Abstract
Introduction
Since legislation in 2009, coroners in England and Wales must make reports in cases where they believe it is possible to prevent future deaths. We categorised the reports and examined whether they could reveal preventable medication errors or novel adverse drug reactions.
Methods
We examined 500 coroners’ reports by pre-defined criteria to identify those in which medicines played a part, and to collect information on coroners’ concerns.
Results
We identified 99 reports (100 deaths) in which medicines or a part of the medication process or both were mentioned. Reports mentioned anticoagulants (22 reports), opioids (17), antidepressants (17), drugs of abuse excluding opioids (12 deaths) and other drugs. The most important concerns related to adverse reactions to prescribed medicines (22), omission of necessary treatment (21), failure to monitor treatment (17) and poor systems (17). These were related to defects in education or training, lack of clear guidelines or protocols and failure to implement existing guidelines, among other reasons. Most reports went either to NHS Hospital Trusts or to local trusts. The responses of addressees were rarely published. We identified four safety warnings from the Medicines and Healthcare Products Regulatory Agency that were based on coroners’ warnings.
Conclusion
Coroners’ reports to prevent future deaths provide some information on medication errors and adverse reactions. They rarely identify new hazards. At present they are often addressed to local bodies, but this could mean that wider lessons are lost.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Coroner’s reports are a potentially rich source of data on fatal medication errors and adverse drug reactions. |
Opiate and anticoagulation medication account for nearly half of fatal medication errors mentioned in coroners’ reports to prevent future deaths. |
Health organisations, professional and regulatory bodies and market authorisation holders could derive wider pharmacovigilance benefits from greater awareness of coroners’ reports. |
1 Introduction
In England and Wales, the cause of deaths other than natural deaths is established at inquest, conducted by a coroner. Since 2009, coroners have had a duty to make reports to a person, organisation, local authority or government department or agency where the coroner believes that action should be taken to prevent future deaths [1].
These ‘Reports to Prevent Future Deaths’ (known as PFDs) are published on the website of the Courts and Tribunals Judiciary [2]. The legal powers underpinning the PFD are set out in paragraph 7, schedule 5 of the Coroners and Justice Act 2009 and regulations 28 and 29 of the Coroners (Investigations) Regulations 2013.
We wished to examine PFDs to establish how often medicines and other drugs (excluding alcohol) were referred to in reports, the nature of the recommendations and the extent to which they revealed preventable medication errors or novel adverse drug reactions.
2 Methods
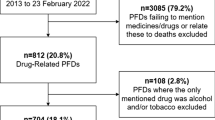
We decided whether reports should be included in our study using the algorithm shown in Fig. 1.
If the PFD mentioned part of the medication process (e.g. administration), or if a medicine was mentioned, or both, and either caused or contributed to death, then we included the report. We included drugs of abuse such as diamorphine (heroin) and cocaine. We excluded those cases in which a medicine or part of the medication process was mentioned, but did not cause or contribute to death, and in which delays in assessment, investigation or diagnosis led to delays in treatment. We also excluded cases where the only drug mentioned was alcohol.
The 500 reports from 24 April 2015 to 7 September 2016 were downloaded from the Courts and Tribunals Judiciary website. Two of the investigators (CE and REF) separately categorised all the PFDs. Where there was uncertainty, the categorisation was resolved by discussion; disagreements were resolved by adjudication (by ARC).
The data from each of the included PFDs were extracted in standard form. After the case reports were categorised into the four separate groups, as described above, the following information was recorded in a Microsoft Excel® spreadsheet for all cases:
-
patient name, age or date of birth and date of death;
-
jurisdiction in which death occurred;
-
catalogue number.
In the case of a medication error, the following information was also recorded:
-
gender and medical and social history of the patient;
-
the medical cause of death/conclusion of the coroners’ inquest;
-
nature of the medication error;
-
circumstances surrounding the medication error and how, if applicable, the error occurred;
-
coroner’s recommendation;
-
coroner’s recommendation classification;
-
whether the report had been classified under the alcohol, drug and medication classification on the Judiciary website;
-
medication error classification;
-
the drug class of the medication involved;
-
setting of patient death, such as hospital/community/care home/state.
