Abstract
Introduction
Medicines cause over 1700 preventable deaths annually in England. Coroners’ Prevention of Future Death reports (PFDs) are produced in response to preventable deaths to facilitate change. The information in PFDs may help reduce medicine-related preventable deaths.
Objectives
We aimed to identify medicine-related deaths in coroners’ reports and to explore concerns to prevent future deaths.
Methods
We carried out a retrospective case series of PFDs across England and Wales, dated between 1 July, 2013 and 23 February, 2022, collected from the UK’s Courts and Tribunals Judiciary website using web scraping, generating an openly available database: https://preventabledeathstracker.net/. We used descriptive techniques and content analysis to assess the main outcome criteria: the proportion of PFDs in which coroners reported that a therapeutic medicine or drug of abuse had caused or contributed to a death; the characteristics of included PFDs; coroners’ concerns; the recipients of PFDs; and the timeliness of their responses.
Results
There were 704 PFDs (18%; 716 deaths) that involved medicines, representing an estimated 19,740 years of life lost (average of 50 years lost per death). Opioids (22%), antidepressants (9.7%), and hypnotics (9.2%) were the most common drugs involved. Coroners expressed 1249 concerns, primarily around the major themes of patient safety (29%) and communication (26%), including minor themes of failures of monitoring (10%) and poor communication between organizations (7.5%). Most expected responses to PFDs (51%; 630/1245) were not reported on the UK’s Courts and Tribunals Judiciary website.
Conclusions
One in five coroner-reported preventable deaths involved medicines. Addressing coroners’ concerns, including problems with patient safety and communication, should reduce harms from medicines. Despite concerns being raised repeatedly, half of the PFD recipients failed to respond, suggesting that lessons are not generally learned. The rich information in PFDs should be used to foster a learning environment in clinical practice that may help reduce preventable deaths.
Clinical Trial Registration
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
One in five preventable deaths identified by coroners in England and Wales in 2013–22 involved a medicine. |
Opioids were the most common medicines involved. |
Problems related to patient safety and communication were coroners’ primary concerns. |
1 Introduction
One in four (26%) individuals of the UK population receives a prescription for at least one medicine yearly [1]. The aging population and corresponding multi-morbidity [2] imply that the use of medicines is expected to increase further. In 2013, drugs introduced since 1981 were estimated to have saved 149 million years of life in 22 countries [3]. However, drugs are also associated with harms, which can result from medication errors, adverse drug reactions, and misuse. They have been implicated in 7–18% of hospital deaths in Spanish studies [4, 5], and avoidable adverse drug events contribute to over 1708 deaths annually in England [6]. Investigating and understanding the issues underlying medicine-related deaths may provide useful information for practitioners and policymakers and help improve patient safety.
In England and Wales, coroners are legally trained, independent judicial officers who investigate deaths that are violent, unnatural, or of unknown cause. Coroners seek to discover the identity of the deceased, and the time, place, cause, and circumstances of death. They carry out necessary enquiries, and take evidence from witnesses, who may include pathologists and toxicologists, to establish the facts on the balance of probabilities [7].
Since 1984, coroners in England and Wales have had a duty to report the details of deaths when the coroner believes that actions should be taken to prevent future deaths [7,8,9]. These reports, named Prevention of Future Deaths reports (PFDs), are mandated under UK law and require recipients to respond within 56 days. A study that examined a sample of PFDs between 24 April, 2015 and 7 September, 2016 showed that medicines contributed to 20% of deaths (99 PFDs of 500 included) deemed preventable by coroners in England and Wales [10]. Other studies of PFDs have provided insights into factors related to deaths related to specific medicine types, including anticoagulants and medicines purchased online [11, 12]. However, an analysis of all available PFDs has not been conducted to determine the overall contributions of medicines to preventable deaths. We therefore aimed to conduct a systematic case series of all available PFDs, to identify and characterize deaths involving medicines and to explore concerns to prevent future deaths.
