Abstract
Background
Fluoroquinolone antibiotics are commonly used to treat infections and are prescribed by general practitioners, medical specialists and surgeons. Tendon injury has been associated with the use of these medications but the risk associated with newer fluoroquinolones has not been established.
Objectives
The aim of this systematic review was to evaluate the evidence from observational studies to determine the strength of the association between fluoroquinolone use and tendinopathy, and to identify risk factors for this complication.
Methods
We searched MEDLINE, EMBASE and the Cochrane Collaboration from inception through May 2013 to identify observational studies focused on tendon injury and fluoroquinolones. Studies with original data were selected for inclusion following the PRISMA guidelines. Of the 560 abstracts screened, 16 relevant studies were independently rated by three authors (WW, AS, DC) using the Newcastle-Ottawa Quality Assessment Scale, and assigned a quality score out of 9. High-quality studies (i.e. scored 4.5 or higher) are summarized in detail in this article. Data were independently extracted by two authors (WW, AS).
Results
Overall, 16 studies were included in our study. Eight were deemed to be of high quality and five specifically evaluated Achilles tendon rupture. In addition, three studies examined Achilles tendinitis, and three included tendon disorders (including any tendon rupture) as an outcome. Results from these studies suggest that individuals exposed to fluoroquinolones are at increased risk for Achilles tendon rupture, particularly within the first month following exposure to the drug (odds ratios ranged from 1.1 to 7.1). One study showed an increased risk of tendon rupture in those over 60 years of age. Five studies stated that individuals taking fluoroquinolones and oral corticosteroids are at increased risk for tendon injury compared with those taking fluoroquinolones alone. Four studies examined the differential effect of a limited number of fluoroquinolones. Ofloxacin had the highest risk of tendon injury in three of the studies.
Limitations
Included studies are observational in nature and rely on self-report, which may lead to misclassification or underestimation of tendon injury.
Conclusions
Observational studies showed an increased risk of tendon injury, including tendon rupture and tendinitis, with exposure to fluoroquinolone antibiotic therapy. Although this complication appears to be rare, concomitant corticosteroids increase the risk for tendon injury, which varies depending on the fluoroquinolone used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Fluoroquinolone-associated tendinopathy has been reported in the literature and the incidence is estimated to be 0.14–0.4 % [1]. The Achilles tendon is often affected; however, case reports of inflammation of other tendons related to fluoroquinolone use have been published [2–8]. The symptoms can range from mild pain around the affected tendon to complete rupture necessitating a surgical intervention. In 2008, mounting public pressure and increased evidence of fluoroquinolone-associated tendinopathy forced ‘black-box’ warnings and ‘Dear Doctor’ letters from manufacturers highlighting this potential complication [9].
Fluoroquinolone antibiotics were first available in the mid-1980s, with norfloxacin, ciprofloxacin and ofloxacin being the earliest introductions of their class [10]. Since then, fluoroquinolones have been used to treat a wide variety of infections, including urinary tract, respiratory tract, gastrointestinal, skin, bone and joint infections. As such, this class of medication is prescribed by a variety of medical specialties ranging from general practitioners to medical specialists and surgeons. The bactericidal activity of the earliest fluoroquinolones involved inhibition of DNA gyrase in gram-negative pathogens, whereas the more recent newer ‘respiratory’ agents (e.g. levofloxacin, moxifloxacin) were expanded to inhibit topoisomerase IV in gram-positive pathogens, and have activity against atypicals and anaerobes [10]. Canadian and American respiratory treatment guidelines for community-acquired pneumonia (CAP) recommend respiratory fluoroquinolones, such as levofloxacin and moxifloxacin, as first-line therapy for CAP and also for the treatment of chronic obstructive pulmonary disease (COPD) exacerbations [11, 12].