We used the information within the PFDs to categorise the role that a drug had played in causing or contributing to a person’s death, and by implication those matters that required attention. We also considered whether the report described a previously recognised hazard, and whether it was of general relevance.
PFDs are directed to specific individuals or organisations, and we also examined whom they were addressed to.
We used only information in the public domain, and did not seek ethics committee approval.
3 Results
We identified 99/500 PFDs that fulfilled the criteria, relating to 100 people. Details are given in the Supplementary Table (see electronic supplementary material).
Forty-two of the hundred people we identified were women; 54 deaths were recorded in 2015 and 46 in 2016. The age was stated in two-thirds of reports, among whom the mean age was 52 years and median age was 50 years (range 1 day to 96 years). The drug classes implicated are shown in Table 1.
Most frequently mentioned drug classes, used alone or in combination, were anticoagulants (in 22 cases) and opioids, whether or not prescribed (in 17 cases). Other drugs of abuse included ecstasy (three deaths), cannabis or cannabinoids (four deaths), and cocaine (two deaths).
The concerns expressed by coroners are categorised in Table 2, which also lists the number of cases in which an adverse drug reaction (ADR) to a prescribed medicine was recorded.
We also categorised the coroners’ concerns according to a pre-determined list of terms. In 58 instances, coroners were concerned about the absence of protocols or guidelines, or the need to update them, or the failure to enforce them. Concerns were centred on education and training in 33 reports and on difficulties in communication in 21 reports. Coroners were also concerned about standards of review or monitoring (24 reports), drug regulation (12 reports), and issues related to staff or equipment (13 reports). We concluded that reports mostly (76) concerned local failure or bad practice, and generally (52) served as a reminder of known risks. Many (57) would be of wide relevance to patients and healthcare professionals who wished to mitigate risks in the health service. One report concerned a possible new risk in the manufacture of slow-release fentanyl patches, and 12 suggested failures in drug regulation, although the failures (principally in the control of novel psychoactive substances) had previously been recognised. The implications of two of the reports were uncertain.
At least 15% of the reports involved patients in care homes, and reflect a change in the regulations for enquiries into cases where people have been deprived of their liberty. Almost a third of all reports concerned drugs of abuse. Of these, several occurred in prison, and in total nearly 10% of the reports related to deaths in police custody or prison, a further issue of current concern.
Table 3 lists the agencies or persons to whom reports were addressed, and who had a duty to respond to the coroner’s report within 56 days of the date on which it was issued.
While there is the opportunity for responses to be posted on the internet, this was rarely done. Some responses were clear. For example, the Medicines and Healthcare Products Agency (MHRA) published warnings about fentanyl patches (Cases 2015-0463 and 2016-0014), emollients (Cases 2015-0317 and 2016-0163), the interaction between cocaine and citalopram (Case 2015-0231) and cardiac effects of hyoscine butylbromide (Case 2016-0308), although reports were only specifically addressed to MHRA in the last two cases.
3.1 Examples of Coroners’ Concerns
-
(a)
An adverse drug reaction that was missed—perforation of a gastric ulcer (Case 2016-0222).
A 16-year-old girl with cerebral palsy and other difficulties was treated with medicines including diclofenac. She appeared to be in pain with abdominal distension. The general practitioner saw her and arranged admission, but the junior doctor who clerked the patient did not see the admission letter, and diagnosed constipation. As the patient’s pulse rate was increased, an electrocardiogram was arranged. This showed only sinus tachycardia. The patient was discharged with a laxative. When she represented the next morning, she was gravely ill, and she died soon afterwards from peritonitis secondary to a perforated gastric ulcer.
