Abstract
Introduction
Although hyperlipidemia is a well known adverse event of atypical antipsychotic (AAP) medication, there are few studies that have quantitatively compared the risks of various AAPs.
Objective
Our aim was to comparatively evaluate the risk of hyperlipidemia associated with the use of AAPs approved in Japan through a consecutive epidemiological study.
Methods
We conducted a sequence symmetry analysis (SSA) using health insurance claims data to analyze the following nine AAPs approved for use in Japan: risperidone, paliperidone, perospirone hydrochloride hydrate, blonanserin, clozapine, olanzapine, quetiapine fumarate, aripiprazole, and zotepine. Exposed cases were identified from drug dispensing records as those who had been administered both AAPs and antihyperlipidemic drugs. The adjusted sequence ratio (ASR) and 95 % confidence interval (CI) for each individual AAP and for all AAPs were calculated while controlling for time trends in dispensing patterns.
Results
Olanzapine was significantly associated with increased hyperlipidemia occurrence (ASR 1.56; 95 % CI 1.25–1.95). The ASRs obtained for risperidone (1.01; 95 % CI 0.80–1.27), perospirone hydrochloride hydrate (0.93; 95 % CI 0.63–1.39), blonanserin (0.83; 95 % CI 0.52–1.33), quetiapine fumarate (0.93; 95 % CI 0.73–1.18), and aripiprazole (1.02; 95 % CI 0.82–1.26) were approximately 1.0. Unstable estimates (wide CIs) were obtained for paliperidone and zotepine due to the small sample sizes.
Conclusions
Among the AAPs used in Japan, only olanzapine was found to have an elevated risk of hyperlipidemia. In contrast, risperidone, perospirone hydrochloride hydrate, blonanserin, quetiapine fumarate, and aripiprazole had relatively low risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Olanzapine initiation is associated with a higher incidence of hyperlipidemia among the atypical antipsychotics approved in Japan. |
Risperidone, perospirone hydrochloride hydrate, blonanserin, quetiapine fumarate and aripiprazole have relatively low risk for hyperlipidemia. |
1 Introduction
Atypical antipsychotics (AAPs) are second-generation antipsychotic drugs primarily used for the treatment of schizophrenia, mood disorders, and other psychiatric disorders. Several studies have reported associations between the use of some AAPs and an increased risk of metabolic disturbances, including weight gain, type 2 diabetes mellitus, and hyperlipidemia [1–4]. Hyperlipidemia is a metabolic disorder characterized by elevated serum cholesterol or triglyceride levels. If left unmanaged, this disorder can lead to atherosclerosis and atherosclerosis-induced conditions such as coronary heart disease, ischemic cerebrovascular disease, and peripheral vascular disease [5].
While the pathogenesis of AAP-induced hyperlipidemia remains to be fully elucidated, the mechanism may involve a direct effect on triglyceride metabolism and an indirect effect on both the central nervous system and adipose tissue, possibly leading to insulin resistance and obesity [1]. Previous studies have suggested that the risk of hyperlipidemia may vary among different AAPs [1, 6]; however, there are few studies that have simultaneously compared the risks of different AAPs using a quantitative epidemiological approach [4, 7].
The AAPs currently approved for use in Japan are risperidone, paliperidone, perospirone hydrochloride hydrate, blonanserin, clozapine, olanzapine, quetiapine fumarate, aripiprazole, and zotepine. In this study, we performed a sequence symmetry analysis (SSA), which is a self-controlled study design to examine asymmetry in the distribution of an outcome before and after an exposure of interest [8], using Japanese health insurance claims data to comparatively evaluate the risk of hyperlipidemia associated with these AAPs.
2 Methods
2.1 Data Source
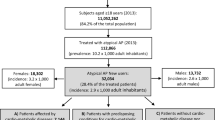
This study was conducted using a commercial database obtained from the Japan Medical Data Center Co. Ltd (Tokyo, Japan). We analyzed monthly administrative claims data between January 2005 and December 2013, which covered approximately 2 million patients. These patients accounted for about 1.5 % of the entire population of Japan. The population in the database consisted of employed workers and their family members belonging to one of 32 health insurance societies operated mainly by large private firms. These insurance societies store detailed information on covered medical services provided to enrollees until they are no longer enrolled.