2 Materials and Methods
2.1 Study Design and Data Sources
We designed a retrospective case series and pre-registered the study protocol on the Open Science Framework (OSF) [13]. Data were acquired from the Courts and Tribunals Judiciary website [14] using web scraping to populate a table for manual screening, as described elsewhere [11, 15]. The web scraper produced a database, called the Preventable Deaths Database (https://preventabledeathstracker.net/database/), which included 3897 PFDs as of 23 February, 2022.
2.2 Data Screening and Eligibility
We screened the 3897 PFDs for medicines using a pre-defined definition developed from previous work (see Electronic Supplementary Material [ESM]) and included PFDs if the coroner reported that a medicine caused or contributed to the death. If there were any discrepancies for inclusion, we consulted with expert clinical pharmacologists (JKA and REF). We excluded PFDs in which the sole agent was alcohol or tobacco, and cases in which a medicine or a component of the therapeutic process did not contribute to or cause death, or when only delays in assessment, investigation, or administration contributed to a death, despite the involvement of a medicine.
2.3 Data Extraction
After screening, data relating to the characteristics of the deceased, circumstances of the death, coroners’ concerns, and responses from recipients were extracted from included PFDs (see Table S1 in the ESM for a full list of fields). The web scraper automatically extracted some information from PFDs and presented it in the Preventable Deaths Tracker [16]. The types of medicines were classified by their generic names under British National Formulary guidance [17]. If a medicine was missing from the British National Formulary, classification was provided by the International Union of Basic and Clinical Pharmacology/British Pharmacological Society Guide to Pharmacology [18] or, failing that, the Misuse of Drugs Act 1971 [19].
2.4 Data Analysis
Frequencies were reported for categorical variables (sex, coroner’s area, type of medicine, source of medicine), and medians and interquartile ranges were calculated for continuous variables (age, latency from death, number of medicines contributing to death). We analyzed the sex distribution with a one-tailed binomial test. We estimated years of life lost (YLL) using the formula \(YLL=EC-\sum A\); where E = life expectancy (years; set using the Office of National Statistics average life expectancy for male individuals [79 years of age] and female individuals [82.9 years of age] [20]); C = the number of subjects with age below the life expectancy; and A = age of the subject at death, adapted from the World Health Organization’s formula [21], for male and female individuals independently and combined.
To identify geographical trends in reporting, we ranked coroners’ areas by deciles, based on the raw number of medicine-related PFDs produced and the proportion of all PFDs from each area that were medicine related, to reduce the influence of differences in coroners’ reporting trends. We used directed content analysis to collate and evaluate coroners’ concerns [22]. Before extraction, we developed a framework of themes of concerns derived from previous publications [10, 11, 23]. During the extraction process, this was used to code and categorize concerns into minor themes by the ideas contained within the reported concerns. If concerns failed to fit into predefined categories, we developed new themes. Minor themes were then grouped together into major themes through shared aspects.
To calculate response rates, we used the 56-day legal requirement to classify responses as “early” (> 7 days before the due date), “on-time” (≤ 7 days before or after the due date), “late” (> 7 days after the due date), or “overdue” (response not available on the Judiciary website as of 23 February). The response rates were grouped by the classes of individuals and organizations to whom PFDs were addressed. Recipient classes were determined through conventional content analysis, identifying common groups.
2.5 Software and Data Sharing
The study protocol was preregistered on the OSF [13] and all study materials, data, and code are openly available via GitHub [24] and the OSF [25]. We used SPSS (version 28) to conduct statistical analyses and Data Wrapper© to create all the figures.
2.6 Protocol Deviations
We did not report analyses on the duration of the inquest, setting of death, or “action should be taken” recommendations from the PFDs, as the information was often incomplete. We also increased the study’s duration and data availability, from 28 June, 2021 to 23 February, 2022.
