Abstract
Purpose
Tendinopathy is a known adverse reaction associated to fluoroquinolones, but a meta-analysis was not yet published. The aim of this study was to conduct a systematic review and a meta-analysis of the scientific evidence evaluating the risk of tendon injury associated with fluoroquinolones.
Methods
A literature search was conducted to identify observational studies which reported results on the risk of Achilles tendon rupture (ATR), risk of Achilles tendinitis (AT), or risk of any tendon disorders (ATD). A meta-analysis was performed by pooling odds ratios (ORs) with their 95% confidence intervals (CIs).
Results
Fifteen studies were included in the meta-analysis. Treatment with fluoroquinolones was associated with an increased risk of ATR (OR 2.52 (95% CI 1.81–3.52), p < 0.001, I2 = 76.7%), an increased risk of AT (OR 3.95 (95% CI 3.11–5.01), p < 0.001, I2 = 0%), and increased risk of ATD (OR 1.98 (95% CI 1.62–2.43), p < 0.001, I2 = 84.5%). The initial risk estimates remained statistically significant among patients aged ≥ 60 years old. Risk estimates did not significantly change after depending on the concomitant use of corticosteroids or studies methodological quality assessment. The analysis according to the type of fluoroquinolones was only possible for ATR, which were ofloxacin and norfloxacin were found to increase the risk of this outcome, but not ciprofloxacin and levofloxacin.
Conclusions
The results of this meta-analysis confirm the risk of tendon injuries associated with fluoroquinolones. Older age and concomitant use of corticosteroids seem to be additional risk factors for tendinopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic fluoroquinolones are commonly used antibiotics, presenting excellent potency in vitro against most Enterobacteriaceae, fastidious gram-negative bacilli including species of Haemophilus and gram-negative cocci [1]. When administered orally, fluoroquinolones have good bioavailability and are generally well tolerated [2]. However, over the years, the use of fluoroquinolones has been associated with serious adverse reactions, leading to the market withdrawal of some of these antibiotics, such as temafloxacin (severe hemolytic–uremic syndrome), trovafloxacin (hepatotoxicity), grepafloxacin (QT interval lengthening), and gatifloxacin (dysglycemia) [2,3,4].
Although rare, tendon injury is a serious adverse effect of systemic fluoroquinolones. In 1995, the US Food and Drug Administration (US FDA) recommended an update of the labeling of all marketed systemic fluoroquinolones to include a warning about the possibility of tendon rupture [5]. In 2008, a boxed warning citing an increased risk of tendinitis and tendon rupture was added to the labeling of systemic fluoroquinolones, based on evidence from post-marketing spontaneous reports and observational studies [6]. The risk of such adverse effects is further increased in patients aged over 60, in transplant recipients, and among individuals on steroid therapy [7].
In 2016, the US FDA reinforced warnings about the risk of tendinopathies associated with the use of systemic fluoroquinolones [6]. Since the risk of these serious adverse effects may outweigh the benefits of systemic fluoroquinolones in the treatment of minor infections, these antibiotics should be reserved for use when no alternative treatment options can be considered [6]. In 2018, the European Medicines Agency (EMA) also recommended new restrictions on the use of systemic fluoroquinolones after reviewing safety information about disabling and potentially long-lasting adverse effects, including tendon injuries [8, 9].
Some systematic reviews of studies evaluating the risk of tendinopathies associated with the use of systematic fluoroquinolones have been carried out [2, 10,11,12]. However, important limitations seem to characterize those works. Not all the observational studies available in the scientific literature were conducted in the previous systematic reviews, particularly the most recently published evidence [9, 13,14,15]. Moreover, although a meta-analysis has been conducted, the results were not stratified according to the different outcomes assessed in the epidemiological studies, different risk windows, studies methodological quality, or type of comparators used [12].
Since fluoroquinolones are essential medicines, it is important, from a public health perspective, to evaluate the consistency of the results from observational studies assessing the risk of tendinopathies. Although meta-analyses may have some limitations, quantitative synthesis is usually preferred over qualitative analysis of the results of a set of studies [16]. Moreover, conducting a meta-analysis may allow assessing the consistency of the results and understanding the influence of risk factors and additional variables in the risk of tendinopathies associated with the use of fluoroquinolones [17, 18].