The database included information on patient’s characteristics (encrypted personal identifiers, age, and sex), prescribed or dispensed medications, and procedures and diagnoses. Medications were coded according to National Health Insurance Drug Codes provided by Japan’s Ministry of Health, Labour and Welfare. Diagnoses were coded according to International Classification of Diseases, 10th revision (ICD-10) codes.
The claims database used in this study included both drug prescription and dispensing data. Dispensing data contain information on specific dispensing dates, but as these data are obtained from pharmacies outside of hospitals, they do not include information on associated inpatient care. Despite this, we used only dispensing data for this study due to the lack of information on dispensing dates in prescription data prior to March 2012.
2.2 Sequence Symmetry Analysis
SSA, a self-controlled study design which was originally proposed by Hallas as a “prescription sequence symmetry analysis” [8], was used in this study. Causal associations between exposure and outcomes were assessed based on asymmetrical distributions in which a disproportionate number of patients experience post-exposure outcomes. In an SSA study, the adjusted sequence ratio (ASR) is estimated as a measure of effect; the ASR can be similar to the incidence rate ratio in exposed to non-exposed person-time if both the exposures and outcomes are uniformly distributed over the observation period [8]. If confounders such as sex, smoking habits, drinking habits, body mass index, genetic factors, and medical history do not vary substantially throughout the observation period, their confounding effects would be minimized in SSA as these factors would not unduly affect the assumption of symmetry. SSA, therefore, can adjust the effect of time-independent confounders even if their information was not obtained from the claims databases used in this study.
2.3 Study Population
The study population for the SSA comprised ‘exposed cases’, referring to patients who had been dispensed any of the target AAPs in addition to any antihyperlipidemic drug during the observation period. We defined the observation start date for each patient as the first day of the month when the health insurance society started providing their data to the database; the 15th day of the month when the patient became a member of the health insurance society; the first day of the month in which the AAP of interest was initially introduced into the Japanese market; or January 1, 2005, whichever was later. The observation end date for each patient was defined as the last day of the month when the health insurance society ceased to provide their data; the 15th day of the month in which the patient dropped out from the health insurance society; or December 31, 2013, whichever was earlier. Since the database vender had obtained claim data from health insurance societies by monthly contract, information on the actual day that the health insurance society started (or ceased) providing patient’s data was not included in the database. On the other hand, they deleted the exact date of when they became (or dropped out from being) a member of the health insurance society from the database because of ethical considerations.
Aripiprazole, blonanserin and paliperidone were introduced into the Japanese market in June 2006, April 2008, and January 2011, respectively; the other AAPs have been available since January 2005. To exclude prevalent users of these drugs, patients who had dispensing records for any of the target AAPs or any antihyperlipidemic drug during the first 3 months of their observation periods were excluded from analysis. We imposed a gap of at least 1 day between the initiation of AAP and antihyperlipidemic drug to evaluate the sequence symmetry and excluded patients who had been dispensed their initial target AAP and initial antihyperlipidemic drug on the same day (gap of 0 days), since the possibility that the hyperlipidemia onset occurred before the usage of AAPs could not be denied.
2.4 Definitions of Exposure and Outcome
We identified patients who had been dispensed the following AAPs: risperidone, paliperidone, perospirone hydrochloride hydrate, blonanserin, clozapine, olanzapine, quetiapine fumarate, aripiprazole, and zotepine. The initial dispensing day of an AAP during each patient’s observation period was designated the date of exposure for that AAP.
The outcome of interest was hyperlipidemia occurrence, which was identified as the dispensing of any antihyperlipidemic drug during the observation period. Antihyperlipidemic drugs included statins, fibrates, ezetimibe, niacin, bile acid sequestrants, probucol, phytosterols, dextran sulfate sodium sulphur, polyene phosphatidylcholine, elastase, and eicosapentaenoic acid. The initial dispensing day of any antihyperlipidemic drug during each patient’s observation period was designated the date of outcome occurrence.