2.7 Patient and Public Involvement
No patients were directly involved in this study. However, the public is continuously engaging and involved with the broader programme of research via the Preventable Deaths Tracker website: https://preventabledeathstracker.net/. The lead author (HSF) presented preliminary findings at the UK Clinical Pharmacology Colloquium (November 2021) and during World Smart Medication Day 2022 for the British Pharmacological Society and the International Union of Basic and Clinical Pharmacology poster competition (May 2022), which was subsequently published in Pharmacology Matters [26], and authors (HSF and GCR) presented some key findings at the EBM Live 2022 Conference for further international engagement (July 2022).
3 Results
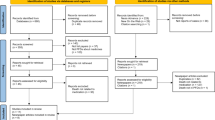
There were 704 medicine-related PFDs eligible for inclusion (18% of all PFDs), representing 716 individual deaths (Fig. 1). Of the 396 deaths that reported ages below life expectancy, 19,740 estimated years of life were lost, equating to an average of 50 years of life lost for every medicine-related preventable death (median age: 42 years, interquartile range 29–63 years, n = 450). Most deaths (68%, n = 480) involved male individuals (p < 0.01) and reporting varied geographically.
On the Judiciary website, PFDs are classified into report types based on the causes of death. For all medicine-related PFDs, only 28% (n = 197) were classified as “Alcohol, drug, and medication related deaths” and 3% (n = 22) were “Product related deaths”.
In 45% (n = 319) of medicine-related PFDs, coroners reported medical histories and information on social histories; in 40% of PFDs (n = 280), coroners reported mental health problems, the most common being depression (11%; n = 79). Previous drug abuse or addiction was mentioned in 23% of PFDs (n = 162) [Fig. S1 and Table S2A–C in the ESM]. In 20% of PFDs (n = 144), medication-related deaths were due to suicide.
Coroners in Manchester, Birmingham, and Inner London wrote the most medicine-related PFDs (Fig. S2 and Table S3 in the ESM). No PFDs were reported in 13 coroner areas: Carmarthenshire and Pembroke; Ceredigion; North Lincolnshire and Grimsby; North Northumberland; North Tyneside; Northwest Kent; Northwest Wales; North Yorkshire (Eastern and Western); Rutland and North Leicestershire; Sefton, Knowsley, St Helens; South Northumberland; and Sunderland.
3.1 Types of Medicines
There were 1172 medicines reported in the 704 PFDs; however, most (65%) reported one medicine (median 1; interquartile range 1–2, range 1–12; Table S4 in the ESM). Opioids (22%), antidepressants (9.7%), hypnotics (9.2%), and anticoagulants (7.3%) were the most common drugs involved in deaths (Fig. 2; Table S5 in the ESM). This was also the case for male and female deaths separately (Fig. S3 in the ESM).
In 93% (n = 656) of PFDs, coroners reported the source(s) of medicine(s), with 723 sources reported in all. Most medicines (63%, n = 454) were prescribed; other common sources included illicitly acquired medicines (28%, n = 202), over-the-counter medicines (5.0%, n = 36), ‘legal highs’ (2.4%, n = 17), and medicines prescribed for someone other than the deceased (1.5%, n = 11). Thirty-two PFDs (5.6%) involved substances obtained using the Internet.
3.2 Coroners’ Concerns
We identified 1249 concerns reported by coroners. We categorized the concerns into six major themes (Patient Safety; Communication; Education & Training; Resources; Regulations; and Failures to Carry Out Necessary Tasks/Protocols/Jobs) plus 44 minor themes. Failure to monitor/observe patients was the most common concern (10%, n = 133), followed by poor communication between organizations (7.5%, n = 99), unsafe protocols (7.3%, n = 96), and failure to keep accurate medical records/care plans (6.9%, n = 90) (Fig. 3).