The Coroner expressed concern that no cause was sought for the tachycardia, that there were failings in the record-keeping, that the general practitioner’s record never reached the junior doctor, and that the possibility of diclofenac-induced ulceration and perforation was not considered.
-
(b)
The danger of high doses of heroin in addicts who have lost tolerance (Case 2016-0058).
A 25-year-old man with a history of depression and substance misuse was referred to a psychiatric team specialising in patients with ‘dual diagnoses’. Appointments were delayed. The man managed to reduce his own opiate usage. He then received a benefits payment, took a large dose of heroin, and died.
The coroner expressed concerns regarding the various agencies involved, and their failure to communicate with each other. The coroner did not explicitly state that addicts should be educated about the dangers of taking large doses after tolerance has lapsed.
-
(c)
An under-appreciated danger from paraffin-based emollients (Cases 2015-0317 and 2016-0163).
Two reports described incapacitated patients who received fatal burns when they dropped smoking materials onto their dressings, which had become impregnated with paraffin-containing emollients and caught fire. Coroners expressed concerns that the dangers were not widely recognised, and warnings insufficient.
-
(d)
Failures of medication control in prisons (Case 2015-0468).
A male prisoner was found collapsed by his cellmate in the early hours of the morning. Toxicological samples showed the presence of prescribed and non-prescribed drugs, including methadone, buprenorphine, diazepam, pregabalin, quetiapine, and a synthetic cannabinoid. Both prescribed and non-prescribed drugs were found hidden in his cell.
The Coroner expressed concern that prison staff lacked awareness and understanding of drugs; that there was a failure to use a multi-disciplinary approach to the problem; that medicines could easily be concealed; that prisoners are not adequately monitored; that positive drug test results were not shared with prison staff; and that drugs could easily be smuggled or thrown into the prison.
-
(e)
Secondary risks of drug therapy—haemorrhage after trauma in a patient taking warfarin.
A woman took warfarin for an established heart condition. She went to meet a friend at a supermarket café and took the lift to the mezzanine. In the lift she leant on the rear wall, which was in fact a door that opened without warning. The woman stumbled, fell, and banged her head. She consequently developed a fatal intracerebral haemorrhage.
The Coroner was concerned that the rear door was not marked in any way, and that no warning sounded when the door opened.
A brief description of each of the 100 included deaths (99 reports) is provided in the Supplementary Table (see electronic supplementary material).
There were several ‘grey’ cases that we excluded from the analysis. For example, in one report, the coroner stated that an elderly woman had been given too much heparin because the dosage calculated had failed to take into account that she weighed only 30 kg. However, the report also explicitly stated that this failure did not cause or contribute to death (Case 2015-0417). In another example, a young man died from abusing helium, breathed from a plastic bag. Although the coroner stated that helium was toxic, it is only toxic in the sense that—like nitrogen—it cannot support life without the addition of oxygen; and nor is it regarded as a medicine (Case 2016-0182). In a third case, a patient died from complications of tracheal stenosis after prolonged intensive care, itself a consequence of severe ketoacidosis from new-onset diabetes. The coroner noted that the patient was treated with clozapine, which can precipitate diabetes, and criticised the failure to monitor blood glucose concentrations during clozapine treatment, but the Summary of Product Characteristics for clozapine recommends periodic measurement of fasting blood glucose concentration only in those with risk factors for diabetes (Case 2015-0194).
4 Discussion
The primary purpose of a coroner’s inquest is to establish who has died in the case of an unexplained death, when and where they died, and what led to death. It is inevitable, in conducting enquiries sufficient to provide this information, that coroners will uncover factors that led to the individual death and which may in future lead to further deaths.
Coroners in England and Wales have, under Rule 43 of the Coroners Rules 1984, been able to make reports with the intention of preventing future deaths. In the updated legislation of 2008 and 2013, Coroners now have a duty to make such reports where they are appropriate, and these reports are available on the internet. Similar provision has been made elsewhere; for example, in New Zealand [3], Australia [4, 5] and Canada [6]. In the United Kingdom, coroners are not permitted to make recommendations of improvements in PFDs, which do not therefore set out explicitly the ways in which improvements might be made.