2.5 Estimation of the Sequence Ratio for Each AAP
The association between each AAP and hyperlipidemia occurrence was examined using SSA. Crude sequence ratios (CSRs) were calculated by dividing the number of patients who had post-AAP hyperlipidemia (i.e., AAP → antihyperlipidemic drug) by the number of patients who had pre-AAP hyperlipidemia (i.e., antihyperlipidemic drug → AAP). To adjust for trends in medication use for either AAPs or antihyperlipidemic drugs over the study period, a null-effect sequence ratio (NSR) was calculated according to the method described in the original proposal for the SSA [8]. For the calculation of NSR, we used information from the patients with dispensing records for either an AAP or any antihyperlipidemic drug in each observation period. Finally, ASR was derived by dividing CSR by NSR, and the 95 % confidence intervals (CI) were calculated based on binominal distributions. When we estimated the ASR of an AAP in patients who had been administered multiple AAPs, the other AAPs were ignored for that calculation.
It has been reported that SSA approaches may lead to false risk estimates if they are used to assess exposure–outcome associations under long time intervals [9, 10]. Therefore, we also conducted sensitivity analyses that restricted the samples to patients for whom the intervals between AAP and antihyperlipidemic drug initiation were less than 90, 180, or 360 days. Since older age is a risk factor for the onset of metabolic syndrome in schizophrenia patients [11], we conducted additional sensitivity analyses for the patients by age group (aged under or over 40 years). To examine effects of olanzapine dosage, we also conducted an additional analysis in which the olanzapine-exposed cases were divided into two groups based on administrated dose (under or more than 5 mg/day of olanzapine at initiation).
2.6 Evaluation of Class Effects of AAP for Hyperlipidemia
We also estimated the ASR for the use of any AAP within the study population to examine the possible class effects of AAP on hyperlipidemia. In addition, because olanzapine was thought to be associated with a higher risk of hyperlipidemia than the other AAPs [3], we identified patients from among the exposed cases who were not prescribed olanzapine throughout the observation period; these patients were designated ‘olanzapine non-users’. The ASR and 95 % CI were calculated for all exposed cases and olanzapine non-users using the methods described above.
All statistical analyses were conducted using SAS 9.3 software (SAS Institute, Cary, NC).
3 Results
3.1 Study Population
Table 1 shows that the characteristics of the study population that had dispensing records for any AAP and any antihyperlipidemic drug after application of the inclusion criteria. The study population was fairly evenly distributed with regard to sex, and consisted mainly of patients aged 20–69 years (approximately 95 % of the sample). The mean and median ages of the study population could not be calculated because the age of patients older than 70 was designated simply as ‘over 70’ in our database.
3.2 Estimation of the Sequence Ratio for Each AAP
According to the results of the SSA (Table 2), olanzapine exposure was significantly associated with increased hyperlipidemia occurrence (ASR 1.56; 95 % CI 1.25–1.95). After restricting the intervals between exposures and outcomes, ASR was observed to increase. The point estimates of ASR obtained for risperidone (1.01; 95 % CI 0.80–1.27), perospirone hydrochloride hydrate (0.93; 95 % CI 0.63–1.39), blonanserin (0.83; 95 % CI 0.52–1.33), quetiapine fumarate (0.93; 95 % CI 0.73–1.18) and aripiprazole (1.02; 95 % CI 0.82–1.26) were approximately 1.0, and their 95 % CI included null values. Even by restricting the intervals between exposures and outcomes, the ASRs for these AAPs were not statistically significant. Wider CIs were obtained from the SSA for paliperidone (ASR 0.46; 95 % CI 0.12–1.53) and zotepine (ASR 0.73; 95 % CI 0.32–1.65) due to smaller sample sizes. Because there was only one exposed case of clozapine in the database, the ASR could not be determined for this AAP. The ASRs for olanzapine in the patients aged over 40 years was relatively high compared with that in the patient aged under 40 years except for the analysis with no restriction of the intervals between exposures and outcomes (Table 3). The ASRs for other AAPs were not different in the patients of both age groups (data not shown). As a result of the additional analysis in which the olanzapine users were divided based on the initial dosage of olanzapine, ASRs with no restriction of the intervals between exposure and outcome were 1.63 (95 % CI 1.27–2.09) and 1.30 (95 % CI 0.76–2.26) in the under 5 mg/day group and over 5 mg/day group, respectively.
Figure 1 shows the distributions of the frequency of antihyperlipidemic drug use before and after initial administration of each AAP (except for clozapine). The results indicated that more patients experienced hyperlipidemia after olanzapine exposure than before (Fig. 1e), whereas asymmetrical distributions were not clearly apparent for the other AAPs.