Coroners’ concerns from 704 included medicines-related Prevention of Future Death reports in England and Wales published between July 2013 and 23 February 2022, grouped into major and minor themes using directed content analysis; the purple sixth (1) represents the failure to carry out necessary tasks/protocols/jobs and the pink sixth (2) represents regulation
3.3 Responses to PFDs
Coroners sent the 704 medicine-related PFDs to 1245 individuals and organizations, representing 564 unique recipients. The most common recipients were National Health Service England (6% of all reports; n = 77), followed by the Department of Health and Social Care (5%; n = 67) and the Medicines and Healthcare products Regulatory Agency (2%; n = 26) [Fig. S4 in the ESM]. Only 49% (n = 615) of recipients responded; of those, 25% (n = 152) were early, 52% (n = 314) on time, and 24% (n = 149) late (Fig. 4; Table S6 in the ESM). At the time of analysis, 630 responses (51%) remained overdue across 268 individual PFDs.
Proportions of responses to medicine-related Prevention of Future Death reports in England and Wales between July 2013 and 23 February 2022, using the 56-day legal requirement to classify responses as “early” (> 7 days before due date), “on-time” (≤ 7 days before or after the due date), “late” (> 7 days after the due date), or “overdue” (response was not available on the Judiciary website as of 23 February) and reporting results by organization type
4 Discussion
The 716 deaths involving medicines that were deemed preventable by coroners in England and Wales reported between July 2013 and 23 February 2022 amounted to 19,740 estimated years of life lost. Previous drug abuse was mentioned in 23% of PFDs, and suicides in 20%. One in five medicine-related PFDs involved opioids and one in ten involved antidepressants. Coroners in Manchester and Inner London wrote the most medicines-related PFDs, whereas many in Northeast England and West Wales wrote no PFDs. Coroners raised repeated concerns, often relating to patient safety and communication. Despite statutory obligations for recipients to respond within 56 days, about half of the legally required responses were overdue at the time of analysis.
4.1 Strengths and Limitations of the Study
Our study built on previous work [10], using a reproducible method to collect all available PFDs [15]. Nevertheless, this PFD population cannot represent all preventable deaths in England and Wales, as 153,008 deaths were deemed avoidable across Great Britain in 2020 [27] and 40% of UK deaths are reported to coroners, 14% progressing to inquest [28]. However, our findings identified repeated concerns that have national importance for preventing future deaths. Although not all lessons are internationally generalizable, similar systems exist in New Zealand [29], Australia [30, 31], and Canada [32]. Thus, our reproducible methods have applications across continents.
There are no quality-control mechanisms to examine the contents of PFDs, which creates heterogeneity and limits the application of our findings. We identified missing information across most variables, including age (missing in 36% of PFDs), types of medicines (missing in 7%), and dates of responses. Some missing information may depend on coroners’ reporting practices, which we controlled for where possible. For example, geographical variation changed after controlling for the total number of PFDs by area. We also found inconsistencies on the Judiciary website. For example, only 28% of medicine-related PFDs were categorized as “Alcohol, drug and medication related deaths”, which increases the time needed to manually screen PFDs for inclusion. The lack of guidance on when a PFD should be written may underlie these problems. Thus, guidelines may help ensure the utility of PFDs in preventing future deaths.
4.2 Comparison with Previous Literature
Compared with previous investigations of PFDs that found anticoagulants as the most common drugs involved in deaths [10, 11], we found that opioids were dominant. This difference can be attributed to the quality of our data collection methods, which allowed us to examine all available PFDs to which medicines contributed. Compared with previous studies [10, 11, 23, 33, 34], concerns reported by coroners were the same, except for those relating to patient safety, which has not been identified in PFDs before. However, in a study examining hospital incident reports, 18% of concerns were related to patient safety [35]. As in previous case series of PFDs, we found a dominance in male-related deaths [10, 33, 36, 37] and identified geographical variations in writing and responding to PFDs [11, 33, 37, 38]. Although responses to PFDs are required by law, research continually highlights low response rates and the need for reform [11, 34, 37, 39] so that PFDs can stimulate significant widespread healthcare changes.