The aim of this systematic review and meta-analysis is to assess the risk of tendon injuries associated with the use of systemic fluoroquinolones, as well as to explore risk variations due to risk factors, study designs, and methodological quality of included studies.
Methods
The current systematic review and meta-analysis were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [19].
Literature search
PUBMED, EMBASE, and Cochrane Library were searched from its inception until November 2018 in order to identify relevant studies evaluating the risk for tendon injury associated with systemic fluoroquinolones. MeSH and Emtree terms, the MedDRA dictionary (v.21.0), and the International Nonproprietary Names (INN) of fluoroquinolones were used in the search equation [20,21,22]. Only the literature published in the English language was considered for inclusion. Bibliographic references list of all relevant studies, meta-analyses, and systematic reviews were hand searched in order to identify additional eligible articles. We did not seek to identify safety data of fluoroquinolones beyond published studies. The electronic databases search strategy is available in Supplemental Table 1.
Study selection, data extraction, and quality assessment
The titles and abstracts of all retrieved citations were screened by two independent reviewers (C.A. and D.M.) to identify potentially relevant publications. Full texts were retrieved for relevant citations. Discrepancies were resolved by majority decision (two out of three) involving a third investigator (F.B.M.). Studies would be included if they fulfilled the following criteria: 1, being a comparative observational study (case-control or cohort studies); 2, included patients of all ages and genders; 3, compared fluoroquinolones with a placebo (or non-use) or active control; and 4, have provided risk estimates (relative risk (RR), odds ratio (OR), or hazard ratio (HR)) or data allowing to calculate such risk estimates. Only peer-reviewed full papers were considered. Basic science studies, reviews, case reports, and studies without a comparison group were excluded.
The following data from the studies was extracted: design, characteristics of participants, treatments under evaluation, risk-window length, and estimated effect measures.
Studies’ methodological quality was assessed through Newcastle–Ottawa scale, which considers the following characteristics: selection of the study groups, comparability of the groups, ascertainment of either the exposure (for case-control studies), or outcome of interest (for cohort studies) [23]. A maximum of one point for each item within the “Selection” and “Exposure/Outcome” categories could be awarded. For “Comparability” category, a maximum of two points could be awarded. The summary score equals the number of points earned by each study, totaling a maximum of 9 points. Studies scoring ≥ 8 points were considered to be of good quality, those scoring < 8 and ≥ 6 points were of moderate quality, and those scoring < 6 points were considered to have poor quality. Two independent reviewers assessed the methodological quality of the studies (C.A. and D.M.). A third investigator (F.B.M.) helped to resolve discrepancies by majority decision. When more than one reference was found for the same study, the methodological quality evaluation was based on the total set of information.
Outcomes assessed
The following outcomes were assessed in this meta-analysis: risk of Achilles tendon rupture (ATR), risk of Achilles tendinitis (AT), and risk of any tendon disorders (ATD). The outcomes selected for this systematic review and meta-analysis were the same as those considered in a previous systematic review [10]. The ATR and AT are the most frequently evaluated tendon injury outcomes in published studies. The ATD is a composite outcome of any tendon rupture and/or any tendinitis.
Statistical analysis
A meta-analysis was performed by pooling odds ratios (ORs) with their 95% confidence intervals (CIs), using the DerSimonian and Laird random-effects model [24]. This model was chosen since the validity of tests of heterogeneity can be limited with a small number of component studies and it is more conservative than a fixed effect model in the presence of between-studies heterogeneity. If the studies presented more than one risk estimate, the most adjusted one would be used. The effect size estimates available for the shortest time intervals between fluoroquinolones prescription and tendon injury diagnosis or treatment were used in the overall risk estimate. The I2 statistic test was used to assess heterogeneity between studies. An I2 estimate > 50% was considered indicative of substantial heterogeneity [25]. The publication bias was visually examined by a funnel plot and statistically evaluated by Egger’s regression asymmetry test [26].
A sensitivity analysis was conducted to explore the influence of the following variables on the summary estimates: study designs, studies’ methodological quality scores, risk windows defined in included studies, age (≥ 60 vs. < 60 years old), type of fluoroquinolone, corticosteroid use, kidney disease, and comparator (fluoroquinolone non-users vs. active comparator). Risk window is the period of time considered after the end of fluoroquinolone treatment course during which patients are at risk of suffering tendon injuries. The influence of each individual study in overall risk estimate was assessed using the “one-study removed” analysis. All reported p values are 2-sided with significance being set as less than 0.05. Stata (version 13.1) was used to perform statistics.