Distributions of the frequency of antihyperlipidemic drug initiation before and after the initial administration of a risperidone, b paliperidone, c perospirone hydrochloride hydrate, d blonanserin, e olanzapine, f quetiapine fumarate, g aripiprazole, and h zotepine. Patients who had hyperlipidemia after atypical antipsychotic (AAP) exposure (AAP → antihyperlipidemic drug) are distributed on the right half of each histogram, whereas patients who had hyperlipidemia before AAP exposure (antihyperlipidemic drug → AAP) are distributed on the left half. The asymmetrical distribution observed in e indicated that antihyperlipidemic drugs were dispensed more often after olanzapine exposure. There were no asymmetrical distributions observed for the other AAPs (a–d and f–h)
3.3 Evaluation of Class Effects of AAP for Hyperlipidemia
As shown in Table 4, the initiation of any AAP was significantly associated with hyperlipidemia occurrence in all exposed cases (ASR 1.29; 95 % CI 1.12–1.50). After restricting the interval between exposures and outcomes, the ASRs in both groups (all exposed cases and olanzapine non-users) became relatively higher. However, the ASR for olanzapine non-users was approximately 1.0, and the 95 % CI included the null value (1.01; 95 % CI 0.84–1.22).
4 Discussion
4.1 The Risk of Hyperlipidemia in Olanzapine Users
This study indicated that the risk of hyperlipidemia occurrence after olanzapine exposure was approximately 1.6 times higher than in the pre-exposure period. The association was robust even after restricting the intervals between exposures and outcomes.
Several studies have reported that patients using olanzapine occasionally experience hypertriglyceridemia, hypercholesterolemia, and elevation of serum low-density lipoprotein with or without weight gain [3, 12, 13]. Two nested case–control studies by Koro et al. [4] and Olfson et al. [7] indicated that olanzapine use was associated with significantly increased odds of developing hyperlipidemia when compared with no exposure (the odds ratios were 4.65 and 1.56 in the two studies, respectively). Olanzapine may cause hyperlipidemia through several mechanisms, such as increasing triglyceride and cholesterol synthesis in hepatocytes, upregulating the expression of several genes involved in hepatic lipid biosynthesis, increasing adipose mass, and causing inflammation in adipose tissue [14–18]. A randomized controlled trial that assessed effectiveness of AAPs indicated that 30 % of the patients allocated to the olanzapine group had developed weight gain equivalent to 7 % of initial body weight and that the risk for weight gain in the olanzapine group was higher than that in the risperidone and quetiapine fumarate groups [19]. Similarly, 21.2 % of Japanese patients with bipolar depression developed more than 7 % weight gain [20]. Thus, hyperlipidemia induced by olanzapine in this study might be associated with weight gain, but such an association could not be examined due to a lack of information regarding body weight of patients in the claims database.
In the sensitivity analysis for olanzapine users, ASR was higher in case of the shorter interval between exposures and outcomes (Table 2). Actually, ASR was found to increase to approximately 3.3 in the interval within 90 days. Although the peak time period after olanzapine initiation of onset of hyperlipidemia is still unclear [3], our result might indicate that the hyperlipidemia associated with olanzapine medication was most likely to occur within 3 months after treatment start.
Although there was the tendency that the risk of hyperlipidemia onset in olanzapine users aged over 40 years was higher than that in users aged under 40 years, these CIs of ASR overlapped each other (Table 3). The risk factors for hyperlipidemia associated with AAPs were still poorly understood whereas older age and illness duration of mental disorder were significant predictors for metabolic syndrome in patients with schizophrenia [11]. Our results indicate that the risk for hyperlipidemia associated with olanzapine was not too different by age group.
Apparent association between hyperlipidemic risk and olanzapine initial dosage was not observed based on the additional analysis in which the olanzapine users were divided based on the initial dosage of olanzapine in our study. However, since the SSA design can assess only sequence symmetry of the initiation of AAP and antihyperlipidemic drugs, effects of dosage of AAPs was difficult to consider. To confirm the effects of olanzapine dosage on the risk of hyperlipidemia, further studies that adopt another epidemiological design that can treat the effects of longitudinal dosage change of olanzapine appropriately, such as cohort or case–control design, would be needed.