4.3 Interpretation and Implications for Clinicians and Policymakers
Our research identifies over 1200 concerns reported by coroners that if not addressed will cause further harms and repeat preventable deaths. The concerns we have identified provide lessons for clinical practice, including the need for better monitoring and observation of at-risk patients in hospitals as well as regular medication reviews in the community. Extra considerations are needed to reduce omissions in record keeping and miscommunications between organizations, particularly when discharging or reviewing patients receiving medications. We also discovered a need for enhanced training and education for those caring for patients taking medicines, especially when controlled substances such as opioids are involved. Coroners also acknowledged a lack of resources that led to understaffing, equipment requirements, and the need for policies and guidelines to help with decision making in complex scenarios involving medicines. However, our study did not explore the contents of responses; thus, whether actions have been taken to address such concerns is largely unknown.
Our study reveals critical problems with the PFD system. Despite the legal requirement to respond to PFDs, there is no process for enforcing and auditing responses to ensure that necessary actions are being taken to prevent similar deaths. Others have suggested the need for sanctions to improve response rates and enforce Regulation 29 of The Coroners (Investigations) Regulations 2013 [9, 37]. However, whether such sanctions would lead to increased actions for reducing preventable deaths has not been tested. In addition, the repeated concerns we identified have national importance for patient safety, which would benefit from creating an environment that encourages lessons to be shared and learnt. Building such an environment could be established using a quarterly summary of coroners’ reports, targeting key stakeholders, including policymakers, clinicians, and the public [39]. Sharing concerns broadly may help disseminate lessons and allow locally relevant PFDs to have national benefits, facilitating changes by local organizations in response to other tragedies, rather than just reactions to their own. This may also require alterations to the contents and recipients of PFDs to facilitate the learning of lessons elsewhere, such that they are also designed to provide general information to third parties.
Our research, alongside other studies [10, 11, 23, 33, 38,39,40,41,42,43], highlights that PFDs contain a wealth of important information that has merit for wide dissemination. However, crucial information in PFDs is often missing, incorrect, or inconsistent. Reporting of information could be improved using technologies that replace the current narrative system with an electronic form, providing specific fields to populate. This would reduce the time spent manually extracting data for research and improve the ability of the Preventable Deaths Tracker to analyze thousands of PFDs to generate summaries for dissemination. However, further action may need to be taken to address the established geographical imbalance in the reporting of PFDs, which is thought to result from differences in coroners’ reporting practices and differences in the interpretation of guidance [36].
Future research should explore the 1245 responses received from medicine-related PFDs to identify previously taken actions that could be shared across healthcare settings. Examining such responses would also identify gaps that have not yet been addressed. With this knowledge, future research could assess the impact and value of PFDs in changing clinical practice, which remain debated [10, 11, 23, 33, 38, 39].
5 Conclusions
One in five PFDs involved medicines and highlighted many areas of concern for patient care. Patients may benefit from changes to clinical practice surrounding medicines such as opioids and antidepressants, especially when related to problems with monitoring, record keeping, and communication. Patients and clinicians may also benefit from improvements to the PFD system, better reporting, and resolution of concerning factors by organizations having the potential to reduce medicine-related deaths across England and Wales.
References
Taylor S, Annand F, Burkinshaw P, Greaves F, Kelleher M, Knight J, et al. Dependence and withdrawal associated with some prescribed medicines: an evidence review. London: Public Health England; 2019.
Kingston A, Robinson L, Booth H, Knapp M, Jagger C, Adelaja B, et al. Projections of multi-morbidity in the older population in England to 2035: estimates from the Population Ageing and Care Simulation (PACSim) model. Age Ageing. 2018;47:374–80. https://doi.org/10.1093/ageing/afx201.
Lichtenberg FR. How many life-years have new drugs saved? A three-way fixed-effects analysis of 66 diseases in 27 countries, 2000–2013. Int Health. 2019;11:403–16. https://doi.org/10.1093/inthealth/ihz003.