To our knowledge, there has been only one published systematic review of the literature evaluating fluoroquinolone-associated tendinopathy, which used data from 1966–2001 [13]. This review primarily included case reports (n = 98) which most commonly involved pefloxacin and ciprofloxacin. From the case reports, the authors concluded that the median duration of onset of tendon injury was 8 days and that 50 % experienced tendon rupture, particularly in individuals receiving oral corticosteroids. Risk of tendon injury was felt to be highest with pefloxacin compared with ciprofloxacin. In addition, older individuals, those with renal impairment, or concomitant use of oral corticosteroids, are at increased risk of tendon injury related to fluoroquinolone use based on the previously published systematic review [13]. However, some unanswered questions remain. The newer respiratory fluoroquinolones (i.e. levofloxacin, moxifloxacin) were not well represented in the previous review, therefore the risk of tendon injury associated with these medications has not been systematically evaluated. In addition, the effect of specific risk factors on tendon injury, such as fluoroquinolone dose, duration of therapy, age and sex, remain uncertain.
Since the systematic review published in 2003 [13], additional observational studies have been published on this topic which may shed light on these unanswered questions. Fluoroquinolone prescribing has significantly increased over the years [14], therefore it is important to increase awareness and expand our knowledge about this association so patients can be monitored closely for this potential adverse event. In this article, we systematically reviewed the evidence from observational studies to determine the strength of the association between fluoroquinolone use and tendinopathy, and to identify risk factors for this complication.
2 Methods
Following PRISMA guidelines, we searched MEDLINE (1948 through to 17 May 2013), EMBASE (1947 through to 17 May 2013) and the Cochrane Collaboration (2005 through to 17 May 2013) for published articles on tendon injuries and quinolones (last searched 17 May 2013). The following search terms were used across all databases: ‘tendon’, ‘tendonitis’, ‘tendon injury’, ‘tendinopathy’, ‘quinolones’, ‘fluoroquinolones’, ‘ciprofloxacin’, ‘gatifloxacin’, ‘levofloxacin’, ‘moxifloxacin’, ‘norfloxacin’, ‘ofloxacin’, ‘enoxacin’ and ‘pefloxacin’ (the search strategy can be found in the electronic supplementary material). Inclusion criteria were observational research studies on human subjects published in the English language. We did not include randomized controlled trials as they have strict selection criteria and often exclude those at highest risk of adverse events. Furthermore, tendon injury in such trials can be very rare. Population-based observational studies reflect real-life clinical practice; therefore, review of observational studies is critical to evaluate the prevalence of adverse events. Review articles, pathophysiology studies, case reports and animal studies were excluded. We included articles where tendon injury was the primary outcome of the study. Article titles and abstracts were screened for inclusion by three authors (WW, AS, DC) independently. If there was uncertainty based on the abstract, the full text was obtained and reviewed. Any disagreements were resolved by consensus. Articles were evaluated and data extracted by two authors (WW, AS) independently on the type of fluoroquinolone studied, fluoroquinolone dosage and duration, time to onset, incidence rate, risk ratio, the type of outcome identified and whether or not specific risk factors identified previously in the literature were evaluated, such as age, sex, corticosteroid use, prior history of fluoroquinolone-associated tendinopathy and renal disease. Disagreements were resolved by consensus.
2.1 Assessment of Risk of Bias
All studies included in the review were assessed for quality using the Newcastle-Ottawa Quality Assessment Scale for assessing the quality of non-randomized studies in meta-analyses and systematic reviews [15]. Two rating scales were used—one for cohort studies and one for case-control studies. The cohort evaluation tool involved rating the study on representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of the study, comparability of the cohorts on the basis of the design or analysis, assessment of the outcome, follow-up length and adequacy of follow-up. Case-control studies were evaluated based on the case definition, representativeness of cases, selection of controls, definition of controls, comparability of cases and controls, ascertainment of exposure, whether the same method was used for cases and controls, and the non-response rate. For studies using an alternative study design (e.g. case-series, correlational, cross-sectional), the evaluation form for case-control studies was utilized to assess the quality of the study [16]. All included articles were independently rated on each of the items by three authors (WW, AS, DC). Any instances of disagreement were resolved by consensus. Each study could receive a maximum rating of nine. High-quality studies were deemed to have a score of 4.5 or greater (the mid-point for the rating scale). Results for high-quality studies are presented in this review.