The other AAPs (excluding olanzapine) were not significantly associated with hyperlipidemia (Table 2). Although the lower limit of the ASR’s 95 % CI for any AAP in all exposed cases was higher than 1.0, the 95 % CI range included the null value of 1.0 in patients who were not administered olanzapine (Table 4). Therefore, the elevated risk of hyperlipidemia was not likely to be a class effect of the AAPs, but instead due to the exposure of olanzapine. This result is consistent with the findings of previous studies that indicated a higher risk of hyperlipidemia for olanzapine than other AAPs, such as risperidone, quetiapine fumarate, and aripiprazole [4, 7, 12, 21].
4.2 Comparison of Our Findings with Other Studies
Contrary to our findings, a nested case–control study conducted by Olfson et al. [7] suggested that both risperidone and quetiapine fumarate have risks for hyperlipidemia that are comparable to that of olanzapine. This discrepancy may be partly explained by the fact that the confounders generally associated with hyperlipidemia were not included in that nested case–control study, except for the matching factors of age, sex, race, and psychiatric diagnosis [7]. In this nested case–control study, the risk of AAP exposure was compared with that of AAP non-exposure without adjustment for the effect of other confounders, although the AAP users could have a different risk profile for hyperlipidemia compared with AAPs non-users. Alternatively, ethnic differences in the risk of hyperlipidemia may also contribute to these findings [22], and the ethnicity of our study population is different from those of previous studies. Since SSA design can control for time-independent confounders by using the patients as their own controls, our results demonstrated that olanzapine had a consistently higher risk of hyperlipidemia occurrence among the AAPs approved in Japan.
Previous studies have indicated that clozapine may also have a higher risk of hyperlipidemia occurrence [7, 12, 23]. However, the hyperlipidemic risk of clozapine could not be assessed in the present study because there was only one patient in the database with dispensing records for both clozapine and antihyperlipidemic drugs. As a result of strict regulation by the ‘Clozaril Patient Monitoring System’ to monitor serious adverse events associated with clozapine such as agranulocytosis [24], this drug is less likely to be administered to outpatients, which would account for the lack of dispensing data. Although the results of this study indicate that risperidone was not associated with hyperlipidemia occurrence, the results for this drug have so far been inconsistent, and its association with hyperlipidemia remains contentious [4, 7, 12, 21, 25]. When the interval between exposure and outcome was restricted to within 90 days, the point estimate of ASR for perospirone hydrochloride hydrate was relatively high [ASR 2.14; 95 % CI 0.76–6.85 (Table 2)], albeit with wide CIs. A recent meta-analysis [26] indicated that perospirone hydrochloride hydrate has a higher risk of hypercholesterolemia than aripiprazole, although the risk for perospirone has not been fully assessed. Further studies that include more users of perospirone hydrochloride hydrate are needed to adequately evaluate its hyperlipidemic risk.
SSA uses a straightforward analytical process with minimal data requirements (only date of exposure and outcome are needed), and has been employed not only for pharmacoepidemiological signal detection, but also for risk estimations for drug safety due to its self-controlled study design. SSA may also minimize the effects of known or unknown time-independent confounders, and generate ASRs that are similar to the incidence rate ratios of outcomes when compared with pre-exposure periods. In fact, several studies have suggested that the sequence ratios calculated from SSA correspond to the relative risks estimated from cohort studies or nested case–control studies using identical data sources [10, 27]. Therefore, the ASRs obtained in this study may represent the relative risks of AAPs for hyperlipidemia between exposed person-time and non-exposed person-time while controlling for time-independent confounders.
Although the point estimates of ASRs tended to be higher when the intervals between exposures and outcomes were restricted (Tables 2, 3, 4), these restrictions did not change the direction of risk or statistical significance. Sample sizes became larger when the intervals were longer, and the CIs for the ASRs became correspondingly narrower [9, 28, 29]. However, it would be specious to declare a causal relationship between exposure and outcome in cases where the patient experienced the outcome long after the exposure. Therefore, sensitivity analyses that restrict the intervals between exposures and outcomes should be an integral component of any SSA study.
