidrug-Resistant Tuberculosis
Treatment of MDR-TB is a major challenge [2]. In the 27 countries with a high MDR-TB burden, it is estimated that 1.3 million patients with MDR-TB will require treatment between 2010 and 2015, with estimated costs of US$16.2 billion [24]. Treatment is costly because of the high number of drugs involved and the long duration of treatment required to achieve relapse-free cure [2, 4]. Each course of current recommended therapy for MDR-TB costs between US$3,000 and $5,000 per patient, compared with $22 for drug-sensitive TB treatment [24]. The cost can be even higher if patients require hospitalization [10].
As drug resistance becomes more extensive, the availability of effective drugs for TB decreases [2]. In fully drug-susceptible TB, two core first-line drugs are usually adequate for cure. However, for MDR-TB, the use of five active drugs is advised. The standard WHO-recommended regimen for MDR-TB consists of pyrazinamide, a fluoroquinolone (preferably a later-generation agent), a second-line injectable drug, a thionamide and another oral bacteriostatic drug (preferably cycloserine) [2]. MDR-TB regimens have several limitations, including poor efficacy and intolerable side effects [10, 24]. Indeed, second-line drugs are less effective and more toxic than first-line medications, and are not always readily available [15]. Patients with MDR-TB have an increased likelihood of treatment failure or death and are more likely to develop additional drug resistance [10]. Therefore, there is a compelling need for new anti-TB drugs that are more effective and better tolerated, and can shorten the duration of therapy [3, 4, 7, 10]. Shorter treatment duration reduces the cost of therapy, improves adherence and helps prevent drug resistance [7]. New MDR-TB regimens should include drugs to which resistance is not expected to have developed, thereby avoiding dependence on drug susceptibility testing [4]. New drug classes have obvious value and benefit in this area [4]. Judicious use of these new drugs and adherence to DOT should be exercised to avoid the development of drug resistance.
Currently, there are several new, re-evaluated or repurposed drugs undergoing phase II or III clinical development for MDR-TB. Delamanid, a member of the nitroimidazole drug class, is approved in the EU and Japan for the treatment of adults with MDR-TB as part of an appropriate combination regimen [11, 19]. Approval was based largely on the results of a robust phase II trial (trial 204) which showed that, when used in combination with an OBR, delamanid 100 mg twice daily for 2 months was associated with an increase in sputum culture conversion (Sect. 4.1). Moreover, two open-label extension studies (trial 208 and study 116) showed that more patients receiving long-term treatment with delamanid had favourable outcomes than those receiving short-term treatment (Sect. 4.2). Long-term treatment with delamanid also reduced unfavourable outcomes and patient mortality (Sect. 4.2). It should be noted that the primary endpoint was measured using liquid culture medium (in an automated MGIT system) in trial 204 and solid culture medium in the open-label extension (trial 208) [10]. Evidence suggests that MGIT is more sensitive than solid culture medium for the detection of viable M. tuberculosis organisms [18].
Delamanid has been shown to be more active in vitro than currently available first-line anti-TB drugs (Sect. 2.2). The fact that delamanid is administered orally may confer an advantage in terms of patient adherence to therapy compared with currently recommended second-line injectable drugs (e.g. amikacin, capreomycin, kanamycin) [4]. A completely oral MDR-TB regimen may be more convenient and acceptable for patients, with improved tolerability. Indeed, injectable agents can cause severe adverse events such as deafness, electrolyte imbalances and renal impairment [4]. Delamanid should be taken orally with food in order to achieve optimum pharmacokinetics (Sect. 6), which may be a disadvantage given that the absorption of some adjunctive anti-TB drugs is inhibited in the fed state [12].
Delamanid has the potential to significantly shorten the 2-year duration of treatment for MDR-TB [4, 12]. In trial 204, increased rates of sputum culture conversion were seen after 2 months of treatment with delamanid plus an OBR compared with placebo plus an OBR (Sect. 4.1). However, evidence suggests that a 6-month treatment duration may be necessary to achieve lasting sputum culture negativity [12]. The optimal duration of treatment with delamanid remains speculative, and additional studies investigating the long-term efficacy of delamanid are currently underway [2].
Evidence suggests that delamanid may have the potential to improve the cure rate of XDR-TB [6]. In a subset of patients with XDR-TB, long-term treatment with delamanid was associated with a higher rate of favourable outcomes than short-term treatment (Sect. 4.2).