In 2015 there were 529,613 deaths in England and Wales, and of these 236,406 (45%) were reported to a coroner [7]. Coroners requested post-mortem examinations in nearly 90,000 deaths, and opened 32,857 inquests. Of 35,473 inquests concluded, death was recorded as non-natural on 24,430 occasions (69%) The number of inquests completed exceeded the number of inquests opened as a consequence of a conscious decision by the Ministry of Justice to clear a historical backlog of cases). Of these deaths, 2% were related to road traffic collision, 6% to drugs or alcohol, 11% to suicide, and 22% to accident or misadventure. During 2015, the website of the Courts and Tribunals Judiciary listed PFDs with serial numbers from 0001 to 0502 [8]; occasional reports concerned more than one death. The next year, the serial numbers of PFDs ran from 0001 to 0467.
The Courts and Tribunals Judiciary website categorises reports. One of the classifications is ‘Alcohol, drug and medication deaths’. During the period of our study, only eight reports were categorised as ‘Alcohol, drug and medication deaths’ on the website; one of these concerned a man who was hit by a train, having probably wandered onto a railway line while drunk (Case 2016-0234), so that only seven fulfilled our criteria. We do not know the criteria by which the reports are classified on the website, but the vast majority of the reports we identified are omitted. This failure to classify deaths as related to medications may impede the use of coroner reports as an additional source of pharmacovigilance data by regulatory authorities and market authorisation holders.
Coroners expressed concern in 33 reports that failures in education and training had contributed to death, and in a further 27 reports that absent or unsatisfactory protocols had contributed. Difficulties in communication (21 reports) and failure to adhere to pre-existing protocols (20 reports) also featured prominently among concerns. The failure to observe pre-existing protocols suggests that introducing new protocols will not always protect against future risk.
The coroners’ reports dealt with a range of drugs and medicines. The medicines involved were largely predictable, and few of the reported problems were novel. The most commonly represented were anticoagulants (22 reports), opioids (17 reports), and antidepressants (17 reports). Drugs of abuse excluding opioids were mentioned in 12 cases. Four of these cases, related to cannabis or synthetic cannabinoids, were among 11 deaths in prison or in police custody. The reports on deaths in custody suggest both that synthetic cannabinoids and other drugs of abuse are readily available in prison, and that there are dangers in the unsupervised medication of prisoners, who are able to hide drugs dispensed to themselves or acquired from others.
Coroners reported failures in the laws governing the misuse of drugs, and the reports have been followed by the introduction of the Psychoactive Substances Act 2016, which controls the production, supply and sale of substances intended for human consumption that are “capable of producing a psychoactive effect”. Parliament discussed both the availability of synthetic cannabinoids (‘legal highs’) in prison, and deaths from legal highs, but it is unclear whether the coroners’ reports influenced the legislators [9].
The influence of coroners’ reports was more certain in the case of the MHRA. The Agency’s bulletin ‘Drug Safety Update’ cited the coroners’ reports in four articles. One warned of the danger of prescribing citalopram or other selective serotonin reuptake inhibitors to patients who are known to abuse cocaine (Case 2015-0231; [10]). A second stated that hyoscine butylbromide posed a risk of serious adverse effects in patients with heart disease (Case-2016-0308; [11]). Drug Safety Update explicitly mentioned the coroner’s report of the angiotensin-converting enzyme–spironolactone interaction that led to fatal hyperkalaemia (Case 2015-0295; [12]). All three coroners’ reports had been addressed to the MHRA. In addition, the coroner’s report was referenced in a Drug Safety Update warning of the dangers of interaction between miconazole gel and warfarin (Case 2016-0096; [13]). A coroner reported a death by fire caused by a paraffin-containing emollient to the National Patient Safety Agency, which is now the National Reporting and Learning System (NRLS), part of NHS Improvement (Case 2015-0317). Drug Safety Update learnt of the incident from NRLS and reiterated earlier warnings of the fire hazard [14].