4.3 Limitations
There are several limitations to this study. First, although we had identified hyperlipidemia through the dispensing of antihyperlipidemic drugs, these drugs may have been used prophylactically against hyperlipidemia, as it is a well known adverse effect of AAPs. To minimize this bias, we excluded cases where an AAP and any antihyperlipidemic drug were initially dispensed on the same day. Second, the effect of time-dependent covariates such as age (maximum 9 years), concomitant drugs, and the occurrence of acute illness could not be eliminated as SSA cannot control for such factors. Third, because SSA includes only exposed cases for analysis, the estimated relative risk may not be applicable to the general population. It is possible that patients with drug dispensing records for AAPs have inherently higher risks of hyperlipidemia, thereby leading to confounding by indication. Fourth, prescribing information during inpatient care was not included in this study as the dispensing data were obtained from pharmacies outside of hospitals. However, most of the medications for hyperlipidemia would be administered on an outpatient basis. Fifth, the effect of the duration of AAP prescription on the risk of hyperlipidemia was not considered in this study, since the SSA design could assess only sequence symmetry of the initiation of AAP and antihyperlipidemic drugs. Finally, the database used in this study comprised employed workers and their family members. Therefore, data for retired elderly patients or patients with severe psychiatric disorders was less likely to be included in this database.
5 Conclusion
In this study, a SSA using Japanese claims data showed that among the AAPs approved for use in Japan, only olanzapine was significantly associated with a higher risk of hyperlipidemia. Our study demonstrated relatively low risks for risperidone, perospirone hydrochloride hydrate, blonanserin, quetiapine fumarate, and aripiprazole. These results provide consistent risk profiles of hyperlipidemia for AAP administration after controlling for time-independent confounding. Although precautions stating the risk of hyperlipidemia are already included in the package inserts (drug labels) of AAPs in Japan, medical professionals should also be more aware of the elevated risks of hyperlipidemia for olanzapine.
References
Yan H, Chen JD, Zheng XY. Potential mechanisms of atypical antipsychotic-induced hypertriglyceridemia. Psychopharmacology. 2013;229:1–7.
Nagamine T. Olanzapine-induced elevation of serum triglyceride levels in a normal weight patient with schizophrenia. Intern Med. 2008;47:181–2.
Koro CE, Meyer JM. Atypical antipsychotic therapy and hyperlipidemia: a review. Essent Psychopharmacol. 2005;6:148–57.
Koro CE, Fedder DO, L’Italien GJ, Weiss S, Magder LS, Kreyenbuhl J, et al. An assessment of the independent effects of olanzapine and risperidone exposure on the risk of hyperlipidemia in schizophrenic patients. Arch Gen Psychiatry. 2002;59:1021–6.
Bersot TP. Drug therapy for hypercholesterolemia and dyslipidemia. In: Brunton LL, editor. Goodman and Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill books; 2011. p. 877–908.
Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70:1–17.
Olfson M, Marcus SC, Corey-Lisle P, Tuomari AV, Hines P, L’Italien GJ. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163:1821–5.
Hallas J. Evidence of depression provoked by cardiovascular medication: a prescription sequence symmetry analysis. Epidemiology. 1996;7:478–84.
Tsiropoulos I, Andersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepidemiol Drug Saf. 2009;18:483–91.
Corrao G, Botteri E, Bagnardi V, Zambon A, Carobbio A, Falcone C, et al. Generating signals of drug-adverse effects from prescription databases and application to the risk of arrhythmia associated with antibacterials. Pharmacoepidemiol Drug Saf. 2005;14:31–40.
Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophrenia Bull. 2013;39:306–18.
Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. J Clin Psychiatr. 2003;64:598–604.
Melkersson KI, Hulting AL, Brismar KE. Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J Clin Psychiatr. 2000;61:742–9.
Victoriano M, de Beaurepaire R, Naour N, Guerre-Millo M, Quignard-Boulange A, Huneau JF, et al. Olanzapine-induced accumulation of adipose tissue is associated with an inflammatory state. Brain Res. 2010;1350:167–75.
Lauressergues E, Staels B, Valeille K, Majd Z, Hum DW, Duriez P, et al. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn Schmiedeberg’s Arch Pharmacol. 2010;381:427–39.
Vik-Mo AO, Birkenaes AB, Ferno J, Jonsdottir H, Andreassen OA, Steen VM. Increased expression of lipid biosynthesis genes in peripheral blood cells of olanzapine-treated patients. Int J Neuropsychopharmacol. 2008;11:679–84.