Montané E, Arellano AL, Sanz Y, Roca J, Farré M. Drug-related deaths in hospital inpatients: a retrospective cohort study. Br J Clin Pharmacol. 2018;84:542–52. https://doi.org/10.1111/bcp.13471.
Pardo Cabello AJ, del Pozo GE, Gómez Jiménez FJ, Mota Rodríguez C, Luna Del Castillo JDD, Puche CE. Drug-related mortality among inpatients: a retrospective observational study. Eur J Clin Pharmacol. 2016;72:731–6. https://doi.org/10.1007/s00228-016-2026-0.
Elliott RA, Camacho E, Jankovic D, Sculpher MJ, Faria R. Economic analysis of the prevalence and clinical and economic burden of medication error in England. BMJ Qual Saf. 2020;30:96–105. https://doi.org/10.1136/bmjqs-2019-010206.
Coroners and Justice Act 2009. Statute Law Database; 2009.
The Coroners Rules 1984. 552 United Kingdom. 1 July, 1984. https://www.legislation.gov.uk/uksi/1984/552/article/6/made. Accessed 28 Jan 2023.
The Coroners (Investigations) Regulations 2013. Queen’s Printer of Acts of Parliament; 2013.
Ferner RE, Easton C, Cox AR. Deaths from medicines: a systematic analysis of coroners’ reports to prevent future deaths. Drug Saf. 2018;41:103–10. https://doi.org/10.1007/s40264-017-0588-0.
Anis A, Heneghan C, Aronson JK, DeVito NJ, Richards GC. Deaths from cardiovascular disease involving anticoagulants: a systematic synthesis of coroners’ case reports. BJGP Open. 2022;6(1):BJGPO.2021.0150.
Aronson JK, Ferner RE, Richards GC. Deaths attributed to the use of medications purchased online. BMJ Evid Based Med. 2022;27:60–4. https://doi.org/10.1136/bmjebm-2021-111759.
France HS, Richards GC, Aronson JK, Ferner R, Cox AR, Heneghan C. Pre-registration of Preventable deaths involving medicines and misused drugs: a systematic analysis of coroners’ case reports in England and Wales 2019–2021. OSF; 2021. https://doi.org/10.17605/OSF.IO/TX3CS. Accessed 11 Sep 2022.
Prevention of Future Deaths | Subjects | Courts and Tribunals Judiciary. 2022. https://www.judiciary.uk/subject/prevention-of-future-deaths/. Accessed 2 Feb 2022.
DeVito NJ, Richards GC, Inglesby P. How we learnt to stop worrying and love web scraping. Nature. 2020;585:621–2. https://doi.org/10.1038/d41586-020-02558-0.
Richards GC. Preventable deaths tracker. https://preventabledeathstracker.net/the-database/. Accessed 28 Jun 2021.
Joint Formulary Committee. British National Formulary. 82nd ed. BMJ Group, editor. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2021.
IUPHAR. IUPHAR/BPS guide to pharmacology. 2021. https://www.guidetopharmacology.org/. Accessed 28 Sep 2021.
Misuse of Drugs Act 1971. Statute Law Database; 1971.
Buxton J. Life expectancy in the UK: 2018 to 2020. Office for National Statistics. 2021.
World Health Organization. Years of life lost (YLL) (per 100 000 population). Global Health Observatory. 2006. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4427. Accessed 25 Nov 2021.
Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88. https://doi.org/10.1177/1049732305276687.
Leary A, Bushe D, Oldman C, Lawler J, Punshon G. A thematic analysis of the prevention of future deaths reports in healthcare from HM coroners in England and Wales 2016–2019. J Patient Saf Risk Manag. 2021;26:14–21. https://doi.org/10.1177/2516043521992651.
Richards GC. Deaths from cardiovascular disease involving anticoagulants. Github; 2021.