3 Results
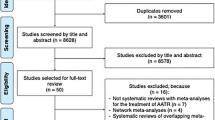
The search identified 469 unique abstracts, from which 16 observational studies were identified (Table 1) for inclusion in our study. Figure 1 shows an overview of the study selection process. Five case-control studies and six cross-sectional/case-series studies [17–27] were identified as well as five cohort studies [28–32]. Two studies examined the post-transplant population (heart, lung) [23, 29] and four studies were surveillance pharmacovigilance studies of self-reported adverse events associated with fluoroquinolone use [18, 22, 25, 27]. The remaining ten studies were population-based database studies using either prescription dispensing and consumption data, health administrative data or general practice data [17, 19–21, 24, 26, 28, 30–32]. The mean age of study subjects varied from 47 to 68 years. Of those studies that reported the follow-up time after exposure (n = 10), the range was from 1 to 18 months. Results from the quality assessment scale for the case-control, cross-sectional and cohort studies are presented in Table 2. All five case-control studies were of high quality [17, 19–21, 24]; however, only three of the five cohort studies [28, 30, 31] met the criteria for high quality. None of the cross-sectional/case-series studies met the criteria for high quality. Of the eight high-quality studies reported in this review, two studies did not specify the names of the fluoroquinolones studied [17, 30]. The remaining six studies included the following fluoroquinolones: ciprofloxacin (six studies), ofloxacin (five studies), norfloxacin (four studies), levofloxacin (three studies), tosufloxacin (one study), moxifloxacin (one study), prulifloxacin (one study), sitafloxacin (one study), garenoxacin (one study), sparfloxacin (one study), fleroxacin (one study) and gatifloxacin (one study). Although a meta-analysis was planned, due to the diversity of the patient populations, study outcomes and fluoroquinolones studied, it was not possible to conduct this analysis.
3.1 Incidence of Tendon Injury
Two studies reported on the incidence of tendon injury, which was found to be rare, ranging from 0.08 to 0.2 % [28, 31].
3.2 Type of Tendon Injury
3.2.1 Tendon Rupture
Four case-control studies and one cohort study assessed the risk of Achilles tendon rupture and one study examined any tendon rupture following fluoroquinolone exposure (Table 3). Four studies found a significantly increased risk of Achilles tendon rupture [20, 21, 24, 30], whereas one study reported an increased risk of any tendon, not limited to the Achilles tendon [odds ratio (OR) 2.0, 95 % CI 1.2–2.3] [17]. A sixth study [19] reported an increased risk (i.e. OR 1.2) but these results were not statistically significant, with a 95 % CI of 0.9–1.7. Of note, the study by van der Linden et al. [24] reported the risk of Achilles tendon rupture stratified by age (<60 vs. ≥60 years), whereas the other studies reported an OR for tendon rupture for the entire cohort without stratification by age.
3.2.2 Achilles Tendinitis
Achilles tendinitis was evaluated by one case-control study, one case-crossover study and one cohort study (Table 3). van der Linden et al. [31] examined 1,841 subjects exposed to fluoroquinolone and 9,406 controls, and compared the risk of developing Achilles tendinitis between the two groups. A non-significant increase in the rate of Achilles tendinitis was reported between the exposed group and reference group [relative risk (RR) 3.7; 95 % CI 0.93–15.14]. A later study by the same group found a statistically significant increased risk of Achilles tendinitis in those ≥60 years of age recently prescribed fluoroquinolones (OR 3.1; 95 % CI 2.0–4.8) [24]. Wise et al. [17] reported an increased risk of Achilles tendinitis in subjects exposed to fluoroquinolones (OR 4.4; 95 % CI 3.3–5.9).
3.2.3 Any Tendon Disorders
One study [20] included ‘tendon disorders’ as an outcome that included synovitis and tenosynovitis (tendon rupture was examined separately in this article). This study reported an increased risk of tendon disorders within 1 month following fluoroquinolone exposure but the risk was no longer statistically significant 1–3 months post-fluoroquinolone exposure (Table 3) [20]. A second study evaluated ‘tendon disorders’ that encompassed tendinitis, tendon rupture and peri-tendinitis. The study results were reported as a composite outcome rather than providing a risk estimate for the three tendon complications separately. There was a statistically significant increased risk of a tendon disorder in those who received a fluoroquinolone compared with individuals receiving cephalosporins (RR 6.29; 95 % CI 2.27–17.46) [28].