Results
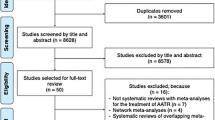
Figure 1 presents the flow of the search strategy criteria. The electronic databases searched returned 1612 references. After excluding duplicates and other studies with inadequate design, 15 studies were included in this meta-analysis comprising seven cohort studies [15, 27,28,29,30,31,32] and eight case-control studies [9, 13, 14, 33,34,35,36,37].
Studies characteristics
The main characteristics of the studies are presented in Table 1. Study design, participants’ demographic characteristics, drugs under evaluation, and a number of subjects evaluated were described. Patients’ mean age varied from 43.5 to 65.0 years and the proportion of female patients included in the studies ranged from 22.1 to 71.4%. Most of the studies elected the population sample from either prescription dispensing data, health administrative data, or general practice data, although one study examined a post-transplant population (heart) and one study evaluated patients with end-stage renal disease [30, 32]. Only one study used propensity score to match participants [31], while the remaining adjusted the analyses for several variables. Five studies compared the risk of tendon injuries in fluoroquinolone users with active controls (other antibiotics), while the remaining compared fluoroquinolone users with non-users [15, 27,28,29, 31]. Thirteen studies defined a risk window of 30 days between fluoroquinolone exposure and outcome development. Two studies adopted a 90-days risk period and the other two did not report the length of time during which the outcome was assessed. Nine studies assessed the risk of ATR, three the risk of AT, and eight the risk of ATD.
The results of the assessment of studies’ methodological quality are also presented in Table 1. The methodological quality was assessed as “good” for three studies, “moderate” for nine, and “poor” for three. Lack of information on confounding variables used to adjust risk estimates was the main methodological impairment found among these studies.
Risk for tendon injuries
The treatment with fluoroquinolones was associated with an increased risk of ATR (9 studies, OR 2.52 (95% CI 1.81–3.52), p < 0.001, I2 = 76.7%) (Fig. 2; Table 2). Fluoroquinolones were also associated with an increased risk of AT (3 studies, OR 3.95 (95% CI 3.11–5.01), p < 0.001, I2 = 0%) and of ATD (8 studies, OR 1.98 (95% CI 1.62–2.43), p < 0.001, I2 = 84.5%) (Fig. 3 and Fig. 4, respectively). The risk estimates did not significantly change when the analysis was stratified according to different study designs, with the exception of the risk of AT among cohort studies (1 study, OR 3.70 (95% CI 0.90–15.16), p = 0.069). The increased risk of all three outcomes remained statistically increased among older patients (aged ≥ 60 years) when the analysis was stratified by age. The risks estimated for any of the three outcomes did not significantly change irrespective of the concomitant use of corticosteroids and fluoroquinolones.
It was not possible to stratify the results according to renal failure status, since only two studies assessed the risk of tendinopathy associated with fluoroquinolones in patients with renal insufficiency, one considering a different outcome from the other (ATR vs. AT) [30, 35]. One identified an increased risk of AT (OR 20.0, 95% CI 2.7–149) and the other increased risk of ATR (RR 1.65, 95% CI 1.32–1.84) [30, 35].
The risk of AT did not significantly change when the analysis was conducted according to the methodological quality of the studies (Table 2). The risk of ATR remained increased, with statistical significance, among the studies with “moderate” methodological quality. Among the studies with “good” methodological quality, the risk of ATR became statistically non-significant but associated with high heterogeneity (OR 1.94, 95% CI 0.76–4.99, p = 0.147, I2 = 91.0%). The risk of ATD became statistically non-significant when only studies with “poor” methodological quality were considered (OR 3.04, 95% CI 0.68–13.52, p = 0.144, I2 = 72.0%).
No statistically significant result was identified among the studies evaluating the risk of ATR more than 30 days after the fluoroquinolone prescription (OR 1.72, 95% CI 0.70–4.25, p = 0.238, I2 = 18.2%) (Table 2). The initial ATR risk estimate did not significantly change among the studies considering a 30-day at-risk window after the fluoroquinolone prescription.
When comparing fluoroquinolone users with non-users, the initial increased risk estimates remained statistically significant (Table 2). Only one study compared fluoroquinolones with an active comparator for both ATR and AT risk analyses (Table 2). The initial ATD risk estimate did not significantly change when the analysis was stratified by different comparators.