One reason why it is difficult to be certain whether other reports have led to action is the relatively rare publication of responses from addressees. The system in England and Wales requires those to whom the report is addressed to respond within 56 days, but the responses are not as a matter of course posted on the internet, and so it is not possible in general to see whether the report has reached the addressee, whether a response has been sent to the coroner, or whether any effective action has been formulated or taken. By contrast, and preferably, the Coronial Service of New Zealand issues 6-monthly summaries of coronial recommendations and responses to them. For example, a New Zealand coroner noted that constipation associated with clozapine treatment could lead to fatal bowel complications, and that Medsafe (the New Zealand medicines regulatory authority) had warned of this for more than a decade prior to the death under investigation, and recommended that the local health board take steps to ensure care-home staff looking after patients prescribed clozapine should be aware of clozapine best practice guidelines, and consider further training [15]. The district health board accepted the recommendations and would “look at how the organisation can strengthen responses in all of the areas noted”. Admittedly, while the response indicates receipt of the recommendations, neither the coroner nor the public can know in the absence of further information whether any action was taken on this occasion.
Coroners differ in the number, range and influence of those to whom they addressed reports. Most of the reports we examined were sent to Local NHS Trusts, NHS Community Commissioning Groups (CCGs) or Hospital Trusts. This limits the opportunities for wider learning from systemic problems that might come from addressing reports to national bodies. For example, a coroner, concerned that a there had been a failure “to address the risk of developing diabetes” during long-term use of olanzapine, addressed the report to the Trust concerned (Case 2015-0264). However, nearly 200,000 prescriptions for olanzapine are issued every month in English CCGs, and more in mental health trusts, so that the message is of wide general relevance. Many of the reports described failures or adverse effects of general relevance. They should be integrated into national systems, and it would be reassuring if healthcare organisations such as the NRLS received copies of these reports so that they could inform an integrated strategy to mitigate harms in healthcare.
Coroners are limited by the rule that they must express their concerns, but are not permitted to make recommendations as to how the concerns should be met. The careful enumeration of concerns by many coroners, but not all, implicitly invites respondents to deal with each concern.
4.1 Strengths and Limitations
We have only considered the UK coronial system. Differences in the legal and medical systems will influence the nature of coroners’ reports, but the principles apply to all countries where coroners, medical examiners or forensic pathologists perform similar roles. The study is also constrained by the working practices of coroners, whose varying level of vigilance to drug-related deaths, and thresholds for writing a PFD report, will have influenced the findings. The interests of coroners and the pharmacovigilance community are only partially aligned, and the reports may contain limited data.
Our study has the strength that a substantial number of coroners’ reports have been made since the obligation to issue PFDs was implemented.
5 Conclusions
This study is the first to demonstrate that coroners’ reports to prevent future deaths include valuable pharmacovigilance data. The reports of coroners to prevent future deaths have led to wider publicity for rare but potentially fatal adverse drug reactions, and to that extent they have been successful. The reports might be more effective if those that related to NHS bodies were addressed as a matter of course to a central authority; probably either the NRLS or the nascent Hospital Safety Investigation Branch, in addition to those persons and organisations currently sent reports. Unless replies are published as a matter of routine (and non-responders pursued), it will be difficult to judge whether responses have been reasonable, proportionate and effective. Future research should focus on increasing the utility and visibility to pharmacovigilance professionals of coroners’ work associated with drug safety issues, and examining if coroners’ reports have led to measurable improvements in patient safety.
References
Coroners and Justice Act 2009. c. 25. http://www.legislation.gov.uk/ukpga/2009/25/pdfs/ukpga_20090025_en.pdf. Accessed 15 Jul 2017.