3.3 Timing of Tendon Injury
The time between the exposure and outcome varied between 15 days and 18 months in the five case-control studies. The three cohort studies followed individuals for either 30 or 90 days for the outcome [28, 30, 31]. The risk of Achilles tendon rupture seemed to be highest in the first month after exposure [17, 20, 21, 24] (Table 3). One study reported a continued statistically significant increased risk up to 6 months (OR 2.4; 95 % CI 1.5–3.7); however, results were not consistent across the studies [21].
3.4 Risk Factors for Tendon Injury
Risk factors for tendon injury are summarized in Table 4. Eight studies evaluated age as a risk factor for the tendon injury with inconsistent results. Three studies compared the risk of tendon disorders (Achilles tendon rupture in one study, composite outcome of tendon rupture, tendinitis or peri-tendinitis in the second study, and Achilles tendonitis in the third study) in those ≥60 years of age compared with those younger than 60 years of age. The risk of Achilles tendon rupture [20] and Achilles tendonitis [17] was significantly higher in those ≥60 years of age; however, the second study did not find a significant difference in tendon disorders in those ≥60 years of age compared with those younger than 60 years of age [28]. The remaining five studies did not specifically compare the risk in older versus younger populations but rather conducted a sub-group analysis based on age categories comparing the risk of tendon injury in exposed and unexposed individuals within a specific age group [17, 19, 21, 24, 30]. These subgroup analyses did not yield statistically significant results in the risk of Achilles tendon rupture in the various age categories, with the exception of van der Linden et al. [21], who found that among those ≥80 years of age, the risk of tendon rupture was increased in those currently exposed to fluoroquinolone (OR 20.4; 95 % CI 4.6–90.1) as well as those recently exposed subjects (OR 7.4; 95 % CI 2.4–22.9). Individuals were considered currently exposed to fluoroquinolone if the index date fell within the prescription duration plus 30 days, and recently exposed to fluoroquinolone if the calculated prescription length was less than 180 days before the index date and the subject did not meet the definition of currently exposed. With respect to Achilles tendinitis and Achilles tendon disorders, van der Linden et al. [24] found a statistically significant increase for those ≥60 years of age for both of these outcomes (Table 4). Corrao et al. [20] found no difference in the risk of tendon rupture (not specific to Achilles) or any tendon disorder between those below 60 years of age compared with those 60 years of age or older (p-value reported as >0.05).
van der Linden et al. [31] examined three fluoroquinolones—ofloxacin, ciprofloxacin and norfloxacin. A differential risk of tendon injury was found depending on the fluoroquinolone used. Ofloxacin appeared to have the highest risk of developing tendinitis [any area] (adjusted RR 4.9; 95 % CI 1.57–15.06) and Achilles tendinitis specifically (RR 10.1; 95 % CI 2.20–46.04), whereas the risk with ciprofloxacin or norfloxacin was not statistically significant compared with the non-fluoroquinolone reference group. Within this same paper, dosage and duration of fluoroquinolone was not evaluated due to the fact that essentially all courses were given for a similar duration and the majority of fluoroquinolone users took the same recommended daily dose. Seeger et al. [19] included ciprofloxacin, levofloxacin, ofloxacin and other non-specified fluoroquinolones and found no association between the type of fluoroquinolone used and the risk of Achilles tendon rupture. The cumulative fluoroquinolone dosage was calculated and the authors found a modestly higher risk (OR 1.5; 95 % CI 1.0–2.3) in the highest cumulative dose category (8000+ mg over 180 days), suggesting a dose-response relationship. van der Linden et al. [21] evaluated the association between tendon injury and both the type and dosage of fluoroquinolone used (ciprofloxacin, norfloxacin and ofloxacin were included in the study). The highest risk of Achilles tendon rupture was noted with ofloxacin (OR 28.4; 95 % CI 7.0–115.3) and this risk increased in those receiving a higher dosage as measured by the prescribed daily dosage equivalent. Finally, Hori et al. [28] found a differential risk of tendon disorders among fluoroquinolones compared with the reference population of subjects who received cephalosporins. Ofloxacin was associated with the highest risk (RR 80.2; 95 % CI 9.5–680.5) followed by moxifloxacin (RR 15.7; 95 % CI 3.1–81.0), tosufloxacin (RR 7.3; 95 % CI 1.4–37.5) and levofloxacin (RR 5.2; 95 % CI 1.7–15.5). An RR could not be estimated for the other fluoroquinolones examined in this study due to the fact that no outcomes were observed in those groups.