France HS, Richards GC. OSF: preventable deaths involving medicines and misused drugs: a systematic analysis of coroners’ case reports in England and Wales 2013-2021. OSF; 2021. https://osf.io/wq7g5/. Accessed 15 Feb 2022.
France H. Pandemics, pharmacology, and preventable deaths. London: Pharmacology Matters, British Pharmacological Society; 2022.
Semple L, Gamblin N. Avoidable mortality in Great Britain: 2020. 2022.
Walters S. Coroners statistics 2019: England and Wales. 2019. https://www.gov.uk/government/statistics/coroners-statistics-2019/coroners-statistics-2019-england-and-wales. Accessed 28 Jan 2023.
Coronial Services of New Zealand. Coronial Services of New Zealand Regulations. 2022. https://coronialservices.justice.govt.nz/coronial-services/. Accessed 3 Feb 2022.
Coroners Court of Victoria. Coroners Court of Victoria Regulations. https://www.coronerscourt.vic.gov.au/. Accessed 3 Feb 2022.
State Coroner’s Court of New South Wales. Coroners Court of New South Wales Regulations. 2022. https://www.coroners.nsw.gov.au/. Accessed 3 Feb 2022.
Office of the Chief Coroner. Ontario Ministry of Community Services and Correctional Services. Coroners Act 1990. 37 Canada. 19 April, 1990. https://www.ontario.ca/laws/statute/90c37. Accessed 28 Jan 2023.
Swift B, Heneghan C, Aronson J, Howard D, Richards GC. Preventable deaths from SARS-CoV-2 in England and Wales: a systematic case series of coroners’ reports during the COVID-19 pandemic. BMJ Evid Based Med. 2022;27(5):296–304. https://doi.org/10.1136/bmjebm-2021-111834.
Ferner RE, Ahmad T, Babatunde Z, Cox AR. Preventing future deaths from medicines: responses to coroners’ concerns in England and Wales. Drug Saf. 2019;42:445–51. https://doi.org/10.1007/s40264-018-0738-z.
Aaronson EL, Brown D, Benzer T, Natsui S, Mort E. Incident reporting in emergency medicine: a thematic analysis of events. J Patient Saf. 2019;15:E60–3. https://doi.org/10.1097/PTS.0000000000000399.
Mclean M, Roach J, Armitage R. Local variations in reporting deaths to the coroner in England and Wales: a postcode lottery? J Clin Pathol. 2013;66:933–6. https://doi.org/10.1136/jclinpath-2013-201640.
Fox AW, Jacobson J. How well do Regulation 28 reports serve future public health and safety? Med Sci Law. 2021;61:186–92. https://doi.org/10.1177/0025802420987431.
King R, Benbow EW. Are the recommendations in coronial prevention of future death (PFD) reports realistic and achievable? Med Leg J. 2022;90:27–31. https://doi.org/10.1177/00258172211059905.
Richards GC, Aronson JK, Heneghan C. Coroners’ concerns to prevent harms: a series of coroners’ case reports to serve patient safety and educate the public, clinicians and policy-makers. BMJ Evid Based Med. 2021;26:37–8. https://doi.org/10.1136/bmjebm-2020-111567.
Richards GC, Anthony G, Aronson JK, Heneghan C, Goldacre B. Preventable suicides: a systematic analysis of coroners’ prevent future death reports in England and Wales. OSF; 2020. https://osf.io/ad4up/. Accessed 14 Jun 2021.
Richards GC, Aronson JK, Ferner RE, Cox AR, Heneghan C, Goldacre B, et al. Preventable opioid-related deaths: a systematic analysis of coroners’ reports. OSF; 2019. https://osf.io/ecz4r/. Accessed 14 Jun 2021.
Karolina Bilip M, Richards GC. Emollients and smoking: a fire hazard that could be prevented to reduce future deaths. BMJ Evid Based Med. 2021;26:131–4. https://doi.org/10.1136/bmjebm-2020-111648.