Courts and Tribunal Judiciary. Reports to prevent future deaths. https://www.judiciary.gov.uk/related-offices-and-bodies/office-chief-coroner/pfd-reports/. Accessed 15 Jul 2017.
Coronial Services of New Zealand. https://coronialservices.justice.govt.nz/. Accessed 15 Jul 2017.
Coroners Court of Victoria. http://www.coronerscourt.vic.gov.au/. Accessed 15 Jul 2017.
State Coroner’s Court of New South Wales. http://www.coroners.justice.nsw.gov.au/. Accessed 15 Jul 2017.
Office of the Chief Coroner. Ontario Ministry of Community Services and Correctional Services. http://www.mcscs.jus.gov.on.ca/english/DeathInvestigations/office_coroner/coroner.html. Accessed 15 Jul 2017.
Ministry of Justice. Coroners statistics annual 2015 England and Wales, 12th May 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/525916/coroners-statistics-annual-2015-england-and-wales.pdf. Accessed 15 Jul 2017.
Ministry of Justice. Case 2015-0502. https://www.judiciary.gov.uk/publications/derek-thomas/. Accessed 15 Jul 2017.
Hansard. Psychoactive substances bill (HL), 23 June 2015. https://hansard.parliament.uk/Lords/2015-06-23/debates/15062355000827/PsychoactiveSubstancesBill(HL). Accessed 15 Jul 2017.
MHRA. Citalopram: suspected drug interaction with cocaine; prescribers should consider enquiring about illicit drug use. Drug Saf Update 2016;9 (12):2. https://www.gov.uk/drug-safety-update/citalopram-suspected-drug-interaction-with-cocaine-prescribers-should-consider-enquiring-about-illicit-drug-use. Accessed 15 Jul 2017.
MHRA. Hyoscine butylbromide (Buscopan) injection: risk of serious adverse effects in patients with underlying cardiac disease. Drug Saf Update 2017;10(7):1. https://www.gov.uk/drug-safety-update/hyoscine-butylbromide-buscopan-injection-risk-of-serious-adverse-effects-in-patients-with-underlying-cardiac-disease. Accessed 15 Jul 2017.
MHRA. Spironolactone and renin-angiotensin system drugs in heart failure: risk of potentially fatal hyperkalaemia. Drug Saf Update 2016;9(7):2. https://www.gov.uk/drug-safety-update/spironolactone-and-renin-angiotensin-system-drugs-in-heart-failure-risk-of-potentially-fatal-hyperkalaemia. Accessed 15 Jul 2017.
MHRA. Topical miconazole, including oral gel: reminder of potential for serious interactions with warfarin. Drug Saf Update 2016;9(11):3. https://www.gov.uk/drug-safety-update/topical-miconazole-including-oral-gel-reminder-of-potential-for-serious-interactions-with-warfarin. Accessed 15 Jul 2017.
MHRA. Smoking or a naked flame could cause patients’ dressings or clothing to catch fire when being treated with paraffin-based emollient that is in contact with the dressing or clothing. Drug Saf Update 2016;9(9):9. https://www.gov.uk/drug-safety-update/paraffin-based-skin-emollients-on-dressings-or-clothing-fire-risk. Accessed 15 Jul 2017.
New Zealand Coroners Court. Waetford. (2015) NZCorC 98. 30 Oct 2015. http://www.nzlii.org/cgi-in/sinodisp/nz/cases/NZCorC/2015/98.html?query=waetford. Accessed on 24 Jul 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
Robin E. Ferner has provided medicolegal reports for coroners and others; Craig Easton and Anthony R. Cox have no conflict of interest directly relevant to the content of this study.
Ethical approval
This study was an analysis of publicly available data. No approval was sought.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferner, R.E., Easton, C. & Cox, A.R. Deaths from Medicines: A Systematic Analysis of Coroners’ Reports to Prevent Future Deaths. Drug Saf 41, 103–110 (2018). https://doi.org/10.1007/s40264-017-0588-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-017-0588-0