Corticosteroid use was evaluated as a risk factor for developing tendon injury in all five case-control studies, but was not specifically examined in the cohort or cross-sectional studies [17, 19–21, 24]. The route of administration of corticosteroids was specified as oral or systemic in two studies and not specified in three studies. All five studies reported an increased risk of Achilles tendon rupture with an OR greater than 1 for those taking fluoroquinolone and corticosteroids; however, one of the studies reported results that were not statistically significant. Corrao et al. [20] showed that the OR was higher in those receiving fluoroquinolones and corticosteroids compared with those subjects receiving fluoroquinolones alone for Achilles tendon rupture and any tendon rupture. Similarly, van der Linden et al. [24] showed that for those aged 60 years or older, the OR for Achilles tendon disorders was 3.2 (95 % CI 2.1–4.9) in fluoroquinolone users compared with non-fluoroquinolone users, and that the OR increased to 6.2 (95 % CI 3.0–12.8) for those receiving fluoroquinolones and corticosteroids compared with non-fluoroquinolone users. van der Linden’s second article published in 2003 [21] confirmed an increased risk for those over 60 years of age who were concomitantly treated with fluoroquinolones and corticosteroids. The risk of Achilles tendon rupture associated with current exposure to fluoroquinolones was increased (OR 5.3; 95 % CI 1.8–15.2) compared with non-fluoroquinolone users. This risk was significantly higher in those currently receiving or who recently took oral corticosteroids (OR 17.5; 95 % CI 5.0–60.9, and 18.4; 95 % CI 1.4–240.2, respectively) compared with non-fluoroquinolone users. Finally, Wise et al. [17] reported an increased risk in individuals with concomitant use of corticosteroids and fluoroquinolones compared with those taking fluoroquinolones without corticosteroids (OR 9.1; 95 % CI 4.6–18.0, and OR 3.2; 95 % CI 2.3–4.4, respectively), again supporting the claim that concomitant corticosteroids and fluoroquinolones increases the risk of adverse tendon events.
Obesity was found to be significantly associated with Achilles tendon rupture in one study [19] (OR 2.0; 95 % CI 1.2–3.1); however, a second study [17] found an increased risk of Achilles tendinitis in the non-obese population (OR 7.7; 95 % CI 4.3–13.7) compared with those who were obese (OR 2.4; 95 % CI 1.1–5.0). One study [19] found male sex to be a significant risk factor for tendon rupture (OR 3.0; 95 % CI 2.6–3.5). Two other studies [21, 24] reported the effect of fluoroquinolones on the occurrence of tendon rupture was not modified by sex, and a third study found a higher risk of Achilles tendinitis in women compared with men [17]. Corrao et al. [20] matched exposed and unexposed subjects on sex; therefore, the effect of sex on the outcome could not be evaluated. One study found the risk of Achilles tendinitis was significantly higher in those with diabetes who were taking fluoroquinolones (OR 7.0; 95 % CI 2.1–23.5) compared with non-diabetics (OR 4.1; 95 % CI 3.0–5.5) and in those with renal failure who were taking fluoroquinolones (OR 20.0; 95 % CI 2.7–149) compared with those without evidence of renal failure (OR 3.9; 95 % CI 2.9–5.2) [17].
4 Discussion
The results of this review suggest that there is a significant association between fluoroquinolone use and tendon injury, in particular Achilles tendon rupture and tendinitis, which has been shown using different study designs and in different populations. Fluoroquinolone-associated tendinopathy is relatively uncommon, but when it occurs it tends to happen soon after exposure (within the first month). High-risk groups include those on concomitant corticosteroids and possibly the elderly population. Despite only a small number of relevant studies, tendon injury may be more common with specific fluoroquinolones, and higher dosages may be associated with higher risk. The current literature is problematic in that published studies examined different tendon outcomes and often relied on self-reporting, the specific fluoroquinolones included in the studies varied, newer fluoroquinolones were not extensively studied and the risk factors evaluated were inconsistent across the published studies, which limits the conclusions that can be drawn. Furthermore, there is a spectrum of disease in terms of tendon injury related to fluoroquinolone use. The majority of studies focused on Achilles tendon injury, although case reports of other tendons being affected by fluoroquinolones have been published [33, 34].