The stratification of the risk estimates according to the types of fluoroquinolones was only possible for the outcome ATR (Table 2). Ofloxacin and norfloxacin were associated with an increased risk of ATR, but not ciprofloxacin and levofloxacin. For AT and ATD, no sufficient data was available to stratify the results according to the type of fluoroquinolone.
Among the results identified for the three outcomes, the “one-study removed” analysis indicates that the overall risk estimates did not significantly change when each study was individually removed from the meta-analysis (Supplemental Figs. 1, 2, and 3).
Publication bias
Egger’s asymmetry test was not statistically significant for the outcomes AT and ATD. Evidence of small-study effects was identified among the studies assessing the risk of ATR (p = 0.024). Subjective evaluation of publication bias was based on the visual inspection of the funnel plot. Most studies did identify increased risk estimates for the three outcomes, although studies with less precise risk estimates have been identified as well (Supplemental Figs. 4, 5, and 6).
Discussion
The benefits of antibiotic therapy with systemically administered fluoroquinolones are well recognized. However, the use of this class of antibiotics is associated with rare but serious adverse effects that may result in significant impairment of patients, namely tendinitis and tendon rupture, particularly of the Achilles tendon. Despite being known for some time, the risk of tendon injuries associated with fluoroquinolones has been continuously investigated by the scientific community, mainly through the conduction of several pharmacoepidemiological studies [9, 13, 15]. Moreover, EMA and US FDA have recently recommended reinforcing the restriction of the use of fluoroquinolones due to their disabling and potentially permanent adverse effects involving muscles, tendons or joints, and the nervous system [6, 8, 9]. This systematic review and meta-analysis identified increased risks of ATR, AT, and ATD associated with fluoroquinolones. The findings are in line with the current knowledge about the safety profile of these drugs.
The mechanism of tendon injury associated with fluoroquinolones is not well clarified but appears to be multifactorial and may result from direct toxicity and degenerative changes in collagen fibers [10, 28]. It was demonstrated that fluoroquinolones can enhance matrix metalloproteinase expression and the inhibition of both fibroblasts proliferation and the synthesis of the matrix, which can result in the development of tendinopathies [38, 39]. A study conducted in dogs assessed the hypothesis of fluoroquinolones having chelate properties [40]. The findings suggested that fluoroquinolone-induced toxic effects on connective tissues may be due to the magnesium-antagonistic effects of these antibiotics [40]. Additionally, there is evidence supporting that fluoroquinolone-associated tendinopathy may result from oxidative stress induction [41].
According to this meta-analysis, patients aged ≥ 60 years old are at an increased risk for ATR and AT. These results are in line with previous warnings issued by regulatory authorities, which recommended that fluoroquinolones should be used with special caution in older patients [6, 8]. The influence of concomitant use of corticosteroids on the risk of tendon injury in patients treated with fluoroquinolones was also evaluated in some pharmacoepidemiological studies. According to the sensitivity analysis, it was observed an increase in risk sizes between 4.68-fold (ATD) and 14.72-fold (ATR) for the three outcomes among patients simultaneously using fluoroquinolones and corticosteroids, compared with the initial estimates. Therefore, the combination of these drugs should be avoided, as previously recommended by drug regulatory agencies [6, 8].
The observational studies included in this meta-analysis presented varying demographic characteristics and methodologies. Two cohort studies investigating the risk of tendon injuries associated with fluoroquinolones included heart transplanted patients and end-stage renal disease patients [30, 32]. It has been suggested that individuals submitted to organ transplant and patients with renal insufficiency are at greater risk of suffering tendon injuries, particularly Achilles tendon ruptures and tendinitis [30, 32]. Gender distribution of the population samples was significantly different among the studies. There is conflicting evidence regarding the influence of patients’ sex in the risk of tendon injuries. There are studies suggesting that one specific gender, male or female, may be a risk factor for tendon injury, while others have not [33,34,35]. Although renal impairment is a known risk factor for tendon injury, it was not possible to conduct a sensitivity analysis due to the scarcity of data. Only two studies assessed the risk of tendinopathy associated with fluoroquinolones in patients suffering from renal insufficiency, but considered two different outcomes (ATR vs. AT), precluding a quantitative synthesis of the results [30, 35].