Eder U, Mangweth B, Ebenbichler C, Weiss E, Hofer A, Hummer M, et al. Association of olanzapine-induced weight gain with an increase in body fat. Am J Psychiatr. 2001;158:1719–22.
Skrede S, Ferno J, Vazquez MJ, Fjaer S, Pavlin T, Lunder N, et al. Olanzapine, but not aripiprazole, weight-independently elevates serum triglycerides and activates lipogenic gene expression in female rats. Int J Neuropsychopharmacol. 2012;15:163–79.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23.
Katagiri H, Tohen M, McDonnell DP, Fujikoshi S, Case M, Kanba S, et al. Efficacy and safety of olanzapine for treatment of patients with bipolar depression: Japanese subpopulation analysis of a randomized, double-blind, placebo-controlled study. BMC Psychiatry. 2013;13:138.
Meyer JM. A retrospective comparison of weight, lipid, and glucose changes between risperidone- and olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry. 2002;63:425–33.
Paramsothy P, Knopp R, Bertoni AG, Tsai MY, Rue T, Heckbert SR. Combined hyperlipidemia in relation to race/ethnicity, obesity, and insulin resistance in the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2009;58:212–9.
Lund BC, Perry PJ, Brooks JM, Arndt S. Clozapine use in patients with schizophrenia and the risk of diabetes, hyperlipidemia, and hypertension: a claims-based approach. Arch Gen Psychiatry. 2001;58:1172–6.
Novartis Pharma K.K.. CLOZARIL-clozapine tablet. Revised Japanese package insert (in Japanese). 2014. http://www.info.pmda.go.jp/downfiles/ph/PDF/300242_1179049F1021_1_10.pdf. Accessed 16 Feb 2015.
Weiden PJ, Daniel DG, Simpson G, Romano SJ. Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J Clin Psychopharmacol. 2003;23:595–600.
Kishi T, Matsuda Y, Iwata N. Cardiometabolic risks of blonanserin and perospirone in the management of schizophrenia: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9:e88049.
Almqvist C, Wettermark B, Hedlin G, Ye W, Lundholm C. Antibiotics and asthma medication in a large register-based cohort study—confounding, cause and effect. Clin Exp Allergy. 2012;42:104–11.
Pouwels KB, Visser ST, Bos HJ, Hak E. Angiotensin-converting enzyme inhibitor treatment and the development of urinary tract infections: a prescription sequence symmetry analysis. Drug Saf. 2013;36:1079–86.
Cher DJ. Myocardial infarction and acute cholecystitis: an application of sequence symmetry analysis. Epidemiology. 2000;11:446–9.
Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20:413–9.
Ministry of Education Culture, Sports, Science and Technology (MEXT), Ministry of Health Labour and Welfare (MHLW). Ethical Guidelines for Epidemiological Research 2002. http://www.niph.go.jp/wadai/ekigakurinri/guidelines.pdf. Accesseed 16 Feb 2015.
Acknowledgments
The views expressed here are based on independent work and do not necessarily represent the official views and findings of the Pharmaceuticals and Medical Devices Agency.
Compliance with ethical standards
Ethical consideration: Personally identifiable information such as patient name, exact date of birth and when they became (or dropped out) a member of the health insurance society (month and year were left in the data) and insurance certification number were removed by the database vendor before providing the data in the claims database used in this study. As an alternative to personally identifiable information, the vendor provided encrypted identifiers generated by hashing algorithms to enable the linkage of claims data to individual patients [30]. As the decryption of this sensitive information is theoretically impossible, we determined that this study did not require ethical committee review in accordance with current ethical standards for epidemiological studies in Japan [31].
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Yoshinori Takeuchi, Kazuhiro Kajiyama, Chieko Ishiguro and Yoshiaki Uyama are employed by the Pharmaceuticals and Medical Devices Agency and have a no financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeuchi, Y., Kajiyama, K., Ishiguro, C. et al. Atypical Antipsychotics and the Risk of Hyperlipidemia: A Sequence Symmetry Analysis. Drug Saf 38, 641–650 (2015). https://doi.org/10.1007/s40264-015-0298-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-015-0298-4