The pathophysiology of tendon injury following fluoroquinolone exposure is not completely understood and it is likely that the negative effect of fluoroquinolones on tendons is multifactorial. Animal models have shown that, following exposure to fluoroquinolones, there was an increase in matrix-degrading activity and a decrease in matrix synthesis and fibroblast cell proliferation, thereby weakening the tendon and increasing the risk of rupture [35]. Transmembrane proteins, called integrins, are involved in the structural stability of a cell and these proteins rely on magnesium as well as other cations. Fluoroquinolone antibiotics are known to chelate cations, thus potentially altering the stability of the cell structure weakening the tendon [36]. Exposure to fluoroquinolones stimulates oxygen radical production and induces matrix metalloproteinases, which are enzymes involved in tendon remodelling [37]. In addition to the potentially direct toxic effects of fluoroquinolones on tendons, increasing age and steroid use can weaken tendons further, which has been shown to increase the risk of tendon injury.
Despite its relatively low incidence, increasing vigilance and awareness of the association between fluoroquinolones and tendon injury is important for several reasons. The apparent disconnect between antibiotics used to treat an infection and seemingly unrelated inflammation of a tendon creates a situation whereby the physician who initially prescribed the drug may not be the same physician who is told about or who treats the complication. As such, it is possible to miss the connection altogether, particularly if the patient and physician are not familiar with this adverse event. Although there is limited evidence to guide therapy of fluoroquinolone-induced tendinopathy, once symptoms of tendinitis occur the antibiotic should be discontinued and anti-inflammatory medications may be prescribed to control symptoms and possibly decrease the risk of a complete tendon rupture [38, 39]. Knowledge of this association is critical to ensure a timely diagnosis and management of this adverse event. Secondly, these medications are commonly prescribed and several treatment alternatives exist which could be considered for individuals at considerable risk for tendon injury. For example, the newer respiratory fluoroquinolones are recommended for management of CAP or treatment of COPD exacerbations. Individuals with COPD are often elderly and may be concomitantly treated with systemic corticosteroids. In these circumstances, alternative antibiotic therapy could be considered since both age and corticosteroid use significantly increases the risk of tendon injury, including rupture.
Strengths of our review include the systematic and comprehensive literature search, the fact that each study was evaluated for quality using a standard scale, and three reviewers independently evaluated the articles for inclusion. There are several limitations of the existing literature on fluoroquinolones and tendon injury. Due to the fact that tendon injury is a relatively rare adverse event related to fluoroquinolone use, and randomized controlled trials exclude those at highest risk of adverse events, we must utilize observational research which often includes self-report data. Self-report is typically an underestimate of the true prevalence of an adverse event since many individuals may not go to the effort of reporting the complication or, alternatively, they may not recognize the condition as an adverse event of a prescribed medication. The majority of observational studies identified in our search were either of low quality to be able to draw firm conclusions, or in a specific sub-population (e.g. transplant) and hence not generalizable. Of the high-quality studies examined, the newer respiratory fluoroquinolones such as moxifloxacin and levofloxacin were under-represented. These antibiotics are broadly used in the respiratory population and more information is needed to quantify the risk of tendon injury with these medications. The literature suggests that corticosteroid use and possibly age increase the risk of fluoroquinolone-associated tendon injury; however, other possible risk factors have not been adequately evaluated, e.g. the impact of renal failure, dose or duration of fluoroquinolone use and recent joint surgery, among others. Recent case reports have suggested that statins increase the risk of tendon rupture [40] and, given the prevalence with which this class of medication is used in the population, it is possible that fluoroquinolone use in an individual taking statins may increase the risk of tendon injury above that of fluoroquinolone alone; however, this has never been formally evaluated.
5 Conclusion
Published observational studies show that there is an increased risk of Achilles tendon rupture and Achilles tendinitis (specifically in individuals ≥60 years of age) with exposure to fluoroquinolone antibiotic therapy. Those taking concomitant corticosteroids are at increased risk for this adverse event. Several unanswered questions remain with respect to the impact of fluoroquinolones on tendons other than the Achilles, which additional high-risk groups exist if any, and the impact of dosage and type of fluoroquinolone on the risk of tendon injury, which are all areas of future research.
References
Mehlhorn AJ, Brown DA. Safety concerns with fluoroquinolones. Ann Pharmacother. 2007;41(11):1859–66.