Most of the studies assessing the risk of ATR due to fluoroquinolones included in this meta-analysis considered risk-window periods of 30 days. Yet, two studies assumed risk-window periods of 90 days and two other studies did not report the length of time during which patients were considered to be at risk. The sensitivity analysis demonstrated that the risk of ATR remained statistically increased when the analysis was restricted to studies considering a 30-day risk window, but not among those considering a risk period of 90 days. A systematic review of case reports concluded that 40% of the cases of tendon rupture were diagnosed within the first 6 days after treatment initiation, while the mean time to onset was estimated at 26 days [2]. Additionally, half of the cases of tendinitis occurred within the first 6 days of treatment and the mean time to onset of symptoms after the initiation of fluoroquinolone therapy was 18 days [2]. The studies included in this meta-analysis assessed the risk of AT and ATD within a risk period of 30 days.
The control groups used by the observational studies included in this meta-analysis differed as well. Although non-use of fluoroquinolones was the most common comparator group, four cohort studies used active controls [15, 27,28,29]. Noteworthy, it is possible that observational studies using fluoroquinolone non-users as control groups may have been affected by confounding-by-indication, namely if the infections were causally related to the tendon injuries events [15]. Therefore, some authors established comparisons with active controls. Yet, the studies included in this meta-analysis used different antibiotics as active controls. Some compared fluoroquinolone users with a composite of multiple antibiotics, such as nitrofurantoin, amoxicillin, tetracyclines, and trimethoprim, which are mostly indicated to treat mild urinary tract infections [15, 27, 28]. Two studies used antibiotics as negative controls in order to assess the specificity of the association between fluoroquinolones and tendinopathy [9, 15]. Nevertheless, the risk of tendon injuries seems to increase due to fluoroquinolones use, regardless of the chosen comparator.
Only three out of the 15 studies were assessed as having “good” methodological quality. Most of them did not take into account all the factors known to modify the risk of tendon injuries, such as trauma, transplant, obesity, diabetes mellitus, kidney failure, or recreational sports. The reduced risk of confounding was deemed to be important in order to attain higher methodological scores. These may have been the reason why previous systematic reviews using the same methodological quality assessment tool awarded higher scores to some of the studies that were also included in this systematic review and meta-analysis [10, 12].
The systematic review and meta-analysis conducted by Yu and colleagues (2019) have identified an increased risk of tendinopathy associated with fluoroquinolones as well [12]. However, some methodological approaches which could strengthen the analysis were not considered by the authors, namely the stratification of the results according to different outcomes (AT, ATR, or ATD), study designs and methodological quality scores and risk windows. Furthermore, some studies have not been included in the meta-analysis, and the results of a retrospective cohort study considering both tendon and joint disorders as an outcome were integrated into the risk estimate analysis, potentially increasing the confounding among the final results [12].
Systematic reviews and meta-analyses of non-experimental studies should be designed to explore eventual sources of heterogeneity among the risk estimates rather than to find evidence of causative associations [18]. With the exception of the subgroup analyses according to different comparators, all the risk estimates were associated with significant between-studies heterogeneity. These observational studies were conducted and published over two decades. Therefore, these results should be taken with precaution due to the inherent methodological limitations faced when comparing studies conducted from different databases and associated with varying size and precision [9]. Moreover, besides the different demographics previously described, there are additional methodological characteristics of the studies included in this meta-analysis which may not be entirely comparable, namely the endpoint’ definition and methods of diagnosis of tendon injuries, the validation of fluoroquinolones’ exposure, and the controlling for confounding variables.
There are additional limitations which should be addressed. Most of the studies did not detail the risk estimates by the type of fluoroquinolone and no sufficient data was provided in order to assess if there is a dose-response relationship associated with the development of tendon injuries. Future research should address these issues, since clinicians could benefit from such information in order to minimize and prevent the risk of these adverse effects [31]. Studies most frequently stratified their results according to patient’s age and concomitant use of corticosteroids. However, additional risk factors for tendon injuries were not considered, precluding additional sensitivity analyses. Only three bibliographic databases were searched. Since tendon injuries have been associated with fluoroquinolones for so long, studies published in journals indexed elsewhere may not have been identified through this search strategy. A previous systematic review considered evidence from case series, cross-sectional, and surveillance pharmacovigilance studies using spontaneous reporting when assessing the risk of tendon injuries associated with fluoroquinolones. However, the methodological quality of such studies was considered to be very low by the authors [10]. Therefore, in order to reduce the risk of bias, other designs than case control and cohorts were not considered for inclusion in this meta-analysis.