Treatment adherence can be influenced by adverse events associated with MDR-TB therapy [4], making it important to consider regimen tolerability in addition to convenience. Delamanid-based therapy was generally well tolerated in the phase II trial and the open-label extension, with the most common adverse events being nausea, vomiting, upper abdominal pain and insomnia (Sect. 5.1). However, the use of delamanid may be limited by the potential risk of serious cardiac adverse events such as QT interval prolongation (Sect. 5.2). Of note, prolongation of the QT interval is also associated with other novel anti-TB agents, including the recently approved bedaquiline [12]. As such, caution should be exercised when considering combining delamanid with these drugs in clinical trials [12].
Patients with HIV infection have an increased risk of developing TB [1], and TB is the leading cause of death among HIV-infected patients [4], with an estimated 0.3 million HIV-associated TB deaths in 2012 [1]. Therefore, new anti-TB regimens need to be appropriate for use in this patient population, with negligible, if any, clinically relevant drug interactions [4]. Unlike other anti-TB and antiretroviral drugs, many of which are metabolized by CYP enzymes and are associated with drug interactions [3], delamanid does not induce or inhibit CYP enzymes (Sect. 3.3) [11]. Co-administration of delamanid with commonly used antiretrovirals was not associated with clinically relevant drug-drug interactions (Sect. 3.3).
The accessibility and affordability of delamanid has not yet been established [24]. According to a Médecins Sans Frontiéres (MSF; Doctors without Borders) report, delamanid and other new anti-TB drugs are likely to be priced at a level that retains the high costs associated with MDR-TB therapy [24]. Evidence suggests that while drug costs for new, shorter MDR-TB regimens containing fewer drugs may be similar to those of the current regimen, they should have the potential to be more affordable in countries with a high MDR-TB burden [4].
To date, there are no published trials comparing delamanid with other new anti-TB drugs or evaluating potential combination regimens involving delamanid and other novel anti-TB drugs. Several established (e.g. the oxazolidinone linezolid and fluoroquinolones such as gatifloxacin and moxifloxacin) or new (e.g. bedaquiline, posizolid, sutezolid, SQ-109) drugs are currently being investigated in phase II and/or III trials for use in the treatment of MDR-TB, as are novel combination regimens involving drugs in the pre-registration phase and/or those currently used in standard first-line treatment [1, 3, 4, 7].
The European marketing authorization for delamanid was conditional upon completion of a confirmatory phase III trial, which is currently underway [5]. Trial 213 is a double-blind, multicentre study designed to investigate the efficacy and tolerability of delamanid for 6 months in adults aged 18–69 years with pulmonary sputum culture-positive MDR-TB [25]. Delamanid 100 mg twice daily for 2 months followed by 200 mg once daily for 4 months or placebo is being added to an OBR. The primary efficacy endpoints are the proportion of patients achieving sputum culture conversion at 2 months and the time to sputum culture conversion during the 6-month treatment period. Although patients with XDR-TB are being excluded, a sub-trial comprised of HIV-infected patients receiving antiretroviral therapy is being conducted at some investigational sites [25].
In conclusion, twice-daily oral delamanid was effective and generally well tolerated when used in combination with an OBR in patients with MDR-TB in a 2-month phase II trial and a 6-month extension study, or treatment for up to 8 months. Delamanid has low potential for drug-drug interactions, including a lack of drug-drug interactions with antiretrovirals, which may offer an advantage in patients co-infected with HIV and TB. In the absence of head-to-head trials, definitive conclusions on the comparative efficacy and tolerability of delamanid versus other recently approved/novel anti-TB agents are not yet possible, and further studies investigating its long-term efficacy are warranted. In the meantime, delamanid is a useful addition to the treatment options for patients with MDR-TB.
Data selection sources:
Relevant medical literature (including published and unpublished data) on delamanid was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) [searches last updated 7 November 2014], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Delamanid, tuberculosis.
Study selection: Studies in patients with tuberculosis who received delamanid. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
World Health Organization. Global tuberculosis report 2013. 2013. http://www.who.int/tb/publications/global_report/en/. Accessed 7 Nov 2014.
Lange C, Abubakar I, Alffenaar JW, et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014;44(1):23–63.
Zumla AI, Gillespie SH, Hoelscher M, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis. 2014;14(4):327–40.
Brigden G, Nyang’wa B-T, du Cros P, et al. Principles for designing future regimens for multidrug-resistant tuberculosis. Bull World Health Organ. 2014;92(1):68–74.
European Medicines Agency. Delamanid assessment report. 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002552/human_med_001699.jsp&mid=WC0b01ac058001d124. Accessed 7 Nov 2014.