Beyer J, Schellong S. Bilateral plantar tendinitis during levofloxacin therapy (Review, 8 refs). Br J. Clin Pharm. 2006;61(5):609.
Karistinos A, Paulos LE. “Ciprofloxacin-induced” bilateral rectus femoris tendon rupture. 17(5):406–7.
Braun D, Petitpain N, Cosserat F, Loeuille D, Bitar S, Gillet P, et al. Rupture of multiple tendons after levofloxacin therapy. Revue du Rhumatisme. 2004;71(6):586–7.
Burkhardt O, Kohnlein T, Pap T, Welte T. Recurrent tendinitis after treatment with two different fluoroquinolones. Scand J Infect Dis. 2004;36(4):315–6.
Mouzopoulos G, Stamatakos M, Vasiliadis G, Skandalakis P. Rupture of adductor longus tendon due to ciprofloxacin. Acta Ortho Belgica. 2005;71(6):743–5.
Khaliq Y, Zhanel GG. Musculoskeletal injury associated with fluoroquinolone antibiotics (Review, 43 refs). Clinics Plastic Surgery. 2005;32(4):495–502.
Ever-Silva WA, Netto Hde B, Pinto JF, Ferry FR, Neves-Motta R. Severe shoulder tendinopathy associated with levofloxacin. Braz J Infect Dis. 2012;16(4):393–5.
Tanne JH. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ. 2008;337:a816.
Zhanel GG, Ennis K, Vercaigne L, Walkty A, Gin AS, Embil J, et al. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs. 2002;62(1):13–59.
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
O’Donnell DE, Hernandez P, Kaplan A, Aaron S, Bourbeau J, Marciniuk D et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care. Can Respir J 2008;15(Suppl A):1A–8A.
Khaliq Y, Zhanel GG. Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clin Infect Dis. 2003;36(11):1404–10.
Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med. 2005;118:259–68.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
Costa SM, Martins CC, Bonfim MC, Zina LG, Paiva SM, Pordeus IA, et al. A systematic review of socioeconomic indicators and dental caries in adults. Int J Environ Res Public Health. 2012;9:3540–74.
Wise BL, Peloquin C, Choi H, Lane NE, Zhang Y. Impact of age, sex, obesity, and steroid use on quinolone-associated tendon disorders. Amer J Med. 2012;125(12):1228.e23–8.
Lapi F, Tuccori M, Motola D, Pugi A, Vietri M, Montanaro N, et al. Safety profile of the fluoroquinolones: analysis of adverse drug reactions in relation to prescription data using four regional pharmacovigilance databases in Italy. Drug Saf. 2010;33(9):789–99.
Seeger JD, West WA, Fife D, Noel GJ, Johnson LN, Walker AM. Achilles tendon rupture and its association with fluoroquinolone antibiotics and other potential risk factors in a managed care population. Pharmacoepidemiol Drug Saf. 2006;15(11):784–92.
Corrao G, Zambon A, Bertu L, Mauri A, Paleari V, Rossi C, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf. 2006;29(10):889–96.
van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HM, Rowlands S, Stricker BH. Increased risk of Achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids. Arch Intern Med. 2003;163(15):1801–7.
Leone R, Venegoni M, Motola D, Moretti U, Piazzetta V, Cocci A, et al. Adverse drug reactions related to the use of fluoroquinolone antimicrobials: an analysis of spontaneous reports and fluoroquinolone consumption data from three Italian regions. Drug Saf. 2003;26(2):109–20.
Chhajed PN, Plit ML, Hopkins PM, Malouf MA, Glanville AR. Achilles tendon disease in lung transplant recipients: association with ciprofloxacin. Eur Respir J. 2002;19(3):469–71.
van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HG, Stricker BH. Fluoroquinolones and risk of Achilles tendon disorders: case-control study. BMJ. 2002;324(7349):1306–7.
van der Linden PD, van Puijenbroek EP, Feenstra J, Veld BA, Sturkenboom MC, Herings RM, et al. Tendon disorders attributed to fluoroquinolones: a study on 42 spontaneous reports in the period 1988 to 1998. Arthritis Rheum. 2001;45(3):235–9.
van der Linden PD, Nab HW, Simonian S, Stricker BH, Leufkens HG, Herings RM. Fluoroquinolone use and the change in incidence of tendon ruptures in the Netherlands (Review, 23 refs). Pharm World Sci. 2001;23(3):89–92.