Conclusion
The results of this systematic review and meta-analysis suggest that fluoroquinolones increase the risk of tendon injuries, which is in line with the current knowledge. The use of these antibiotics should be restricted since several subgroups of patients are at increased risk to develop serious tendon injuries, particularly older patients and individuals under concomitant treatment with corticosteroids. Moreover, patients should discontinue the treatment with fluoroquinolones and seek help from healthcare professionals when they start feeling tendon pain and inflammation.
References
Wolfson JS, Hooper DC (1991) Fluoroquinolone antimicrobial agents. N Engl J Med 324:384–394
Khaliq Y, Zhanel GG (2003) Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clin Infect Dis 36:1404–1410
Tomé AM, Filipe A (2011) Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf 34:465–488
Poluzzi E, Raschi E, Motola D, Moretti U, De Ponti F (2010) Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA adverse event reporting system. Drug Saf 33:303–314
Pierfitte C, Gillet P, Royer RJ (1995) More on fluoroquinolone antibiotics and tendon rupture. N Engl J Med 332:193
US US Food and Drug Administration. FDA News Release. U.S. Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm612995.htm. Accessed 14 May 2019
US Food and Drug Administration. Postmarket Drug Safety Information for Patients and Providers. http://wayback.archive-it.org/7993/20170112032310/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm. Accessed 14 May 2019
European Medicines Agency. Disabling and potentially permanent side effects lead to uspension or restrictions of quinolone and fluoroquinolone antibiotics. https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products. Accessed 14 May 2019
Morales DR, Slattery J, Pacurariu A, Pinheiro L, McGettigan P, Kurz X (2019) Relative and absolute risk of tendon rupture with fluoroquinolone and concomitant fluoroquinolone/corticosteroid therapy: population-based nested case-control study. Clin Drug Investig 39:205–213
Stephenson AL, Wu W, Cortes D, Rochon PA (2013) Tendon injury and fluoroquinolone use: a systematic review. Drug Saf 36:709–721
van der Vlist AC, Breda SJ, Oei EHG, Verhaar JAN, de Vos RJ (2019) Br J Sports Med. https://doi.org/10.1136/bjsports-2018-099991
Yu X, Jiang DS, Wang J, Wang R, Chen T, Wang K, Cao S, Wei X (2019) Fluoroquinolone use and the risk of collagen-associated adverse events: a systematic review and meta-analysis. Drug Saf. https://doi.org/10.1007/s40264-019-00828-z
Persson R, Jick S (2019) Clinical implications of the association between fluoroquinolones and tendon rupture: the magnitude of the effect with and without corticosteroids. Br J Clin Pharmacol 85:949–959
Nyyssönen T, Lantto I, Lüthje P, Selander T, Kröger H (2018) Drug treatments associated with Achilles tendon rupture. A case-control study involving 1118 Achilles tendon ruptures. Scand J Med Sci Sports 28:2625–2629
Daneman N, Lu H, Redelmeier DA (2015) Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open 5:e010077
Ioannidis JP, Patsopoulos NA, Rothstein HR (2008) Reasons or excuses for avoiding meta-analysis in forest plots. BMJ 336:1413–1415
Smith TC, Spiegelhalter DJ, Thomas A (1995) Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med 14:2685–2699
Berlin JA (1995) Invited commentary: benefits of heterogeneity in meta-analysis of data from epidemiologic studies. Am J Epidemiol 142:383–387
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
U.S. National Library of Medicine. Medical Subject Headings 2019. U.S. National Library of Medicine. https://meshb.nlm.nih.gov/search. Accessed 14 May 2019
ICH MedDRA Maintenance and Support Services Organization (MSSO). International Council for Harmonisation of technical requirements for Pharmaceuticals for Human use (ICH). https://www.meddra.org/. Accessed 14 May 2019
Elsevier. Embase Indexing and Emtree. https://www.elsevier.com/solutions/embase-biomedical-research. Accessed 14 May 2019