Matteelli A, Roggi A, Carvalho ACC. Extensively drug-resistant tuberculosis: epidemiology and management. Clin Epidemiol. 2014;6(1):111–8.
Dooley KE, Nuermberger EL, Diacon AH. Pipeline of drugs for related diseases: tuberculosis. Curr Opin HIV AIDS. 2013;8(6):579–85.
Kwon Y-S, Jeong B-H, Koh W-J. Tuberculosis: clinical trials and new drug regimens. Curr Opin Pulm Med. 2014;20(3):280–6.
Reviriego C. Delamanid antimycobacterial agent treatment of tuberculosis. Drug Future. 2013;38(1):7–12.
Field SK. Safety and efficacy of delamanid in the treatment of multidrug-resistant tuberculosis (MDR-TB). Clin Med Insights Ther. 2013;5:137–49.
European Medicines Agency. Delamanid (Deltyba): summary of product characteristics. 2014. http://ec.europa.eu/health/documents/community-register/2014/20140428126881/anx_126881_en.pdf. Accessed 7 Nov 2014.
Diacon AH, Von Groote-Bidlingmaier F, Donald PR. Delamanid, a new 6-nitro-2,3-dihydroimidazo[2,1-b]oxazole for the management of tuberculosis resistant to at least isoniazid and rifampicin. Expert Opin Orphan Drugs. 2014;2(1):87–94.
Hurdle JG, Lee RB, Budha NR, et al. A microbiological assessment of novel nitrofuranylamides as anti-tuberculosis agents. J Antimicrob Chemother. 2008;62(5):1037–45.
Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3(11):e466.
van den Boogaard J, Kibiki GS, Kisanga ER, et al. New drugs against tuberculosis: problems, progress, and evaluation of agents in clinical development. Antimicrob Agents Chemother. 2009;53(3):849–62.
Saliu OY, Crismale C, Schwander SK, et al. Bactericidal activity of OPC-67683 against drug-tolerant Mycobacterium tuberculosis. J Antimicrob Chemother. 2007;60(5):994–8.
Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15(7):949–54.
Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–60.
Otsuka Pharmaceutical Co. Ltd. Deltyba (delamanid): Japanese prescribing information. Tokyo: Otsuka Pharmaceutical Co., Ltd.; 2014.
Paccaly A, Petersen C, Patil S, et al. Absence of clinically relevant drug interaction between delamanid, a new drug for multidrug-resistant tuberculosis (MDR-TB) and tenofovir or lopinavir/ ritonavir in healthy subjects [abstract no. WEPE043]. In: 19th international AIDS conference. Washington, DC; 22–27 Jul 2012.
Petersen C, Paccaly A, Kim J, et al. Delamanid, a new drug for multi-drug resistant tuberculosis (MDR-TB), and efavirenz do not show clinically relevant drug interactions in healthy subjects [abstract no. A-1255]. In: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. San Fransisco; 9–12 Sep 2012.
Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41(6):1393–400.
Zhang Q, Liu Y, Tang S, et al. Clinical benefit of delamanid (OPC-67683) in the treatment of multidrug-resistant tuberculosis patients in China. Cell Biochem Biophys. 2013;67(3):957–63.
Medecins Sans Frontieres. DR-TB drugs under the microscope: sources and prices for drug resistant TB medications. 2013. http://www.msfaccess.org/content/dr-tb-drugs-under-microscope3rd-edition. Accessed 7 Nov 2014.
Otsuka Pharmaceutical Development & Commercialization Inc. Safety and efficacy trial of delamanid for 6 months in patients with multidrug resistant tuberculosis [ClinicalTrials.gov identifier NCT01424670]. US National Institutes of Health, ClinicalTrials.gov. 2011. http://clinicaltrials.gov/show/NCT01424670. Accessed 7 Nov 2014.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit. Hannah Blair and Lesley Scott are salaried employees of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: S. K. Field, Division of Respirology, University of Calgary, Calgary, AB, Canada; D. Goletti, National Institute for Infectious Diseases “L. Spallanzani”, Rome, Italy; J-P. Zellweger, TB Competence Center, Swiss Lung Association, Berne, Switzerland; Q. Zhang, Tuberculosis Diagnosis and Treatment Center, Shanghai Pulmonary Hospital, Shanghai, China.
Rights and permissions
About this article
Cite this article
Blair, H.A., Scott, L.J. Delamanid: A Review of Its Use in Patients with Multidrug-Resistant Tuberculosis. Drugs 75, 91–100 (2015). https://doi.org/10.1007/s40265-014-0331-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0331-4