Pierfitte C, Royer RJ. Tendon disorders with fluoroquinolones. Therapie. 1996;51(4):419–20.
Hori K, Yamakawa K, Yoshida N, Ohnishi K, Kawakami J. Detection of fluoroquinolone-induced tendon disorders using a hospital database in Japan. Pharmacoepidemiol Drug Saf. 2012;21:886–9.
Barge-Caballero E, Crespo-Leiro MG, Paniagua-Martin MJ, Muniz J, Naya C, Bouzas-Mosquera A, et al. Quinolone-related Achilles tendinopathy in heart transplant patients: incidence and risk factors. J Heart Lung Transpl. 2008;27(1):46–51.
Sode J, Obel N, Hallas J, Lassen A. Use of fluroquinolone and risk of Achilles tendon rupture: a population-based cohort study. Eur J Clin Pharmacol. 2007;63(5):499–503.
van der Linden PD, van de Lei J, Nab HW, Knol A, Stricker BH. Achilles tendinitis associated with fluoroquinolones. Br J Clin Pharmacol. 1999;48(3):433–7.
Wilton LV, Pearce GL, Mann RD. A comparison of ciprofloxacin, norfloxacin, ofloxacin, azithromycin and cefixime examined by observational cohort studies. Br J Clin Pharmacol. 1996;41(4):277–84.
Le Huec JC, Schaeverbeke T, Chauveaux, Rivel J, Dehais J, Le Rebeller A. Epicondylitis after treatment with fluoroquinolone antibiotics. J Bone Joint Surg Br. 1995;77(293):295.
Schwald N, Debray-Meignan S. Suspected role of ofloxacin in a case of arthralgia, myalgia, and multiple tendinopathy. Revue du Rhumatisme. 1999;66:419–21.
Williams RJ III, Attia E, Wickiewicz TL, Hannafin JA. The effect of ciprofloxacin on tendon, paratenon, and capsular fibroblast metabolism. Am J Sports Med. 2000;28(3):364–9.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87.
Pouzaud F, Bernard-Beaubois K, Thevenin M, Warnet JM, Hayem G, Rat P. In vitro discrimination of fluoroquinolones toxicity on tendon cells: involvement of oxidative stress. J Pharmacol Exp Ther. 2004;308(1):394–402.
Maffulli N, Sharma P, Luscombe KL. Achilles tendinopathy: aetiology and management. J R Soc Med. 2004;97(10):472–6.
McLauchlan GJ, Handoll HH. Interventions for treating acute and chronic Achilles tendinitis. Cochrane Database Syst Rev 2001;(2):CD000232.
Marie I, Delafenetre H, Massy N, Thuillez C, Noblet C, et al. Tendinous disorders attributed to statins: a study on ninety-six spontaneous reports in the period 1990–2005 and review of the literature. Arthritis Rheum. 2008;59(3):367–72.
Acknowledgments
This study was funded by Team Grant OTG-88591 from the Canadian Institutes of Health Research (CIHR). The authors are grateful for the work of Corinne Holubowich and Pamela Richards, Information Specialists at the Health Sciences Library, Li Ka Shing Knowledge Institute, St. Michael’s Hospital and who conducted the literature searches for this review.
Author Contributions
Dr. Stephenson had full access to all of the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and had final responsibility for the decision to submit for publication. Study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content was carried out by Drs Stephenson and Rochon, Mr. Wu and Mr. Cortes; acquisition of data was undertaken by Drs Stephenson, Wu and Cortes; statistical analysis was carried out by Drs Stephenson and Wu; funding was obtained by Dr Rochon; and study supervision was carried out by Dr Rochon.
Role of the funding source
The study sponsor did not participate in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the paper for publication.
Conflicts of interest
Drs Stephenson and Rochon, Mr. Wu and Mr. Cortes do not have any conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stephenson, A.L., Wu, W., Cortes, D. et al. Tendon Injury and Fluoroquinolone Use: A Systematic Review. Drug Saf 36, 709–721 (2013). https://doi.org/10.1007/s40264-013-0089-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-013-0089-8