Wells GA, Shea B, O’Connell D et al (2014). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 14 May 2019
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
B Publication bias (2009) In: Borenstein M, Hedges LV, JPT H, Rothstein HR (eds) Introduction to meta-analysis. Wiley, Chichester, pp 277–291
van der Linden PD, van de Lei J, Nab HW, Knol A, Stricker BH (1999) Achilles tendinitis associated with fluoroquinolones. Br J Clin Pharmacol 48:433–437
Sode J, Obel N, Hallas J, Lassen A (2007) Use of fluroquinolone and risk of Achilles tendon rupture: a population-based cohort study. Eur J Clin Pharmacol 63:499–503
Hori K, Yamakawa K, Yoshida N, Ohnishi K, Kawakami J (2012) Detection of fluoroquinolone-induced tendon disorders using a hospital database in Japan. Pharmacoepidemiol Drug Saf 21:886–889
Humbyrd CJ, Bae S, Kucirka LM, Segev DL (2018) Incidence, risk factors, and treatment of Achilles tendon rupture in patients with end-stage renal disease. Foot Ankle Int 39:821–828
Jupiter DC, Fang X, Ashmore Z, Shibuya N, Mehta HB (2018) The relative risk of Achilles tendon injury in patients taking quinolones. Pharmacotherapy 38:878–887
Barge-Caballero E, Crespo-Leiro MG, Paniagua-Martín MJ, Muñiz J, Naya C, Bouzas-Mosquera A, Piñón-Esteban P, Marzoa-Rivas R, Pazos-López P, Cursack GC, Cuenca-Castillo JJ, Castro-Beiras A (2008) Quinolone-related Achilles tendinopathy in heart transplant patients: incidence and risk factors. J Heart Lung Transplant 27:46–51
van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HM, Rowlands S, Stricker BH (2003) Increased risk of Achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids. Arch Intern Med 163:1801–1807
Seeger JD, West WA, Fife D, Noel GJ, Johnson LN, Walker AM (2006) Achilles tendon rupture and its association with fluoroquinolone antibiotics and other potential risk factors in a managed care population. Pharmacoepidemiol Drug Saf 15:784–792
Wise BL, Peloquin C, Choi H, Lane NE, Zhang Y (2012) Impact of age, sex, obesity, and steroid use on quinolone-associated tendon disorders. Am J Med 125:1228.e23–1228.e28
Corrao G, Zambon A, Bertù L et al (2006) Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf 29:889–896
van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HG, Stricker BH (2002) Fluoroquinolones and risk of Achilles tendon disorders: case-control study. BMJ 324:1306–1307
Williams RJ 3rd, Attia E, Wickiewicz TL, Hannafin JA (2000) The effect of ciprofloxacin on tendon, paratenon, and capsular fibroblast metabolism. Am J Sports Med 28:364–369
Tsai WC, Hsu CC, Chen CP et al (2011) Ciprofloxacin up-regulates tendon cells to express matrix metalloproteinase-2 with degradation of type I collagen. J Orthop Res 29:67–73
Shakibaei M, de Souza P, van Sickle D, Stahlmann R (2001) Biochemical changes in Achilles tendon from juvenile dogs after treatment with ciprofloxacin or feeding a magnesium-deficient diet. Arch Toxicol 75:369–374
Pouzaud F, Bernard-Beaubois K, Thevenin M, Warnet JM, Hayem G, Rat P (2004) In vitro discrimination of fluoroquinolones toxicity on tendon cells: involvement of oxidative stress. J Pharmacol Exp Ther 308:394–402
Author information
Authors and Affiliations
Contributions
Carlos Alves conceived and designed the study, performed research, analyzed data, and wrote the paper. Diogo Mendes and Francisco Batel Marques conceived the study and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Figure 1
(DOCX 21 kb)
Supplemental Figure 2
(DOCX 17 kb)
Supplemental Figure 3
(DOCX 21 kb)
Supplemental Figure 4
(DOCX 15 kb)
Supplemental Figure 5
(DOCX 15 kb)
Supplemental Figure 6
(DOCX 16 kb)
Supplemental Table 1
(DOC 64 kb)
Supplemental Table 2
(DOCX 17 kb)
Supplemental Table 3
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Alves, C., Mendes, D. & Marques, F.B. Fluoroquinolones and the risk of tendon injury: a systematic review and meta-analysis. Eur J Clin Pharmacol 75, 1431–1443 (2019). https://doi.org/10.1007/s00228-019-02713-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02713-1