Richards GC. Alcohol-based hand sanitisers: a warning to mitigate future poisonings and deaths. BMJ Evid Based Med. 2021;26:65–8. https://doi.org/10.1136/bmjebm-2020-111568.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was obtained for this study. An Engagement and Dissemination grant (2020) and Seedcorn funding (2021) was obtained from the National Institute of Health Research (NIHR) School for Primary Care Research to develop the Preventable Deaths Tracker website: https://preventabledeathstracker.net/.
Conflicts of Interest/Competing Interests
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare no support from any organization for the submitted work. HSF has received scholarships (2020–22) from Brasenose College, University of Oxford and Fidelity National Information Services for undergraduate study, from the British Pharmacological Society (2022) for meritorious performance in a research competition and received payments (2022) from Brasenose College, University of Oxford for undergraduate teaching. JKA has published papers in bioscience journals and edited textbooks on adverse drug reactions; he has often acted as an expert witness in civil actions relating to suspected adverse drug reactions and in coroners’ courts. CH holds grant funding from the NIHR, the NIHR School of Primary Care Research. CH has received expenses and fees for his media work, for teaching EBM and is also paid for his GP work in the National Health Service out of hours (contract Oxford Health National Health Service Foundation Trust). CH is the Director of the Centre for Evidence-based Medicine. REF has undertaken research and published on adverse drug reactions and medication errors. REF has acted as an expert witness in coronial and other legal cases related to these. ARC holds grant funding from Cancer Alliance. ARC has received fees for media work, a scientific advisory committee at IQVIA, and external examinations at UK Universities. ARC is the Head of the School of Pharmacy at the University of Birmingham and is an honorary pharmacovigilance pharmacist at the West Midlands Centre for Adverse Drug Reaction Reporting. GCR is the director of a limited company that is independently contracted to work as an epidemiologist and teach at the University of Oxford. GCR received scholarships (2017–20) from the National Health Service NIHR School for Primary Care Research, the Naji Foundation, and the Rotary Foundation to study for a DPhil at the University of Oxford. The study guarantor affirms that the article is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Ethics Approval
This study exclusively uses publicly available information, for which ethics committee approval is not required.
Consent to Participate
Patient consent was not sought for this study, which exclusively used publicly available information regarding deceased individuals.
Consent for Publication
Not applicable.
Availability of Data and Material
All study materials, data, and statistical code are openly available via online repositories. The study protocol was preregistered on the OSF (https://doi.org/10.17605/OSF.IO/TX3CS); the code to generate the database and the Preventable Deaths Tracker is openly available via GitHub (https://github.com/georgiarichards/georgiarichards.github.io); individual Prevention of Future Deaths reports are available on the Courts and Tribunals Judiciary website (https://www.judiciary.uk/prevention-of-future-death-reports/); and all other study materials are openly available via the OSF project page (https://doi.org/10.17605/OSF.IO/WQ7G5).
Code Availability
The code to generate the database and the Preventable Deaths Tracker is openly available via GitHub (https://github.com/georgiarichards/georgiarichards.github.io).
Author contributions
HSF updated the study protocol, carried out screening of PFDs, extracted remaining data, conducted all analyses, produced all figures and tables, and wrote the first draft of the manuscript. GCR conceptualized, designed, and initiated the study; provided supervisory support, and edited the first draft of the manuscript. REF and ARC contributed to the study conceptualization. CH and JKA provided supervisory support and oversight. REF and JKA provided clinical pharmacological advice and interpretation. All study authors read, contributed to, and approved the final manuscript. HSF is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Additional information
Preprint uploaded to MedRxiv. Available at: https://doi.org/10.1101/2022.11.01.22281803.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
France, H.S., Aronson, J.K., Heneghan, C. et al. Preventable Deaths Involving Medicines: A Systematic Case Series of Coroners’ Reports 2013–22. Drug Saf 46, 335–342 (2023). https://doi.org/10.1007/s40264-023-01274-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01274-8